Clavatulidae Gray, 1853
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5123.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:036F6B4D-CDCC-4CD7-A914-9A1D8C7A097A |
|
DOI |
https://doi.org/10.5281/zenodo.10723113 |
|
persistent identifier |
https://treatment.plazi.org/id/039487D1-FF89-FFBC-FFBA-F8886C2FFC2D |
|
treatment provided by |
Plazi |
|
scientific name |
Clavatulidae Gray, 1853 |
| status |
|
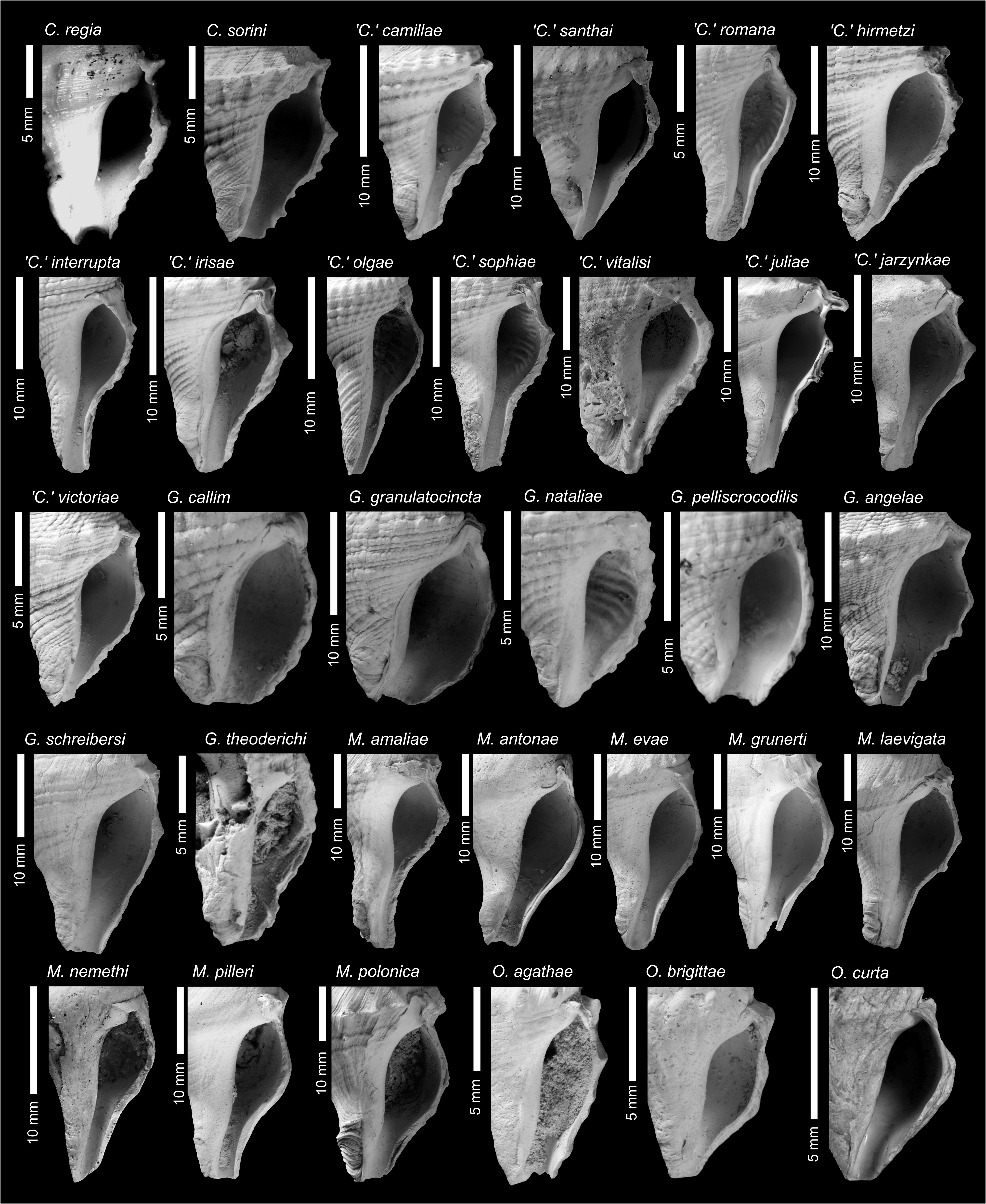
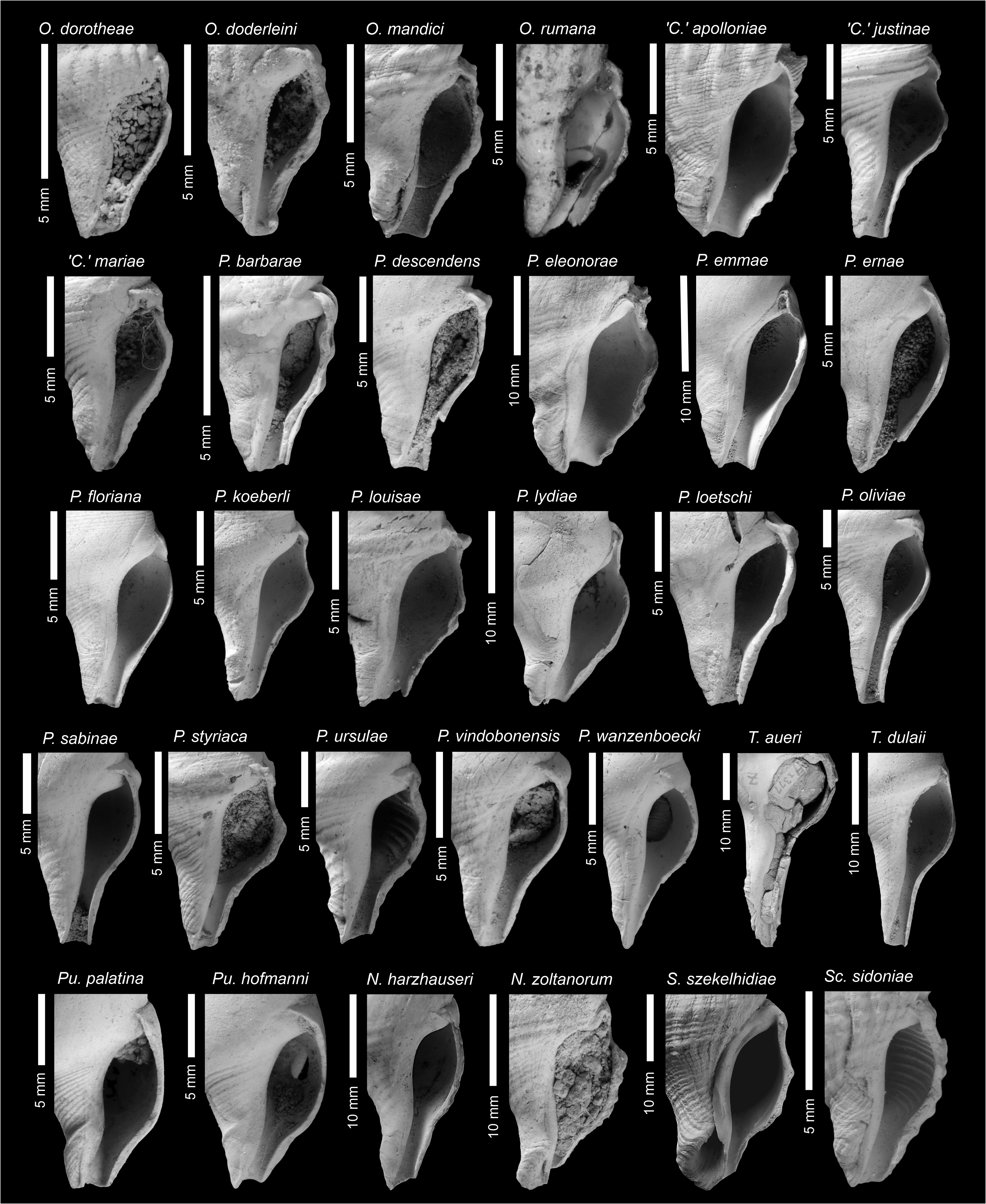
