Abstract
Introduction: Hepatocellular adenoma (HCA) is a rare noninvasive adenoma of the liver, occasionally observed in childbearing-aged women who use contraceptive pills (85% of which contain steroids) and is infrequent in children. HCA can cause several complications such as hemorrhage (20?25%) or proceed to malignancy (4?10%), especially when the tumor size is greater than 5 cm. The clinical manifestation of HCA is variable, from asymptomatic to tumor rupture. Most cases admitted to hospitals are due to mild atypical abdominal discomfort. HCA is classified into four types, based on molecular behavior, HNF-1a inactive, b -catenin activated, inflammatory HCA, and unclassified HCA. Among them, the b -catenin activated subgroup carries the highest risk of hemorrhage and malignant transformation.
Case presentation: We report a 16-year-old boy who presented with right upper quadrant pain. Abdominopelvic computed tomography revealed a large hepatocellular adenoma located in lobules V, VI, VII and VIII, with normal laboratory tests. A neovascularized liver tumor, 18x20x22 cm in size, situated in lobules V, VI, VII, and VIII was detected and removed surgically. Immunopathology results indicated a b -catenin activated hepatocellular adenoma.
Conclusion: HCA is a very rare noninvasive adenoma in children. The b -catenin activated subgroup comprises 15? 20% of HCA cases, and is related to male hormone exposure. Surgical resection is typically recommended due to their high tendency to hemorrhage or become malignant. This case is exceptionally rare and can be challenging to diagnose.
Introduction
Although liver tumor in children can be malignant, some tumors remain benign, including the rare hepatocellular adenoma, which has an incidence of only 3–4 cases per 100,000 people1, 2. Interestingly, hepatocellular adenoma is much more common in females, with a male-to-female ratio of 1:103.
Due to the potential for complications such as bleeding and metastasis, the diagnosis and management of hepatocellular adenoma are critical. Ruptured HCAs can cause stabbing abdominal pain, hypotension and/or shock, potentially leading to increased mortality rates1. The recommended treatment for male and female patients whose tumor size is larger than 5 cm is surgical resection2, 4.
CASE REPORT
A 16-year-old male patient was admitted to Children Hospital 2 on January 1st 2022, due to severe right upper quadrant abdominal pain that had been ongoing for two days. He vomited twice a day, with no bile or blood present in the vomit, and had no fever or weight loss. His medical record indicated that he suffered similar abdominal pain in November 2021, was diagnosed with a liver tumor, and underwent a biopsy procedure resulting in a diagnosis of hepatocellular adenoma. The family history revealed that his aunt had hepatocellular carcinoma and his uncle had colon cancer. Upon examination, the patient presented with mild jaundice, yellow eyes, an enlarged liver, and was tender to the touch.
Laboratory results were 33, 31,95, and 56 U/L for AST, ALT, ALP, and GGT respectively, 22.7 and 13.0 mmol/L for total and conjugated bilirubin, 2.12 mg/L for AFP, 189 U/L for LDH, and 484 mmol/L for uric acid, and both complete blood count and C-reactive protein were within normal ranges. Abdominal ultrasonography revealed a large mass in the right portion of the liver, 171x148x220 mm in size, which was compressing the hepatic and portal veins, and pushing down the right kidney. A contrast abdominopelvic computed tomography scan revealed a large, well-demarcated solid mass involving Couinaud segments V, VI, VII, and VIII, the dimensions of which were 120x170x220mm. The mass was infiltrated and heterogeneous, but absent of hemorrhaging, fat, or calcification. The tumor was also delineated and unruptured. In the arterial phase, there were some areas of hyperattenuation, resembling normal liver, while other regions were hypoattenuating. In the portal phase, most of the tumor had similar density to the normal liver, except for areas of necrosis, which showed faint enhancement in both phases. Large subcapsular feeding vessels surrounded the tumor, and large penetrating vessels rising from the proper hepatic artery were also present. The mass compressed and pushed down the right kidney, shifting the right renal vein and inferior vena cava to the left, but did not invade the portal vein or inferior vena cava. A post-contrast chest CT scan revealed no evidence of lung lesions, and there were no enlarged lymph nodules around the liver hilum or evidence of any extrahepatic disease contiguous with the tumor. The celiac trunk, common hepatic artery, and proper hepatic artery were dilated, consistent with the enlargement of the feeding artery of the tumor (Figure 1).
