INTRODUCTION
Gingival biotype refers to the quality of soft tissue profile surrounding the tooth. Biotype was first defined by Oschenbein and Ross in 1969 describing the anatomy of gingival contour.1 The term periodontal biotype was first used by Seibert and Lindhe.2 Olsson et al referred it as periodontal morphotype.3 Later the term was replaced by gingival or periodontal phenotype coined by Muller, as they felt phenotype is appropriate word to use as it describes the shape of the teeth and alveolar process along with soft tissue.2
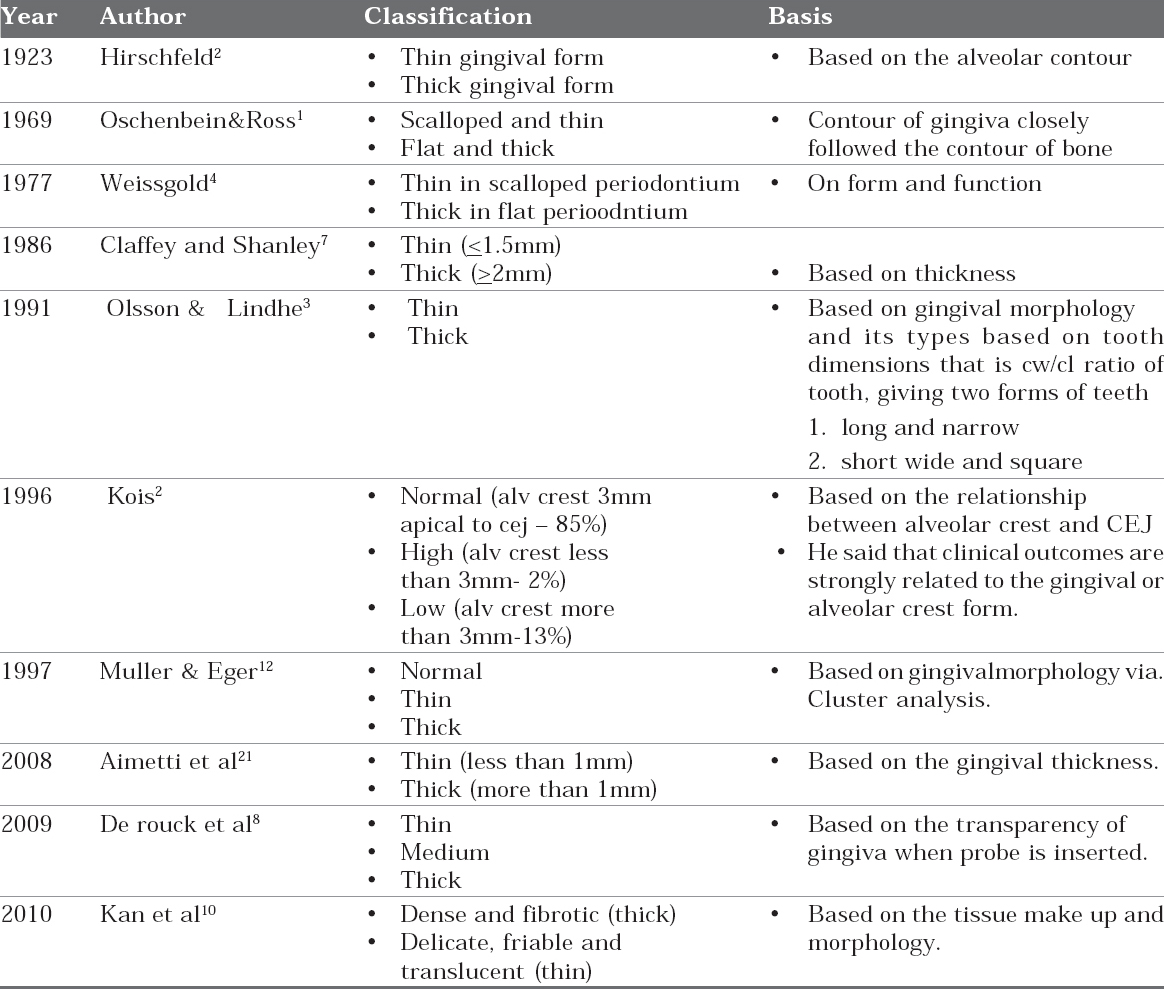
Going back to history, in 1923 Hirschfeld first reported that the thin alveolar bone contour is probably covered with a thin gingival form.2 In 1940, the dental anatomist wheeler noted in his study in extracted teeth that there is a cervical ridge; this ridge helps in holding gingiva with a definite tension. In 1958 Morris described tooth position in relation to gingiva more the buccal prominence more apically the gingival margin located.4 Oscheinbein and Ross postulated that anatomy of gingiva is dictated by underlying bone architecture.1 Weisgold emphasized on form and function, he stated that scalloped periodontium has thinner gingiva when compared to a flat periodontium.4 Olsson & Lindhe (1991) found that individuals with long narrow form of central incisors have a thin periodontium and show more recession compared to subjects with a wide, square form.3 So by definition periodontal or gingival biotype/ periodontal morphotype/ gingival or periodontal phenotype include bone morphotypes, shape of the teeth, morphologic characteristics of gingiva and periodontium. Various authors have put forward various forms of gingival biotype which are described in table 1.