Family Clavatulidae Gray, 1853 View in CoL
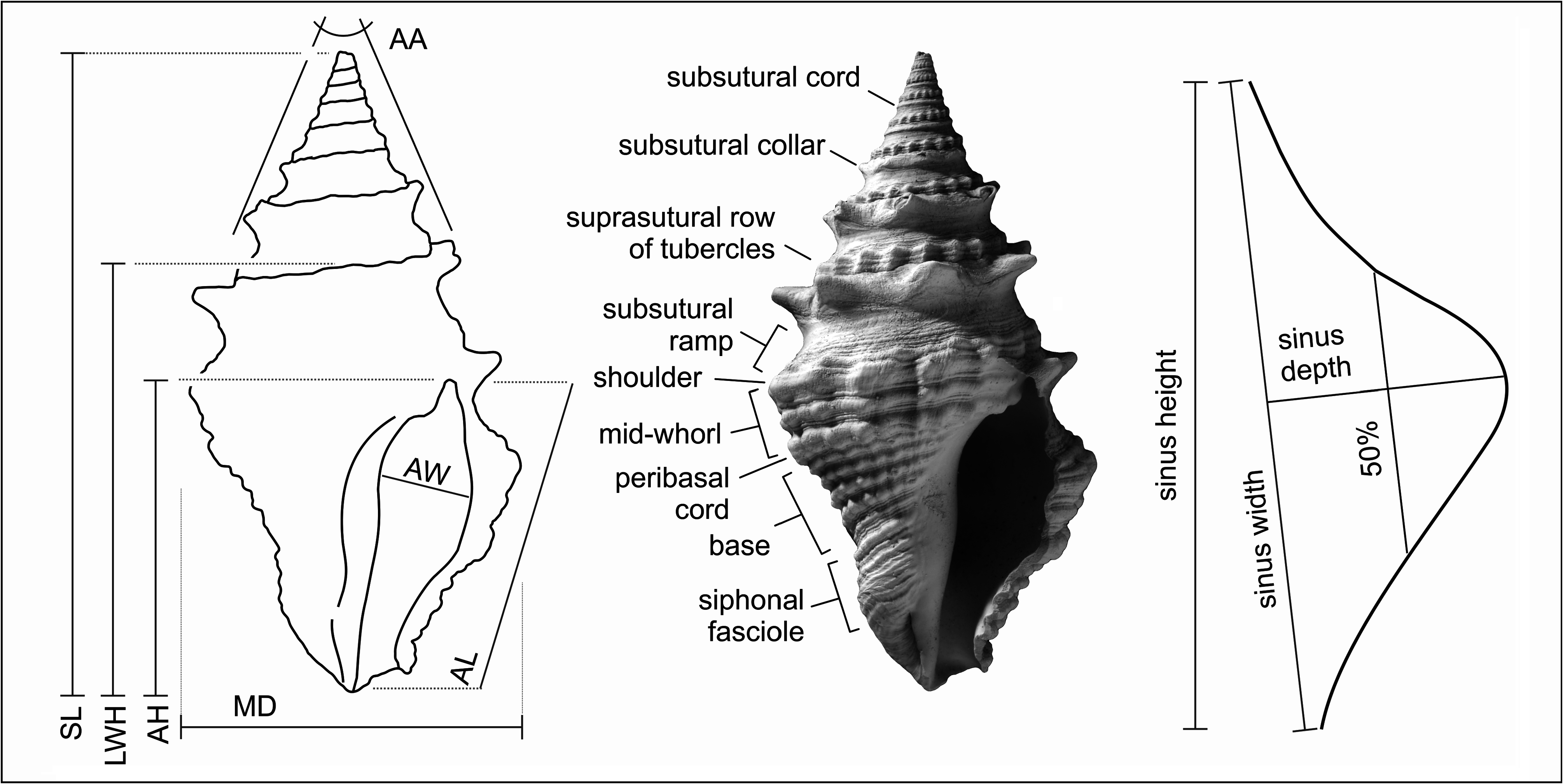
Although all extant clavatulid species are superficially similar, which has led all authors until now to divide them into two genera ( Clavatula Lamarck, 1801 and Perrona Schumacher, 1817 ), on closer examination there are also characters that are shared by some groups of clavatulids and not others. In order to clarify the descriptions given in the systematic section, some discussion on clavatulid shells is required.