The patient underwent a liver tumor excision on January 4th, 2022, four days after being admitted to the hospital.
During the operation, a Mercedes-line skin incision measuring 30 cm was made. The tumor was 18 x 20 x 22 cm in size, occupied lobules V, VI, VII, and VIII, and was neovascularized. We proceeded to snare the right liver hilum vessels, dissect and excise the entire right section of the liver, and ligate the right liver veins, right portal veins, and right bile duct. To remove the tumor, we used a cavitron ultrasonic surgical aspirator and bipolar techniques to cut out the portion of the liver containing the tumor, while also clipping the transverse vessels (Figure 2). Following the procedure, subhepatic drainage was placed, and the patient was transfused a 250 mL pack of red blood cells during the procedure.
After the operation, the patient was carefully monitored in the intensive care unit for a period of four days. During this time, the patient experienced a fever with temperatures reaching up to 38.9°C. However, the patient reported no nausea or vomiting and did not experience any abdominal discomfort. The subhepatic drainage contained reddish fluid, and the volume gradually decreased from 310 mL to 173 mL, and eventually to 90 mL, indicating a positive recovery. The drainage was successfully removed six days after the operation.
Lab tests:
AST/ALT: 414/482 → 201/408 → 100/276 → 24/119 → 18/38 U/L (POD 10)
Bilirubin total/conjugated: 35/18.8 → 56.4/25.7 → 39.1/19.6 → 9.7/5.3 umol/L (POD10) C — reactive protein: 100 → 40 mg/L
Ultrasonography revealed a reduction in the volume of right subdiaphragmatic fluid accumulation. The patient experienced elevated levels of liver enzymes and bilirubin post-operation, which gradually returned to normal levels within 10 days.
The patient was provided with supportive treatment, including ventilation support for two days followed by insertion of a nasal cannula, fluid optimization, and antibiotic therapy with cefotaxime, metronidazole, and vancomycin. The patient was given internal feeding three days after the operation, did not require any blood transfusions during treatment, and was discharged after 13 days.
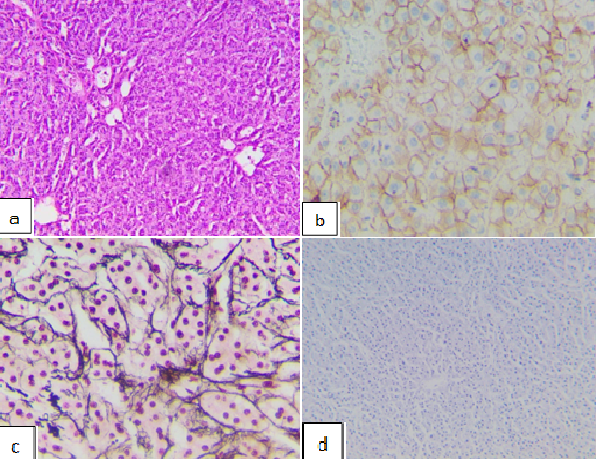
Post-operative histological results revealed the arrangement of hepatocytes in cellular plate structures, scattered biliary infiltration within and between hepatocytes, and no portal veins or central lobular veins, indicating hepatocellular adenoma. Additionally, immunohistochemistry results showed that the tumor was recticulin (+), CD34 (+) diffused, glypican-3 (−), β-catenin (+), and Ki67 (−), indicating the β‑catenin activated subgroup of hepatocellular carcinoma (Figure 3).
DISCUSSION
Hepatocellular adenoma is a relatively asymptomatic condition, often detected incidentally during imaging studies. In some cases, patients may experience mild abdominal pain with no known cause. Changes in biochemical tests include elevated alkaline phosphatase and gamma-glutamyl transferase. In the case of HCC transformation, α-fetoprotein (AFP) may be elevated5. Hepatomegaly and tenderness may be present. When a hepatocellular adenoma is ruptured, acute bleeding occurs, and the patient may experience a throbbing abdominal pain with hypotension and/or shock. The liver becomes enlarged with a smooth surface but does not become rigid. Ruptured tumors are occasionally larger than 5 cm, and may be close to Glisson’s capsule2.