|
TABLE 1: Forms of Gingival Tissues Described By Various Authors
Click here to view |
PREVALANCE
The thicker biotype is more prevalant in general population. Males have thicker biotypes when compared to females and younger individuals showed thicker biotypes when compared to middle age or older groups. Some authors demonstrated that maxilla has thicker biotype than mandible. Maxillary canines and mandibular 1st premolars usually have thinnest biotype.5
In a study done by Vandana KL and Savitha to determine the thickness of facial gingiva among Indians and its association with age, gender and dental arch it was observed that the younger age group had significantly thicker gingiva than that of the older age group. The gingiva was found to be thinner in females than males and, in the mandibular arch than the maxilla.6
METHODS USED TO ASSESS GINGIVAL BIOTYPE
Many methods (invasive and non-invasive) have been used to evaluate the thickness of facial gingival and other parts of the masticatory mucosa. These methods include conventional histology on cadaver jaws, injection needles, transgingival probing, calipers, histologic sections, cephalometric radiographs, probe transparency, ultrasonic devices, and CBCT.
VISUAL EXAMINATION
It is a simple and non invasive method where in the tissue is visually examined and assessed it is routinely used in clinical practice. Disadvantages being it are not considered as a reliable method as it cannot assess the degree of gingival thickness. Eghbali et al (2009) in their study concluded that simple visual examination cannot be relied as an effective method for assessment of biotype irrespective of the clinicians’ experience.7
TRANSGINGIVAL PROBING
In this method tissue thickness is measured using a periodontal probe. Biotype was categorized as thick and thin based on thickness. Thick being more than 1.5 mm and Thin is less than 1.5 mm. Its advantages are Simple and inexpensive. Disadvantages being it are invasive and requires local anesthesia. It has inherent limitations such as precision of the probe during probing which is to the nearest 0.5 mm and the angulation of the probe during probing and distortion of tissue during probing may play a significant role. To avoid these limitations of distortion and angulation In transgingival probing method, some authors used injection needles with rubber stoppers, endodontic pluggers with stoppers.
TRAN METHOD (transparency)
In this method probe is inserted into gingival sulcus and checked for transparency of gingival. If the probe is visible through the gingival tissue, biotype is considered asthin if the probe is not visible then biotype isthick. Advantages of this method are it is Simple, rapid and minimally invasive. This method was found to be highly reproducible with 85% of intraexaminer repeatability for gingival thickness assessment in a clinical trial of 100 periodontally healthy subjects. It was thus validated as a simple, rapid and minimally invasive method.8
MODIFIED CALLIPER TECHNIQUE
Kan et al in 2010 first used a tension free calliper to measure the gingival thickness in the facial aspects of maxillary anterior teeth and compared the results with that of obtained using probe and reported no statistically significant difference. But the disadvantage with this procedure is tension-free caliper can only be used at the time of surgery and cannot be used for pretreatment evaluation.9
CONE BEAMED COMPUTED TOMOGRAPHY
CBCT is known for its superior diagnostic ability and it is extensively used for hard tissues. Fu et al used CBCT to measure the labial thickness of gingiva and bone and compared the results with those obtained by using caliper and no statistically significant difference was noticed, but it is considered more objective method when compared to visual examination and caliper method. But the only disadvantage using CBCT is it requires technical expertise and expensive.10
ULTRASONIC DEVICES
A 1971 study by Kydd et al was the first to measure the thickness of palatal mucosa using an ultrasonic device. Ultrasonic devices appear to be the least invasive and offer excellent validity and reliability. However, such devices are no longer available commercially in addition, they make it difficult to both determine the correct position for accurate measurement and successfully reproduce measurements.