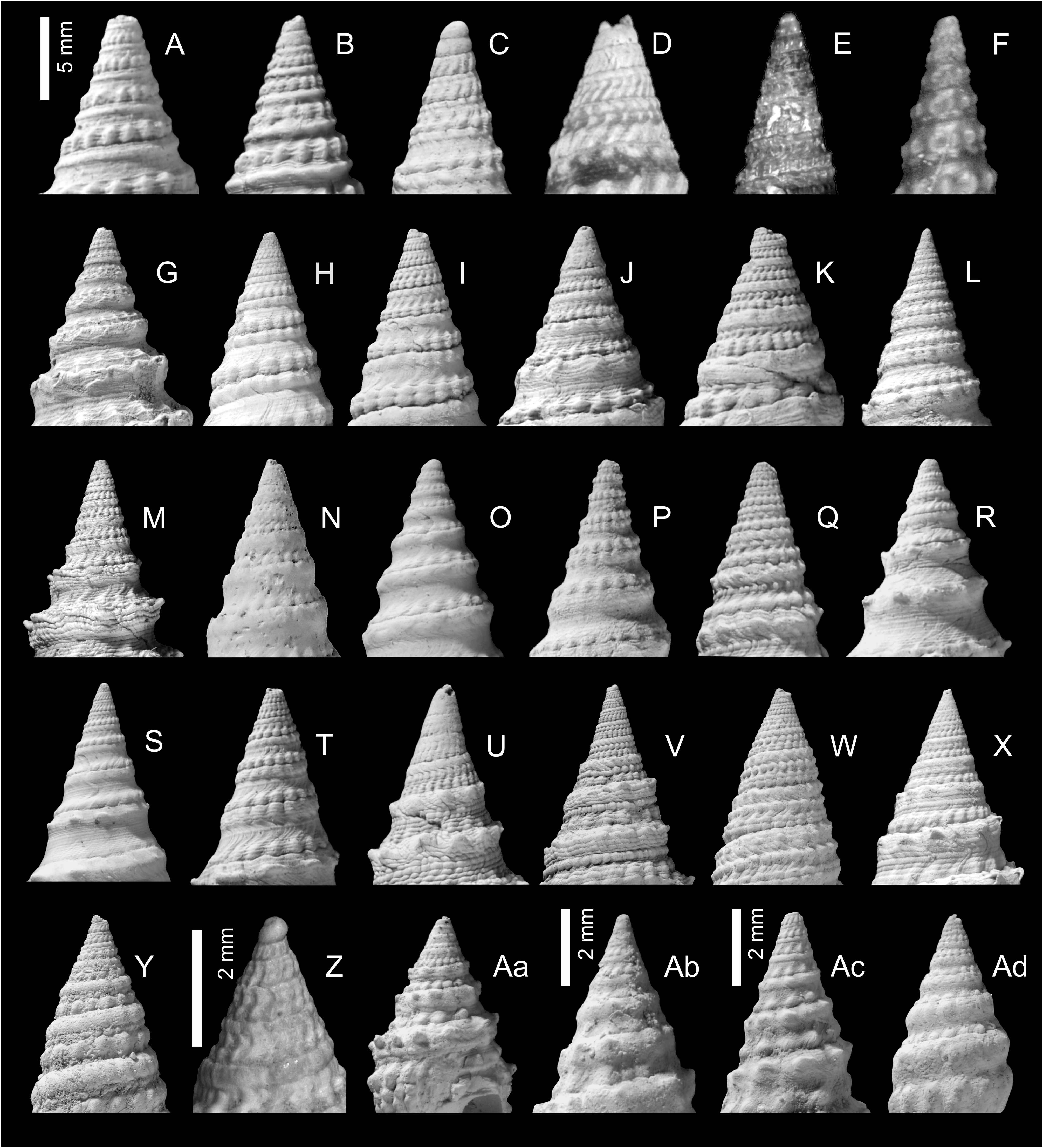
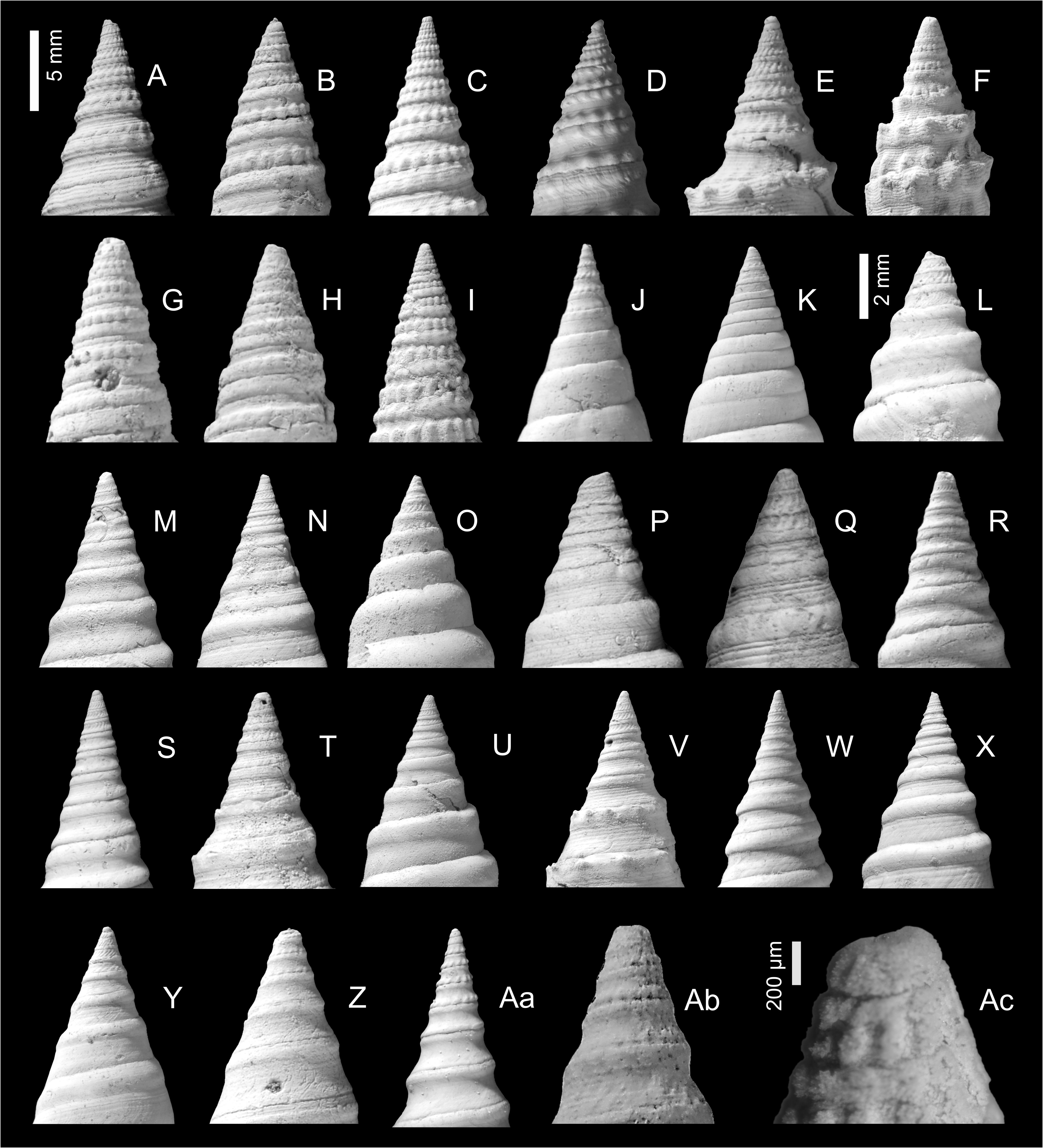
The protoconch of almost all known clavatulid species is paucispiral and almost always unsculptured (exceptions are Scaevatula Gofas, 1990 , which has a ribbed protoconch and Tomellana aueri nov. sp., which has a protoconch of 2.5 whorls). This paucispiral type of protoconch is suggestive of direct development, or at least a short planktotrophic phase, which tends to lead to stratigraphically short lived and geographically restricted species ( Jablonski & Lutz 1980). This is certainly the pattern seen in both the fossil and present-day record and reaches its zenith in the Miocene Paratethys. Unfortunately, the protoconch is rarely preserved in adult specimens in the Paratethys record, however, based on the experience of other clavatulid populations (e.g., Pliocene Atlantic and Mediterranean), it is of limited taxonomic use in distinguishing species (own data B.L.).
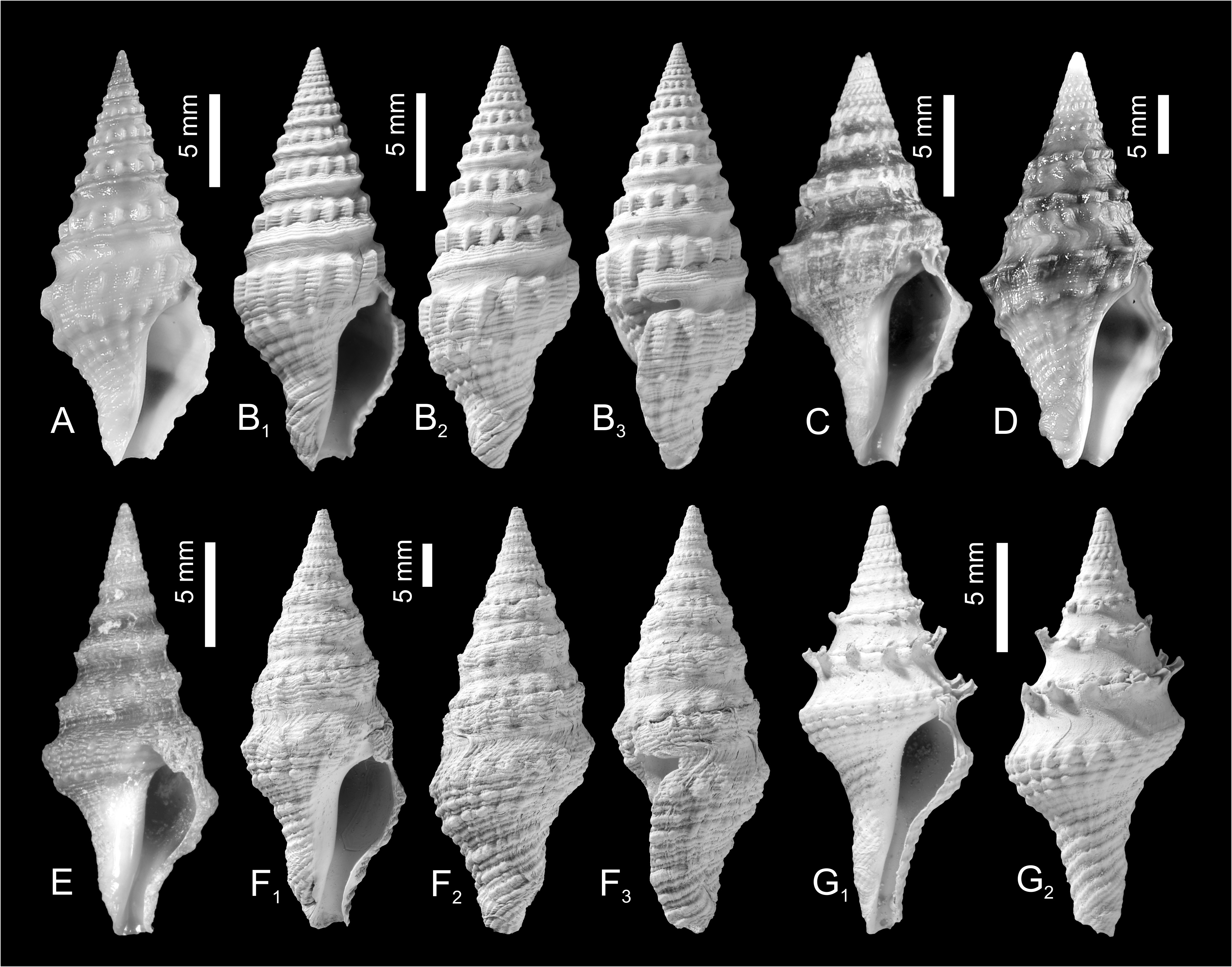
The spire in all species is relatively raised and conical, although the outline may be made irregular or somewhat gradate by a strongly developed raised subsutural collar and made coronate by spines or tubercles developed either on the adapical part of the collar or mid-collar. In many species sculpture changes markedly with ontogeny on about the fourth to sixth whorls. Early teleoconch whorls are often divided into two beaded cords separated by a narrow groove or concave area (bipartite), or bear three beaded portions, a subsutural cord, mid-cord and suprasutural cord (tripartite) ( Figs 3–4 View FIGURE 3 View FIGURE 4 ). In some species the early teleoconch sculpture is neither bipartite nor tripartite but consists of very narrow adsutural cords and a wide concave mid-portion (e.g., ‘Clavatula’ jarzynkae nov. nom.), or a welldeveloped, but smooth, subsutural cord and a suprasutural row of beads separated by a concave portion, as is the case with many of the species placed in Clavatula s.l. herein.