Hepatocellular adenoma is usually a solitary tumor which is brownish to yellowish in color, soft, and has a smooth edge but no capsule. Hepatocellular adenoma has a pseudocapsule formed by hepatocytes which are compressed by the normal surrounding hepatic tissue. The tumor connects to arterioles and contains no bile ducts; these characteristics help to differentiate the tumor from normal hepatic tissue.
Ultrasonography cannot differentiate between benign and malignant tumors, although Doppler ultrasound can reveal hyperactive arteriolar flow along the tumor’s borders. Contrast MRI is the best imaging modality to diagnose hepatocellular adenoma and can differentiate between hepatocellular adenoma and other benign or malignant liver tumors. The tumor may have a central edge that is well delineated, with parallel peripheral vessels6. There may also be many peripheral vessels with central necrosis. CT scans are also a useful imaging modality.
Hepatocellular adenoma categorization2, 3, 5, 7, 8:
Conservative management is the first-choice treatment for solitary or contraceptive-related tumors that are less than 5 cm in size. Cessation of contraceptive pills and imaging study follow up are the main stays of management, which often reduces the size of the tumor9.
Tumor removal surgery is recommended for all male patients regardless of the size of the tumor, and for female patients whose tumors are larger than 5 cm6. The removal does not require large scale excision or local lymphadenectomy. Selectional dissection has a mortality rate below 1%. Emergency surgery due to a hemorrhagic ruptured tumor has a mortality rate of 5–10%10.
Transarterial embolization (TAE) is recommended for complicated hemorrhagic hepatocellular adenoma. Patients who have intraabdominal bleeding rarely have unstable hemodynamics, which allows transarterial embolization before adenomectomy. TAE is administrated within 2–3 days of the hemorrhagic event2, 10.
Radiofrequency ablation is only suitable for selected patients. This treatment is not favored for hormone-sensitive tumors, patients with underlying liver disease, or those who wish to bear a child. This method should be used only for tumors smaller than 4 cm2.
Hepatocellular adenoma prognosis does not have definite criteria, but depends on whether the patient uses contraceptive pills or complications such as hemorrhage or malignant transformation. We found that reports of the β-catenin activated subgroup of HCA are extremely rare.
In 2015, Richard A. Rosencrantz et al. reported a case of giant hepatocellular adenoma in a thirteen-year-old boy that had some similarities to our case11. The liver tumor was revealed by ultrasound without significant symptoms and was non-tender but firm on physical examination.
Laboratory results were within normal range and MRI showed a large heterogenous mass in the right lobe of the liver which was inferiorly displacing the right kidney. A liver biopsy showed features consistent with hepatocellular adenoma. The patient underwent an extended right lobe resection and hepatectomy, which successfully removed the tumor in its entirety. The immunohistochemical stains supported the diagnosis of the β-catenin activated subtype.
A recent case report by Young Kwon Koh et al. in 2022 described a rare case of a large hepatocellular adenoma presenting with iron deficiency anemia in a fourteen-year-old boy12. The patient was admitted to hospital due to nonresponse to iron therapy and weight loss. Laboratory examination revealed low hemoglobin levels although other tests were normal, and imaging studies revealed an arterial enhanced mass in the left lateral segment with peripheral hypervascularity. He underwent an open left lateral segmentectomy, and immunohistochemical analysis confirmed a diagnosis of β-catenin activated hepatocellular adenoma.
CONCLUSION
Hepatocellular adenoma is a very rare, noninvasive tumor of the liver that is predominantly diagnosed in women of reproductive age who are taking oral contraceptives. It is much less common in children. HCA categorization based on phenotype and genotype appears useful. The β-catenin activated subtype comprises 15–20% of HCA, and is related to male hormone exposure. Since this subtype has a high tendency to become malignant, surgical resection is recommended for these tumors. In cases of solitary tumors or tumors less than 5 cm in size, cessation of oral contraceptive pills can lead to self-resolution of the tumor, and regular imaging studies are recommended to monitor the size of the tumor. Surgical resection is recommended for all tumors in males and all tumors larger than 5 cm, but lymphadectomy and wide edge dissection are not necessary.