DEVICE AND ITS MECHANISM
The ultrasonic measuring device is SDM® (Austenal Mcdizintechnik, Koln, Germany).
It was extensively described by Knapp & Nentwig 1991, Eger et al. 1996, Muller et al. 1999, 2000. It contains a piezo-electric crystal which is set oscillating at a pulse of 5 MHz transmitting waves through mucosa at a velocity of 1520 m/s and at a rate of 1000 signals/sec which are received and analysed. By timing of the echo with respect to the pulse of transmission the thickness of mucosa is determined within 2-3 secs. The transducer probe has a diameter of 4 mm with a precision of 0.1 mm.
In 2005 a study was done by Savitha and KL Vandana comparing assessment of gingival thickness using trans-gingival probing and ultrasonic device and concluded that transgingival probing method significantly over estimated the thickness of gingiva than the ultrasonographic method and the thickness of gingiva varies with morphology of the crown. It was concluded that compared to transgingival probing ultrasonographic method assesses gingiva thickness more accurately, rapidly and atraumatically.6
PUFFED CHEEK METHOD
Dvorak et al (2013) described this method in a case series where they assessed the thickness of mucosa by using computed tomography with splint placed to localize the exact position by a marker points. Marker points were placed at four sites. The Four sites were evaluated two central incisors and two first molars. Patients were asked to puff out cheeks because, Computed tomography scans with distended cheeks provide a more detailed evaluation of mucosal surfaces of the oral cavity than conventional CT scans do.11
CHARACTERISTICS OF GINGIVAL BIOTYPES
THICK BIOTYPE
Thick biotypes include flat soft tissue, bony architecture, denser and more fibrotic soft tissue curtain, large amount of attached masticatory mucosa. When seen from the occlusal view, the alveolar housing of the teeth forms a broad, even ridge and are resistant to acute trauma and respond to disease with pocket formation and infra bony pocket.12
THIN BIOTYPE
Thin biotypes are delicate, highly scalloped and translucent in appearance. The soft tissue appears to be delicate and friable with minimal amount of attached gingiva and thin labial plate with possible presence of dehiscence and fenestrations. Thin scalloped biotypes are considered to be at risk as they have been associated with compromised soft tissue response following surgical or restorative treatment.12
VARIOUS DEFINING FACTORS OF GINGIVAL BIOTYPE
Tooth Dimensions: Long and slender teeth usually show a thinner biotype where as short and wider teeth show thicker form. Hence, lesser the overall crown area thick is the biotype.13
Papillary Height and Area: Long and thin interdental papilla usually associated with thin biotype whereas wider and shorter papilla shows a thicker form. Hence, lesser the overall papillary area thick is the biotype.13
Bone Morphotpye: The gingival contour follows that of bone contour so more scalloped the bone thinner the biotype. Thick biotype is usually associated with thick labial plate.14
Keratinised Mucosa: Thick tissue phenotype with flat gingival architecture has a wider keratinised mucosa compared to that of thin and scalloped one.4
Palatal Mucosa: Is usually thick in thick tissue biotypes, thickest in the 1st premolar region and thinnest in the 1st molar region.8
Tooth Position and Movement: If the tooth is placed or moved buccally the thickness of the facial gingiva decreases. Hence, care should be taken while orthodontic movement in cases of thin gingival biotypes.4
BOP and Biotype: Thin and vulnerable gingiva of insufficient width was not more likely to bleed after probing than thicker tissue (Muller 2001).
All the above mentioned define the biotype but the most uniform parameter was found to be tooth dimensions (TD). According to Sammut E (2013) soft tissue biotype is a aggregate or composite of 4 features of soft tissue sand the teeth those are:15
-
Gingival width (keratinised gingiva width).
-
Gingival thickness (thin or thick).
-
Papillary complex proportion.
-
Crown width/height ratio.
CLINICAL CONSIDERATIONS
It was suggested that since the two tissue biotypes have different gingival and osseous architectures, they exhibit different pathological responses when subjected to inflammatory, traumatic, or surgical insults. These factors dictate the disease progression, treatment outcome and prognosis, hence proper knowledge of it helps to choose an appropriate treatment modality.16
Here are some of the clinical situations where in the biotype response is discussed.