On later teleoconch whorls the subsutural cord swells to form the subsutural collar, the mid-portion beads fade and become smooth or finely spirally striate, and the beads on the suprasutural cord swell and become tubercles. In some species the subsutural cord is not visible, obscured by the succeeding whorl. On the last whorl it becomes clear that the mid-portion on the spire whorls represents the subsutural ramp and the suprasutural row of tubercles coincide with the shoulder ( Fig. 2 View FIGURE 2 ).
In all Conoidea the anal sinus is an important character, and often useful for separation at both generic and species level. In clavatulids the sinus is well-developed and clearly preserved in all species ( Fig. 5 View FIGURE 5 ). On early teleoconch whorls the sinus can be represented by comma-shaped axial riblets but these structures are strong growth lines but do not represent true ribs. Ribs are uncommon in clavatulids, and when present are poorly defined and formed by axially aligned rows of tubercles (i.e., ‘ Clavatula ’ apolloniae ), and are only present on the last whorl below the shoulder.
Spiral elements on the spire whorls are composed of sub- and suprasutural cords on the early whorls, which later become the subsutural collar and suprasutural row of tubercles (as discussed above). Many species have secondary spiral cords that cover the entire surface including the collar and suprasutural tubercles. Spiral sculpture on the last whorl is more variable. The shoulder cord is tubercular (occasionally spinous) in Clavatula species and usually smooth or rarely weakly tubercular in Perrona species. Usually, a peribasal and perifasciolar cord is more prominent, and less often a mid-whorl cord, all of which are more or less tubercular. Rarely a species may have five primary cords below the shoulder (i.e., ‘ Clavatula ’ apolloniae ). Secondary and tertiary spiral sculpture tends to be rather irregular in clavatulids and is often not only present in the interspaces, but overrides the primary cords.