Abbreviations
CTscan: Computed tomographyHCA: Hepatocellular adenoma TAE: Transarterial embolization
Acknowledgments
We are grateful for our colleagues at the Department of Hemato-Oncology and Department of General Surgery, Children’s Hospital 2, Ho Chi Minh City for their great assistance.
Author’s contributions
Truong Dinh Khai and Tran Thi Phuong carried out the experiment. Tran Thi Phuong wrote the manuscript with support from Truong Dinh Khai and Phung Nguyen Viet Hung. Le Minh Huy contributed to the interpretation of histological result. Nguyen Ngoc Minh Khanh interpreted abdominopelvic computed tomography result. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
None.
Ethics approval and consent to participate
This study was conducted in accordance with the amended Declaration of Helsinki. The institutional review board approved the study, and all participants provided written informed consent.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
References
-
Romailler
É.,
Moradpour
D.,
Sempoux
C.J.R.M.S.,
Hepatocellular adenoma: update 2020. Revue Medicale Suisse.
2020;
16
(704)
:
1554-1559
.
PubMed Google Scholar -
Shreenath
A.P.,
Kahloon
A.,
Hepatic Adenoma. In: StatPearls. StatPearls Publishing, Treasure Island (FL); 2022.
.
-
T. Mounajjed,
Hepatocellular adenoma and focal nodular hyperplasia. Clinical Liver Disease.
2021;
17
(4)
:
244
.
View Article PubMed Google Scholar -
Tsilimigras
D.I.,
Rahnemai-Azar
A.A.,
Ntanasis-Stathopoulos
I.,
Gavriatopoulou
M.,
Moris
D.,
Spartalis
E.,
Current Approaches in the Management of Hepatic Adenomas. Journal of Gastrointestinal Surgery.
2019;
23
(1)
:
199-209
.
View Article PubMed Google Scholar -
Torbenson
M.,
Hepatic Adenomas: Classification, Controversies, and Consensus. Surgical Pathology Clinics.
2018;
11
(2)
:
351-66
.
View Article PubMed Google Scholar -
Fodor
M.,
Primavesi
F.,
Braunwarth
E.,
Cardini
B.,
Resch
T.,
Bale
R.,
Indications for liver surgery in benign tumours. European Surgery.
2018;
50
(3)
:
125-31
.
View Article PubMed Google Scholar -
Dharmana
H.,
Saravana-Bawan
S.,
Girgis
S.,
Low
G.,
Hepatocellular adenoma: imaging review of the various molecular subtypes. Clinical Radiology.
2017;
72
(4)
:
276-85
.
View Article PubMed Google Scholar -
Dhingra
S.,
Fiel
M.I.,
Update on the new classification of hepatic adenomas: clinical, molecular, and pathologic characteristics. Archives of Pathology & Laboratory Medicine.
2014;
138
(8)
:
1090-7
.
View Article PubMed Google Scholar -
Thapar
M.,
Grapp
O.,
Fisher
C.,
Management of hepatic adenomatosis. Current Gastroenterology Reports.
2015;
17
(3)
:
12
.
View Article PubMed Google Scholar -
Karkar
A.M.,
Tang
L.H.,
Kashikar
N.D.,
Gonen
M.,
Solomon
S.B.,
Dematteo
R.P.,
Management of hepatocellular adenoma: comparison of resection, embolization and observation. HPB : The Official Journal of the International Hepato Pancreato Biliary Association.
2013;
15
(3)
:
235-43
.
View Article PubMed Google Scholar -
Rosencrantz
R.A.,
Wu
Y.,
Sonke
P.Y.,
Yusuf
Y.,
Giant hepatocellular adenoma in a previously obese thirteen-year-old boy. Annals of Hepatology.
2015;
14
(4)
:
559-63
.
View Article PubMed Google Scholar -
Koh
Y.K.,
Yoon
S.H.,
Kang
S.H.,
Kim
H.,
Im
H.J.,
Ha
S.,
Large Hepatocellular Adenoma Presenting with Iron Deficiency Anemia: A Case Report. Journal of Pediatric Hematology/Oncology.
2023;
30
(1)
:
25-9
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 10 No 7 (2023)
Page No.: 5757-5762
Published on: 2023-07-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 2197 times
- PDF downloaded - 507 times
- XML downloaded - 42 times
 Biomedpress
Biomedpress