Tissue Biotype and Extraction of Teeth
Thick bony plates associated with thick biotypes and thin bony plates with thin biotypes respond differently to extraction. Thick biotypes are associated with minimal ridge atrophy following extraction when compared to thin biotypes so, possible strategies that should be followed in case of extraction of teeth with thin biotypes
-
Minimizing leverage forces towards labial plate.
-
Sectioning the roots from teeth when possible
-
Using periotomes to expand and elevate the tooth and root tips
-
Using ratchet device to remove root tips (safest and atraumatic method in extraction of broken root tips).
Tissue Biotype and Implant Treatment Planning
If osseous and gingival tissues are different for thick and thin tissue biotypes, it seems logical that these distinctions would significantly influence implant site preparation and treatment planning. This is consistent with the observations that the stability of the osseous crest and soft tissue is directly proportional to the thickness of the bone and gingival tissue. Thick biotypes with thick bony plates provide a better environment for implant placement as osseous remodeling among thick and thin biotype differs for a thin biotype case, practitioner must be aware that there is always risk of alveolar bone resorption.
For a thin biotype the tissue over the implant will be more thinner and translucent reflecting the color of implant thus compromising the esthetics. A delayed implant approach might be taken when there is minimal periodontal support or in case of thin biotypes to avoid alveolar resorption. In case of thick biotypes immediate implant placement can be done with predictable results. Berglundh et al 1996 reported marginal bone loss in thin biotypes after implant placement. Huang et al reported angular bone defects in thin biotypes after implant placement. Abrahamsson et al said that thick tissues can avoid significant crestal bone loss after implant placement.17
Tissue Biotype and Root Coverage Procedures
According to McFall thickness at donor & recipient sites are key factors in predicting tissue coverage. An initial gingival thickness was found to be the most significant factor that influences the prognosis of a complete root coverage procedure. A flap thickness of 0.8-1.2 mm produced predictable results. A thick tissue has an increased blood supply that will enhance the revascularization of grafts, leading to increased healing and graft incorporation and hence there are more chances of complete root coverage in thick biotype.18 Nisapakultorn et al (2010) reported a significant association of thin biotype with increased risk of facial mucosal recession.19
Gingival Biotype and Crown Lengthening
Thick gingival tissues are more resistant to mucosal recession or mechanical irritation and are capable of creating a barricade to conceal restorative margins. It is said that there is atleast 0.5-0.8 mm bone loss each time when flap is reflected hence, 6 months of healing period is desirable in case of anterior restorations. In case of thin biotypes soft tissue grafting is recommended 6-8 weeks prior the restoration. Pontoriero & Carnevale (2001) showed in a study that on crown lengthening there wassoft tissue regain in patients with thick periodontal biotypes than in thin periodontal biotypes.20
Gingival Biotype and Sinus Lift Procedures
Aimetti et al in 2008 took maxillary mucosal biopsies from the sinus floor during otorhinolaringologic surgical interventions, and measured gingival thickness in the area of the maxillary anterior teeth. He found out that thick gingival biotypes has thick schnerderian membrane and this could be a reliable factor in predicting and planing sinus lift procedures.21
Blood supply to the tissue and underlying bone, in case of thin biotypes is inadequate or less when compared to that of thicker ones this effects the post surgical revascularization due to which compromise in the blood supply may occur leading to underlying bone resorption and loss of soft tissues in the form of recession, dehiscence.
Methods to Improve Tissue Thickness
First carefully assess the soft tissue and underlying bone and determine the biotype. In case of thin biotype soft tissue, graftings can be done to enhance soft tissue quality. The best way to convert thin soft tissue biotype into thick is by using a sub-epithelial connective tissue graft. Other methods include roll technique; finger split technique and acellular dermal matrix. Tissue keratinisation can be improved by recommending oral physiotherapy.22
CONCLUSION
Periodontal biotype evaluation is an important parameter in establishing patient expectations in many complex esthetic procedures by allowing the clinician to predict therapeutic outcome. By understanding the nature of biotypes clinician can employ appropriate PDL therapy and minimize unwanted treatment outcomes. New technologies for assessment of periodontal biotype allow clinicians for accurate diagnosis and predictable treatment outcome. Therefore to achieve success clinician has to properly asses the soft tissue parameters like keratinised tissue, periodontal biotype and vestibular depth which play a vital role in decision making process and ultimately will help in maintaining the balance between the white and the pink.