Clavatulids often have rows of beads, tubercles, and spines. In this work beads are defined as small round, spirally-aligned structures in apposition to each other. Tubercles are larger, not in contact with each other and can be rounded, subtrigonal or subquadrate in outline and low or pointed, if sharp here called spinous. Spines are also present in some species, which is when the pointed structure, usually on the subsutural collar, is open on the abapical side (e.g., Perrona eleonorae ).
The aperture is pyriform to ovate in clavatulids, the outer lip does not possess a varix, and is usually smooth within ( Figs 6–7 View FIGURE 6 View FIGURE 7 ). Species with lirae within the outer lip do occur (e.g., Granulatocincta granulatocincta , ‘ Clavatula ’ irisae, ‘ Clavatula ’ sophiae , inter alia), and if present are considered a species-specific character in most species (‘ Clavatula ’ romana is an exception). The siphonal canal is open in all species and usually weakly to moderately notched (exceptions exist). The length of the canal is species specific.
The columella is more or less deeply excavated in the upper third or upper half, more or less twisted at the fasciole, and smooth in all species. The columellar callus is weakly to moderately developed, at most expanded onto the medial side of the venter, but never greatly thickened. The parietal portion of the callus is never thicker than the abapical portion and never develops a callus pad or fold.
No color pattern is present in the Paratethyan specimens, nor is it enhanced by UV.
What is Clavatula ?
MolluscaBase (Eds.) (2021a) lists 37 species of Clavatula , including also Clavatula xanteni Nolf & Verstraeten, 2006 , which was placed in Perrona by Kantor et al. (2018a). Clavatula pyramidata ( Kiener, 1839) is missing from that list, although Kantor et al. (2018a) placed that species in Clavatula based on molecular data. Kilburn (1985) and Boyer & Hernández (2004) stated that Clavatula is a poorly characterized genus, lacking well-defined diagnostic features, but did not provide solutions.
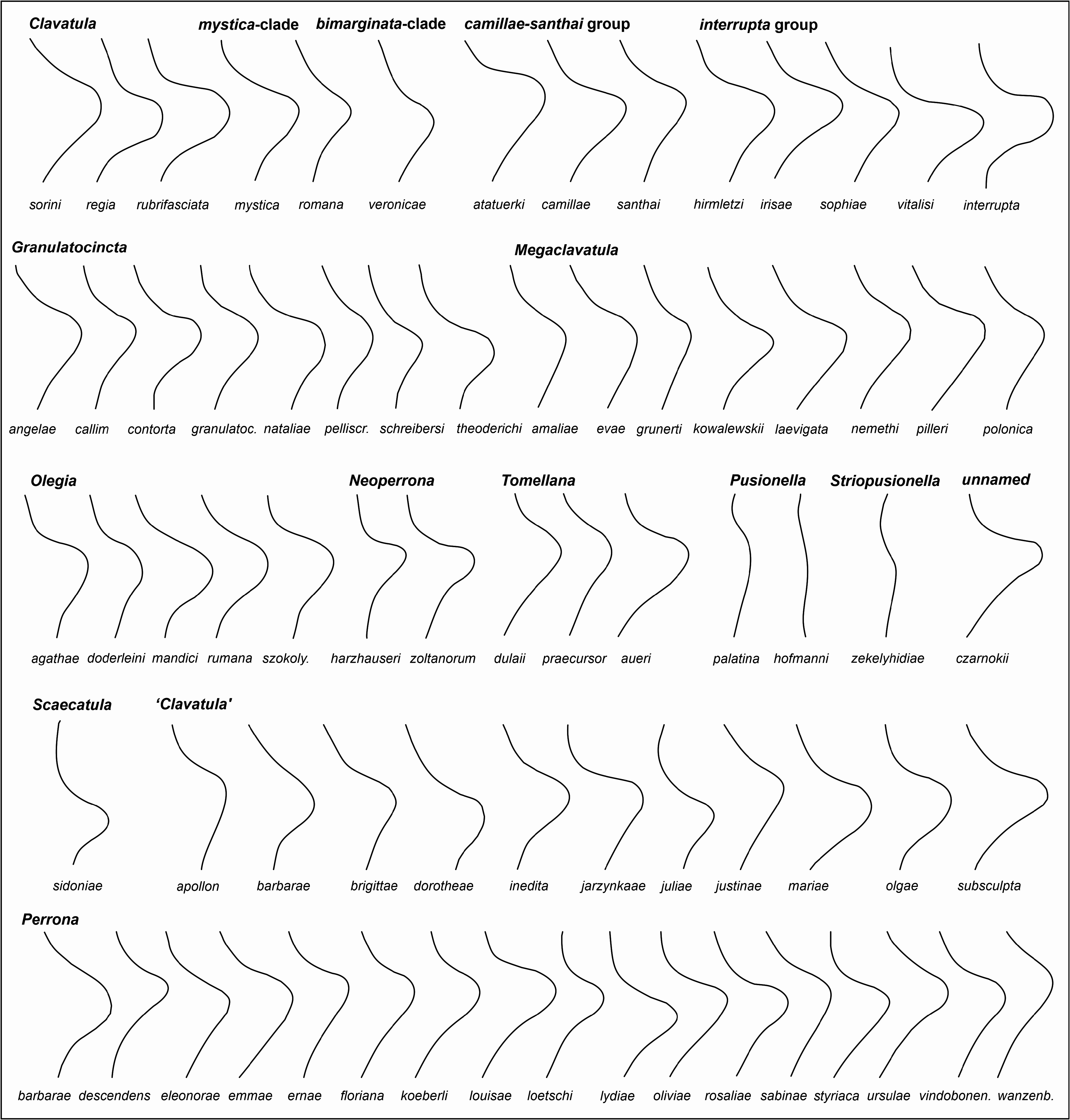
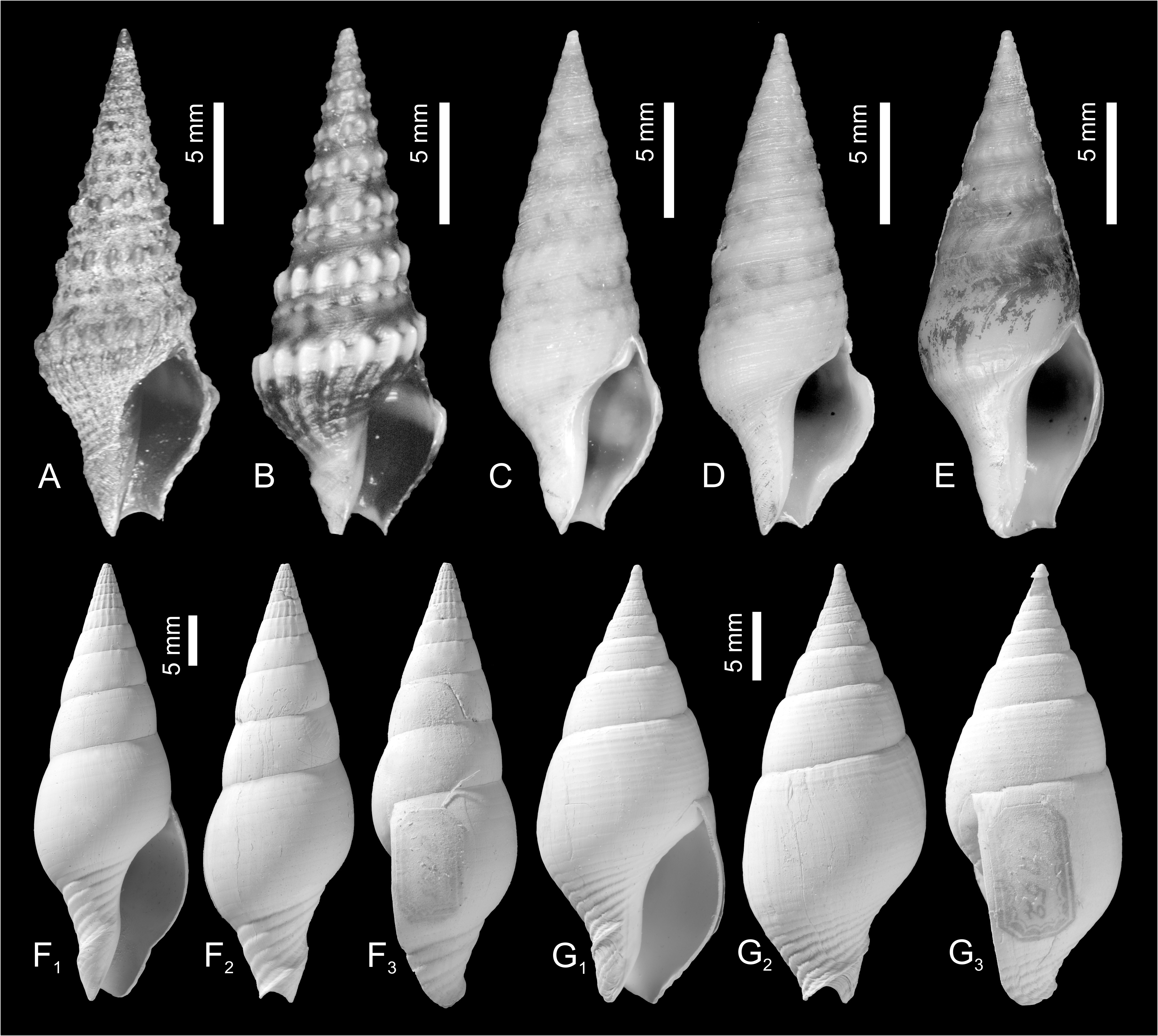
New unpublished molecular data confirmed Perrona , Tomellana and Scaevatula as genus-level taxa, but are in conflict with Clavatula as currently perceived (Nicolas Puillandre, pers. comm., October 2021). These results suggest that Clavatula is not monophyletic, which is also suggested by the morphologies of the early teleoconch whorls. We observe peculiar morphologies in certain species groups ( Figs 3A–E View FIGURE 3 ), which we discuss herein as clades. Clavatula muricata ( Lamarck, 1822) (probably a synonym of C. regia ) and C. regia ( Röding, 1798) ( Figs 8A–H View FIGURE 8 ) have a bipartite sculpture with a weak, smooth subsutural cord and a prominent suprasutural cord of large beads (overrun by weak spiral cords) ( Fig. 3A View FIGURE 3 ). The same early teleoconch sculpture is developed by C. rubrifasciata (Reeve, 1845) ( Figs 3B View FIGURE 3 , 9A–B View FIGURE 9 ) and C. lelieuri ( Récluz, 1851) (see Nolf 2008: pl. 8, figs 42–49, pl. 9). The muricata + regia clade contains the type species and therefore, these species represent Clavatula sensu stricto.
A close relation of ‘ Clavatula ’ caerulea (Weinkauff in Weinkauff & Kobelt, 1875) and ‘ Clavatula ’ pyramidata ( Kiener, 1839) ( Figs 3F View FIGURE 3 ) with Clavatula is suggested by the early teleoconch sculpture with a prominent suprasutural cord of large beads. Both species, however, differ from typical Clavatula in their slender fusiform shell with high conical spire, the comparatively short last whorl, and the wider and shorter siphonal canal ( Figs 10A, B View FIGURE 10 ) (see Nolf and Verstraeten 2008: pls 1–2 for ‘ C. ’ caerulea). ‘ Clavatula ’ martensi von Maltzan, 1883 and ‘ C. ’ matthiasi Nolf and Verstraeten, 2008 belong also to this group, based on conchological features (see Nolf and Verstraeten 2008: pls 3–5). Herein, we treat these species as caerulea -clade.
‘Clavatula’ mystica ( Reeve, 1843) differs from these species by its tripartite sculpture on the first four teleoconch whorls ( Figs 3D View FIGURE 3 , 9C–D View FIGURE 9 ). It starts with weakly opisthocline axial ribs, which grade into three spiral rows of beads, with the suprasutural spiral row being most prominent. The whorl profile is almost flat-sided.A comparable sculpture is developed by ‘ C.’ filograna Odhner, 1923, which has a tripartite sculpture on early teleoconch whorls consisting of three beaded spiral cords of similar strength. These species are grouped herein as mystica -clade
‘ Clavatula’ bimarginata ( Lamarck, 1822) has a bipartite sculpture with a granulose subsutural cord and suprasutural spiral cord of equal strength with indistinct, bifid beads ( Fig 3F View FIGURE 3 , 9E–F View FIGURE 9 ). The moderately concave interspace bears prominent, comma-shaped axial riblets formed by growth lines. Spiral threads form a somewhat cancellate pattern especially in the abapical half of the whorls. This species is placed in the bimarginata -clade.
‘Clavatula’ tripartita (Weinkauff, 1876) ( Figs 10C–D View FIGURE 10 ) and ‘ C.’ taxea ( Röding, 1798) ( Fig. 10E View FIGURE 10 ) are neither bipartite nor tripartite, but develop close-set spiral cords on flat-sided early teleoconch whorls. This morphology differs greatly from other Clavatulidae discussed herein and are placed herein in the taxea -clade. A separation of the ‘ C. ’ taxea- clade from Clavatula is also suggested based on the comparatively slender, smooth and high spired shells, and the weak subsutural collar, which lacks spines. Consequently, already Kilburn (1985: 424) doubted that these South African species were congeneric with the western African Clavatula s.s. In addition, the anal sinus allows for a separation of ‘ C.’ mystica , which has a moderately deep, widely V-shaped anal sinus, from Clavatula muricata and C. rubrifasciata , which have a deep, U-shaped anal sinus ( Fig. 5 View FIGURE 5 ). An additional type of early teleoconch sculpture is represented by the elongate biconic, prominently spinose ‘ C.’ diadema ( Kiener, 1839) ( Figs 3C View FIGURE 3 , 9G View FIGURE 9 ), which has weakly opisthocline axial ribs on the first teleoconch whorl and a weak subsutural spiral cord on the second teleoconch whorl. The beaded suprasutural spiral cord evolves from the abapical tips of the axial ribs on subsequent whorls. In addition, this species is characterized by very long spines.
Thus, within the species, formerly referred to as Clavatula we distinguish:
1. Clavatula (‘ C.’ regia , ‘ C.’ muricata , ‘ C.’ rubrifasciata , ‘ C.’ lelieuri )
2. caerulea -clade (‘ C.’ caerulea, ‘C.’ pyramidata )
3. mystica -clade (‘ C.’ mystica )
4. bimarginata -clade (‘ C.’ bimarginata)
5. taxea -clade (‘ C.’ taxea, ‘C.’ tripartita)
6. diadema -clade (‘ C.’ diadema)
As we focus on fossil species from the Neogene of Europe, we refrain from introducing genus names for these clades. Nevertheless, these data allow a refined systematic treatment of Paratethyan species, based on the hypothesis that early teleoconch sculpture is a useful trait to delimit genera within Clavatulidae View in CoL . Only three of the Paratethyan Clavatulidae View in CoL can be placed in one of these clades: Clavatula sorini View in CoL nov. sp. is placed in Clavatula View in CoL s.s., ‘ C. ’ veronicae Hoernes & Auinger, 1891 is placed in the bimarginata -clade and the ‘ Clavatula View in CoL ’ interrupta group and ‘ Clavatula View in CoL ’ romana ( Defrance, 1826) View in CoL might be placed in the mystica View in CoL -clade.
Consequently, most of the remaining Paratethyan species should be placed into new genera, which we propose in the following. Nevertheless, some species remain, which do not fit in our scheme. These are listed provisionally as ‘ Clavatula View in CoL ’.
Distribution and stratigraphy. The oldest records from the Eocene of Italy and France, placed in Clavatula by Vinassa de Regny (1898) and Boussac, (1911) represent other Conoidea families, such as Pseudomelatomidae or Borsoniidae (see Vinassa de Regny 1898, pl. 7 (20), figs 40–45). Clavatula praegotica Vinassa de Regny, 1898 is reminiscent of Perrona , but consists only of a fragment of the last whorl and lacks distinctive features (see Vinassa de Regny, 1898, pl. 7(20), figs 39a, b). Similarly, the numerous species from the Paleogene of Kazakhstan, placed in Clavatula by Amitrov (1973) are clearly not Clavatulidae and belong to other conoidean families (see Amitrov 1973, pls 9–11).
Species belonging to the ‘ Clavatula ’ species group first appear during the Oligocene. Von Koenen (1890) described ‘ Clavatula ’ species, such as ‘ Clavatula ’ barthi, ‘ C.’ bifrons and ‘ C.’ roeveri, from the Rupelian of the North Sea. Several North Sea species such as ‘ Clavatula ’ struckmanni and ‘ C.’ millegranosa, however, are clearly unrelated to Clavatula based on their morphologies (see von Koenen 1890, pl. 33, figs 8, 12). Similarly, ‘ Clavatula ’ mogenstrupensis Schnetler in Schnetler & Beyer, 1990, from the Chattian of Denmark, is unrelated to Clavatula based on its convex whorls and broad axial ribs (see Schnetler & Beyer 1990, pl. 3 fig. 5). ‘ Clavatula ’ apenninica Bellardi, 1877, from the Rupelian of Italy, seems to represent a Clavatulidae , but does not fit in Clavatula due to its comparatively slender biconic shell with high last whorl and weak subsutural collar (see Ferrero Mortara et al. 1981:77, pl. 15, figs 1a–b).
During the Chattian, the ‘ Clavatula ’ species group was widespread along European coasts, documented from the northeastern Atlantic [‘ Clavatula ’ concatenata ( Grateloup, 1832) ] and the Central Paratethys Sea (‘ Clavatula ’ danuvii Vicián, Kovács & Stein, 2019) ( Lozouet 2017; Vicián et al. 2019). The North Sea species Clavatula chattica R. Janssen, 1978 differs from Clavatula in having convex whorls and axial ribs (see R. Janssen 1978).
Species from the Miocene of the Western Atlantic, placed by Gabb (1873) and Gardner (1947) in Clavatula , are clearly unrelated with Clavatulidae and represent rather members of the Pseudomelatomidae or other Conoidea families. Similarly, ‘ Clavatula ’ species described from the Neogene of the Indo-West Pacific, by Cox (1936), Oostingh (1938) and Beets (1941, 1942) represent other genera such as Paradrillia and Turricula .
Therefore, the ‘ Clavatula ’ genus group is strictly Tethyan. It was widely distributed along the European Atlantic and Proto-Mediterranean coasts during the Oligocene. The group peaked in distribution and diversity during the Miocene, documented by numerous species from all European biogeographic regions except for the Eastern Paratethys (e.g., Bellardi 1877; Kautsky 1925; Glibert 1954; Peyrot 1931; Landau et al. 2013, 2020; hoc opus).
After the late Pliocene cooling event, the group suffered a gradual range contraction, and today Clavatula and other ‘ Clavatula ’-clades are restricted to tropical waters off West Africa, where they are represented by a relatively small number of genus groups and species compared to their older historical diversity.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |