Scyliorhinus haeckelii
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4066.5.1 |
|
publication LSID |
lsid:zoobank.org:pub:34FD1E55-D28E-481C-B10B-F149392BDC11 |
|
DOI |
https://doi.org/10.5281/zenodo.6089784 |
|
persistent identifier |
https://treatment.plazi.org/id/0570016C-FFAF-FFEC-0ADB-F94BFC6D759D |
|
treatment provided by |
Plazi |
|
scientific name |
Scyliorhinus haeckelii |
| status |
|
Scyliorhinus haeckelii View in CoL Miranda Ribeiro, 1907
( Figs. 9–20 View FIGURE 9 View FIGURE 10 View FIGURE 11 View FIGURE 12 View FIGURE 13 View FIGURE 14 View FIGURE 15 View FIGURE 16 View FIGURE 17 View FIGURE 18 View FIGURE 19 View FIGURE 20 , 22 View FIGURE 22 , 23 View FIGURE 23 , 25–28 View FIGURE 25 View FIGURE 26 View FIGURE 27 View FIGURE 28 ; Tabs. 1–4 View TABLE 1 , 6 View TABLE 6 )
Common names: freckled catshark, polkadot catshark, cação-pinto.
Catulus haeckelii Miranda Ribeiro, 1907, pp. 163–165, fig. 8 (original description, figured). Scyliorhinus boa: Bigelow & Schroeder, 1948 View in CoL , pp. 204–207, fig. 32 (photograph of holotype of S. haeckelii included in species account).
Scyliorhinus fernandezi Weibezahn 1953 View in CoL , pp. 3–7, fig. 1 (original description).
Scyliorhinus haeckelii: Springer, 1966 View in CoL , pp. 601–602, fig. 15A (revision, figured); Springer, 1979, pp. 135–137, fig. 86 (revision, figured); Gomes & de Carvalho, 1995, pp. 232–236, fig. 2 (egg-cases); Gadig & Gomes, 2003, pp. 22 (listed); Compagno et al., 2005, p. 250, fig. 42 (brief account, illustrated); Ebert et al., 2013, pp. 374, 381, pl. 52 (brief account, illustrated)
Scyliorhinus retifer haeckelii: Springer & Sadowsky, 1970 View in CoL , pp. 92–93 (description).
Scyliorhinus retifer besnardi Springer & Sadowsky, 1970 View in CoL , pp. 94–97, fig. 2 (described as a new subspecies, figured). [New synonymy]
Scyliorhinus retifer: Figueiredo, 1977 View in CoL , p. 13, fig. 16 (general account, illustrated).
Scyliorhinus besnardi: Springer, 1979 View in CoL , pp. 126–128, figs. 79, 80 (revision, taxonomic account, illustrated); Compagno, 1984, p. 357 (general account, illustrated); Compagno et al., 2005, p. 246, fig. 42 (brief account, illustrated); Ebert et al., 2013, pp. 374, 377, pl. 52 (brief account, illustrated).
Scyliorhinus haeckeli: Compagno, 1984 View in CoL , pp. 362–363 (general account, illustrated).
Scyliorhinus haeckelii View in CoL / besnardi View in CoL group Gadig, 2003, pp. 147–148 (compilation); Gomes et al., 2010, pp. 84–85, fig. 109 (brief account, illustrated); Soares, 2014, pp. 71–79, figs. 2, 10, 15, 16, 56 (revision, illustrated); Soares et al., 2015, p. 1 (compared to S. ugoi View in CoL , described as new).
Holotype. MNRJ 494, immature male, 316 mm TL ( Ilha Rasa, Rio de Janeiro, Brazil).
Additional material examined. 124 specimens (see Appendix). Specimens cited by Springer & Sadowsky (1970), including the paratypes of Scyliorhinus retifer besnardi , originally part of the Instituto Oceanográfico of Universidade de São Paulo, were not found and are presumed lost.
Diagnosis. A species of Scyliorhinus from the southwestern Atlantic distinguished by having a triangular or squared tipped first dorsal fin (vs. first dorsal fin never squared tipped in S. ugoi and S. cabofriensis ), neurocranium with a narrow basal plate (vs. broad basal plate in S. cabofriensis ), color pattern with saddles not delineated by light or dark spots (vs. saddles delineated by light or dark spots in S. boa ), saddles darker than background color and lacking sharp median projections (vs. saddles indistinct in S. cabofriensis and S. boa and with sharp median projections in S. ugoi ), and spots large and small, sometimes sickle-shaped or lunate and with clear centers on the back and sides, arranged in approximate bilateral symmetry, and sometimes present in intersaddle regions (vs. spots spiracle-sized, randomly distributed, not sickle-shaped or lunate in S. cabofriensis ). The following combination of characters, although less conspicuous, also distinguishes these species: snout rounded and short, preoral length 4.5% TL (vs. 5% TL in S. ugoi and in S. cabofriensis ); head short and depressed, its length 17.5–19.2% TL (vs. 19.7–20.8% in S. cabofriensis and 19.5–20.3% in S. ugoi ); interdorsal space 1.2–2 times dorsal-caudal space (vs. 2 times in S. cabofriensis and 2.1–2.5 in S. ugoi ); claspers with ventral terminal cartilage 2 slender and positioned above ventral terminal cartilage, its length 1.8 times in ventral terminal cartilage (vs. 1.5 times in S. cabofriensis ); ventral terminal cartilage without a prominent groove posteriorly or with shallow and poorly developed groove (vs. groove well developed in S. cabofriensis ); small-sized, adult males at about 353 mm TL and adult females at about 415 mm TL (vs. 445 mm and 500 mm TL, respectively, in S. ugoi ).
Description. Morphometric data are given in Table 1 View TABLE 1 and neurocranial measurements in Table 6 View TABLE 6 . Modes of meristic counts are given, as well as their range, in parentheses, when values differ.
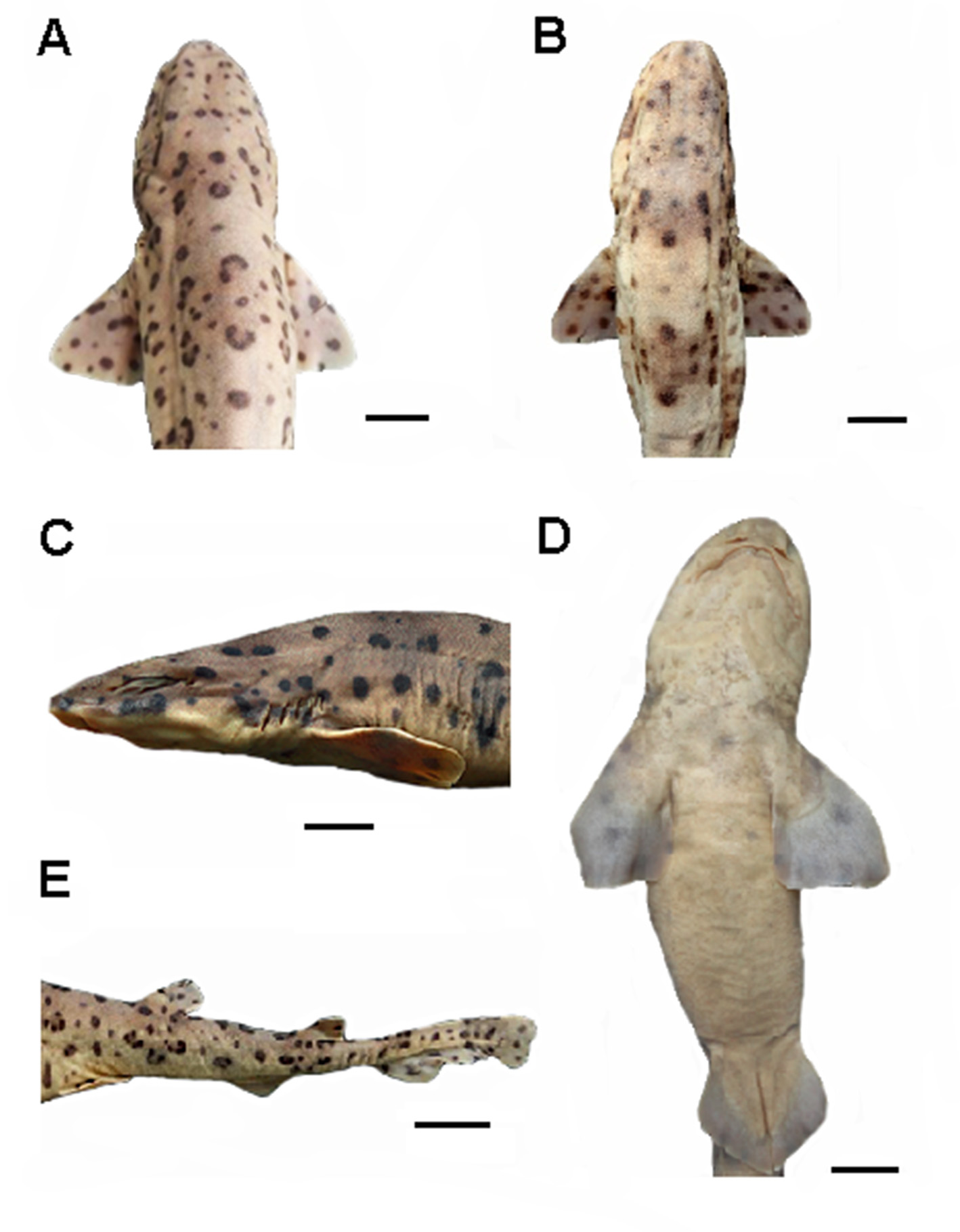
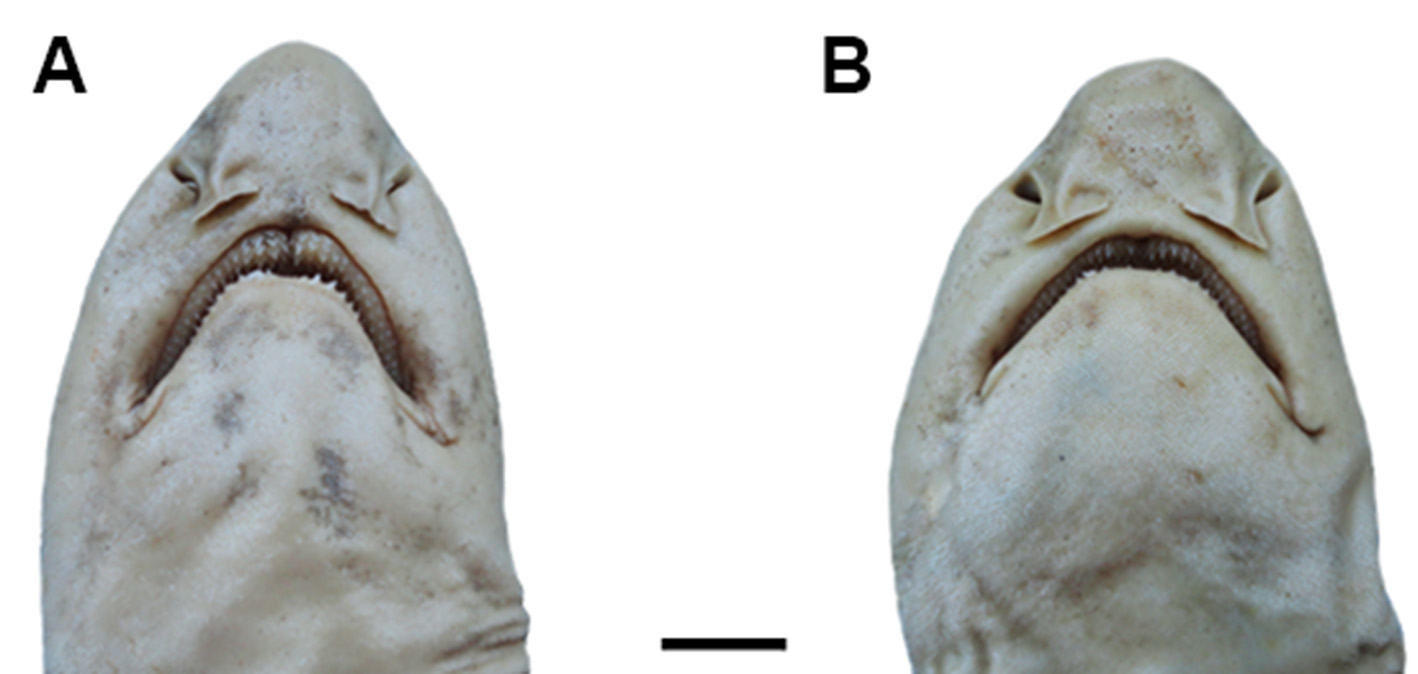
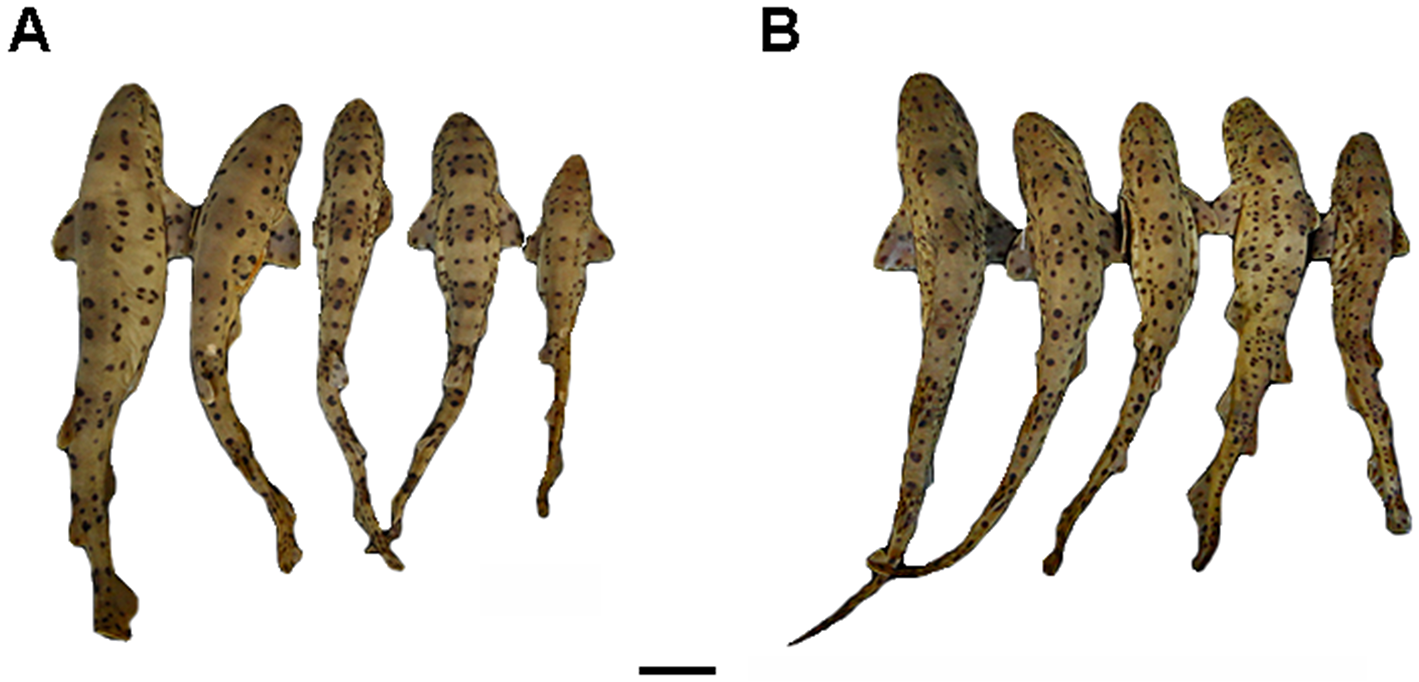
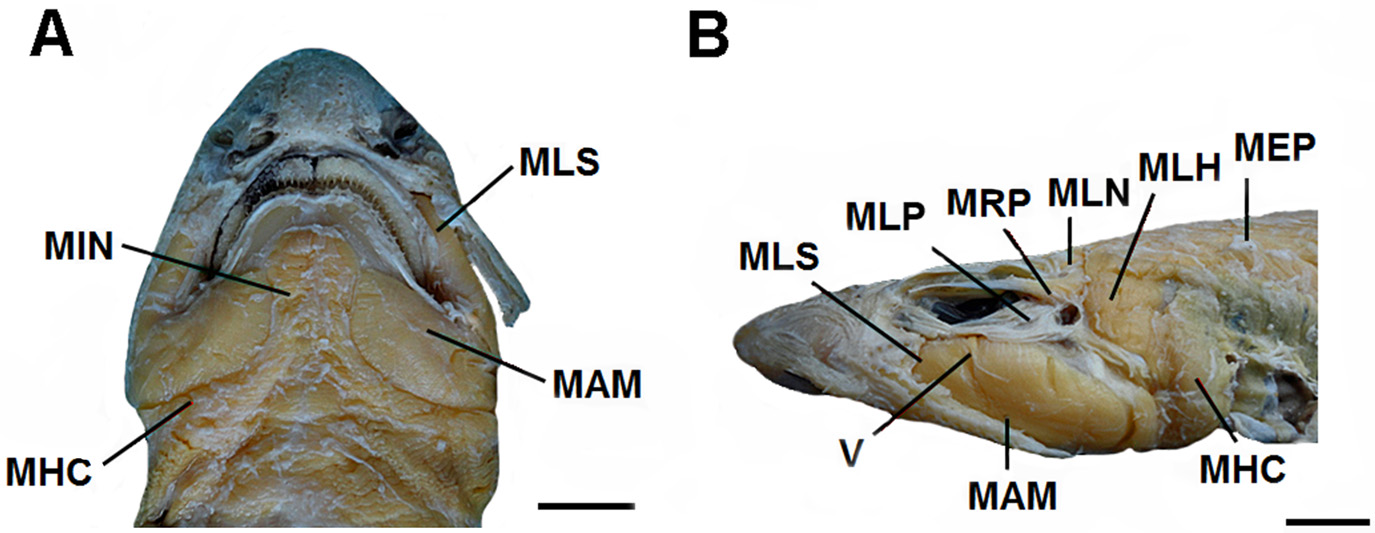
Body slender and depressed at head region, tapering considerably to caudal fin ( Figs. 9 View FIGURE 9 , 10 View FIGURE 10 ). Prepectoral length 16.1–18.2% TL; prepelvic length 34.9–43.7% TL. Snout-vent length 0.8 times vent-caudal length. Pectoral-pelvic space 0.8–1.4 times pelvic-anal distance. Interdorsal space 2.2 times dorsal-caudal space ( Tab. 1 View TABLE 1 ). Head moderately broad and depressed, greatest width 0.58 times head length ( Figs. 10 View FIGURE 10 , 11 View FIGURE 11 ). Snout relatively short; preoral length 3.7–4.9% TL and 0.6 times mouth width. Head length 17.5–19.2% TL and 1.7 times head width. Preoral length 1.3–1.4 times in preorbital length ( Tab. 1 View TABLE 1 ). Sexual dimorphism in snout and mouth shape evident ( Fig. 11 View FIGURE 11 ).
Eyes large and slender, dorsolateral on head ( Figs 9 View FIGURE 9 , 10 View FIGURE 10 ); eye length 2.9 times its height and 0.2 times head length. Nictitating lower eyelids of rudimentary type (sensu Compagno, 1970), with shallow subocular pouches and secondary lower eyelids free from upper eyelids. Prespiracular length 9.6–11% TL ( Tab. 1 View TABLE 1 ). Nostrils with broad incurrent apertures, without nasoral grooves or nasal barbels ( Fig. 11 View FIGURE 11 ). Anterior nasal flaps large, covering posterior nasal flaps and excurrent apertures, not extending to mouth. Internarial space 2.6 times interorbital space. Mouth moderately arched and long, its length 4.7–5.6% TL and 1.8 times in mouth width ( Fig. 11 View FIGURE 11 ). Lower labial furrows moderately long and narrow, its length 4.2 times in mouth width; upper labial furrows absent. Dorsal labial cartilages 1.5 times ventral labial cartilage; anterior tip of dorsal cartilages reaching orbital processes of palatoquadrate. First gill opening 2 times greater than fifth ( Fig. 10 View FIGURE 10 c). All gill openings slightly concave and not elevated on dorsolateral surface of head; gill filaments not visible externally.
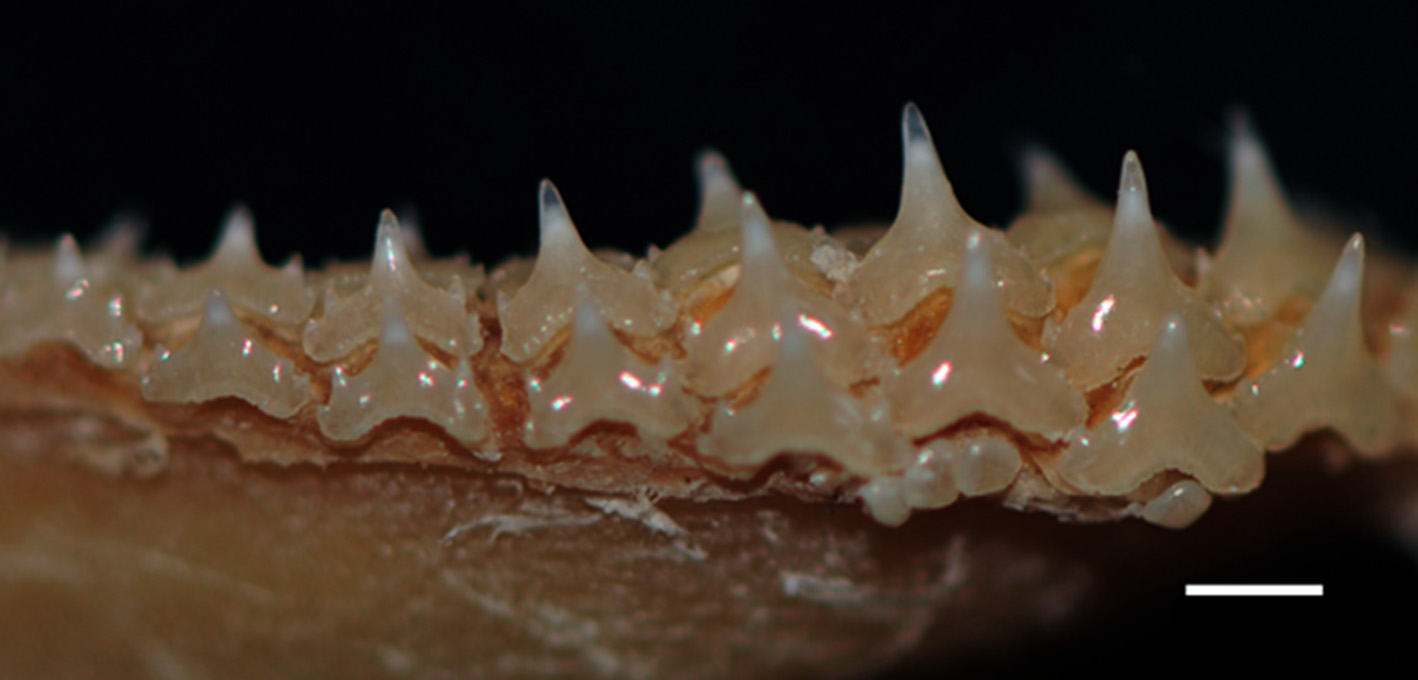
Teeth morphologically similar in both jaws; tooth row counts 52/49 (48–54/43–53), in 3/3 (2–3/2–3) functional series ( Tab. 2). Sexual heterodonty weak; adult males with longer teeth and undeveloped lateral cusps on central portion of lower jaw. Upper teeth with slightly higher crowns than lower teeth, and with longer, stronger transverse ridges. Medial teeth and poorly differentiated symphysial teeth with higher crowns, and smaller than anteroposterior teeth, with erect or semi-erect cusps and one weak cusplet on either side of main cusp. Anteroposterior dentition in both jaws larger than medial teeth and symphysials, with semi-oblique cusps, and usually one strong high cusplet on either side. Gradient monognathic heterodonty well developed in anteroposterior teeth; anteroposteriors smaller distally, with thicker and more oblique cusps, and lower cusplets ( Fig. 12 View FIGURE 12 ). Sexual dimorphism of teeth described by Gomes & Tomás (1991).
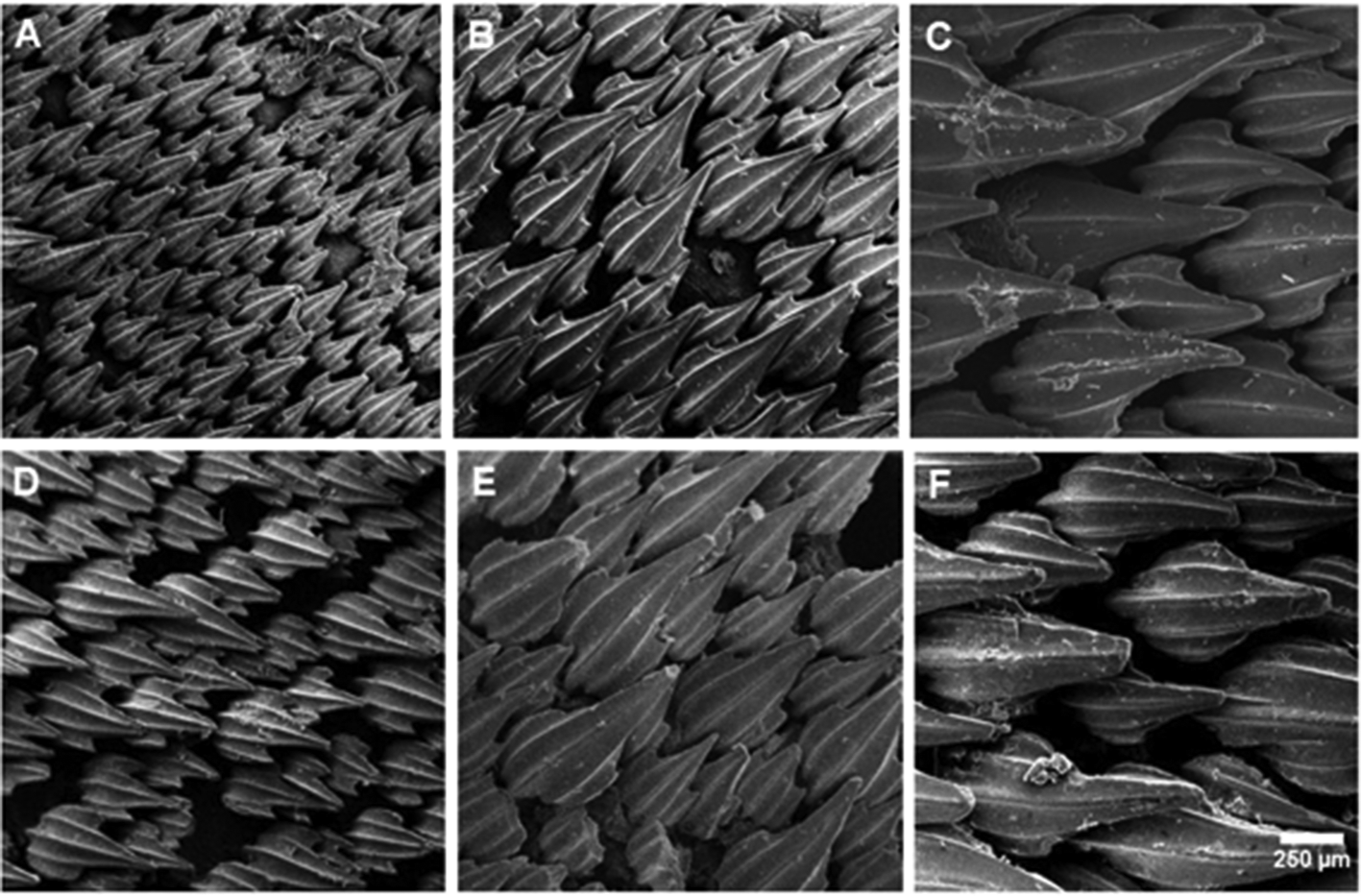
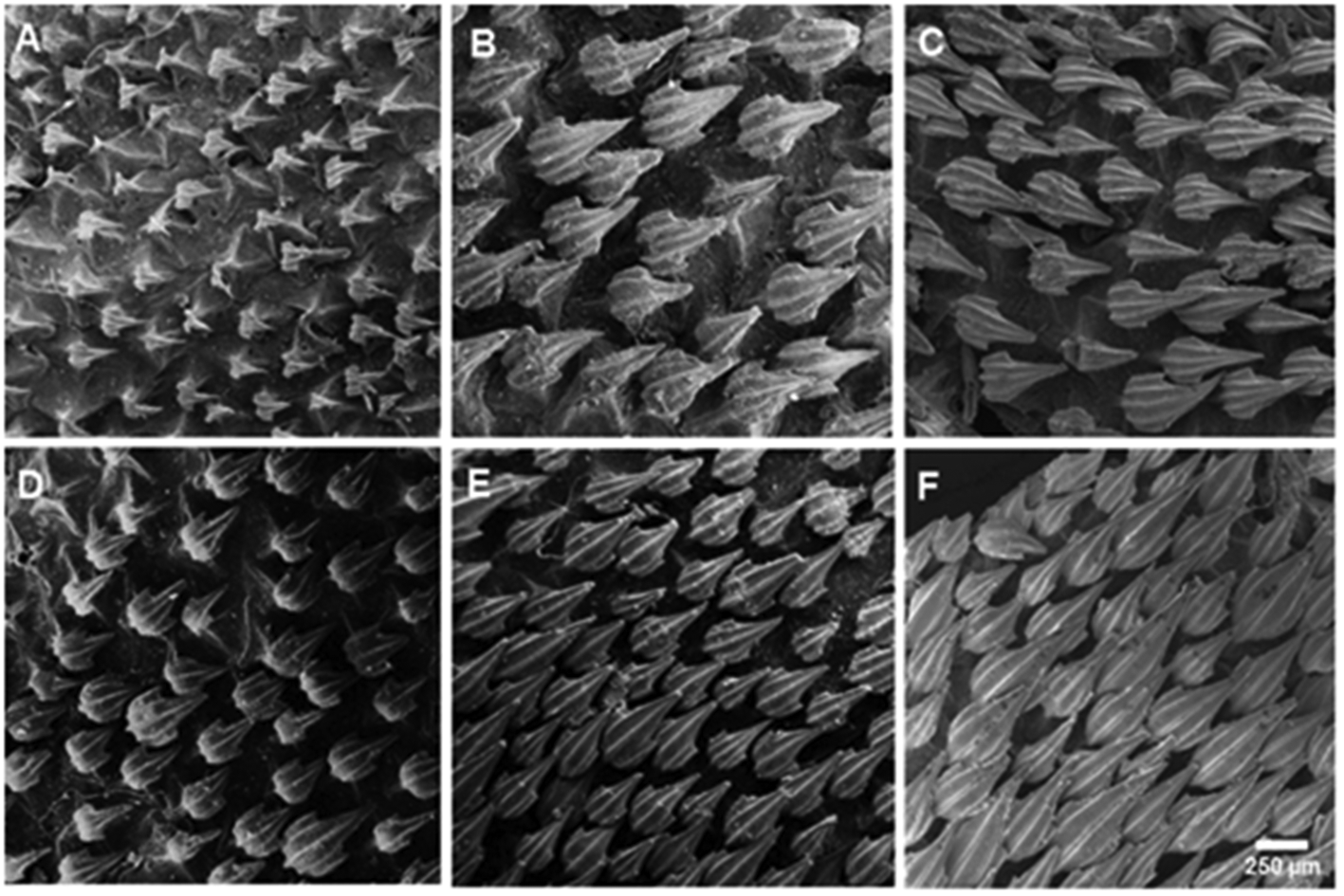
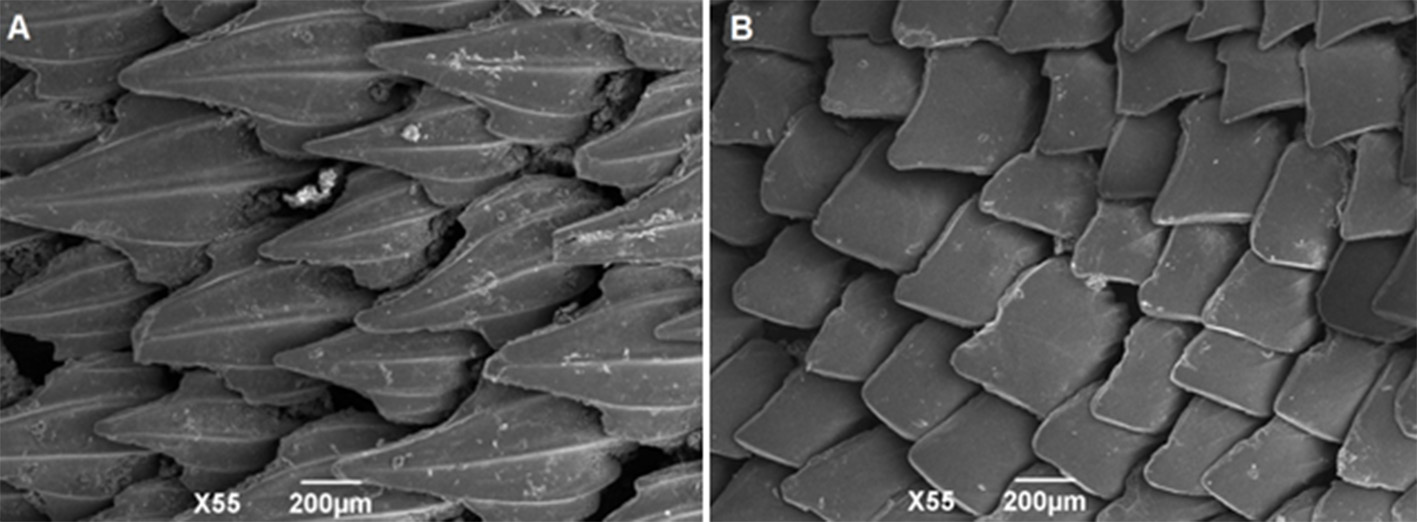
Lateral trunk denticles with flat, elongated, teardrop-shaped crowns, 1.5–2.1 times as long as wide; their anterior portion covered with ectodermal pits (sensu Muñoz-Chápuli, 1985) ( Fig. 13 View FIGURE 13 ). Crown with a strong medial ridge that extends entire length of the crown onto the long main cusp. Lateral cusps not well developed, 0.3–0.4 times main cusp; lateral ridges anterior to these very short or absent. Females with dermal denticles proportionately longer than males. Density of dermal denticles on posterior regions lower than on anterior regions ( Tab. 3 View TABLE 3 ).
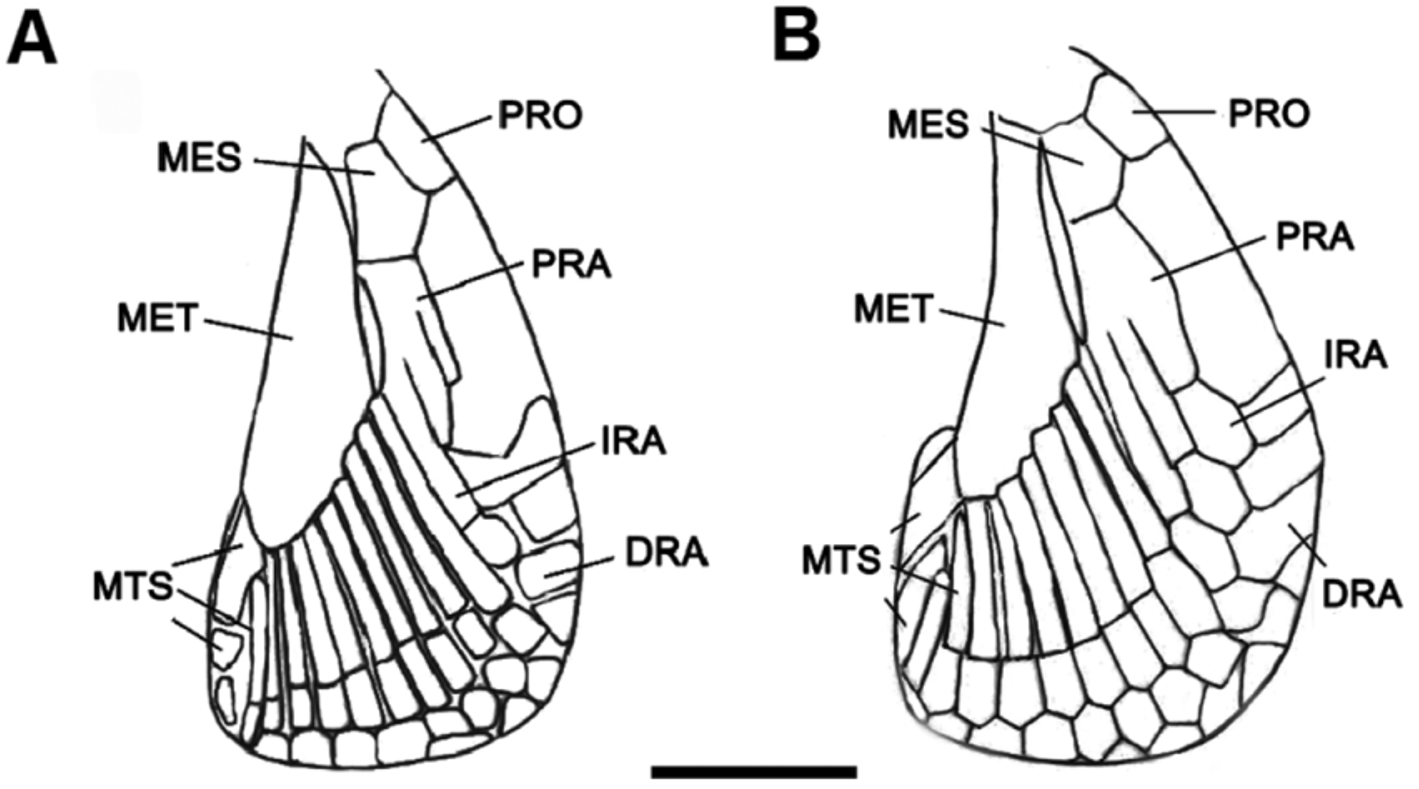
Pectoral fins large and rounded-triangular, not falcate, with narrowly rounded apices and broad bases ( Figs. 9 View FIGURE 9 , 10 View FIGURE 10 ). Origin of pectorals under interspace between third and fourth gill openings. Pectoral base 0.8 times mouth width. Pectoral anterior margin 1.7–2.3 times its base and 1.6 times its posterior margin. Pectoral fin skeleton aplesodic with radials mostly divided into three segments; longest distal segment (DRA) 1.6 times length of proximal segment (PRA). Total pectoral fin radial counts 14 (13–17) ( Table 2).
Pelvic fins broadly triangular ( Figs. 9 View FIGURE 9 , 10 View FIGURE 10 ); pelvic anterior margins 0.5 times pectoral anterior margins. Pelvic anterior margin about same length as its posterior margin. Total pelvic fin radial counts 15 (13–16) ( Tab. 2).
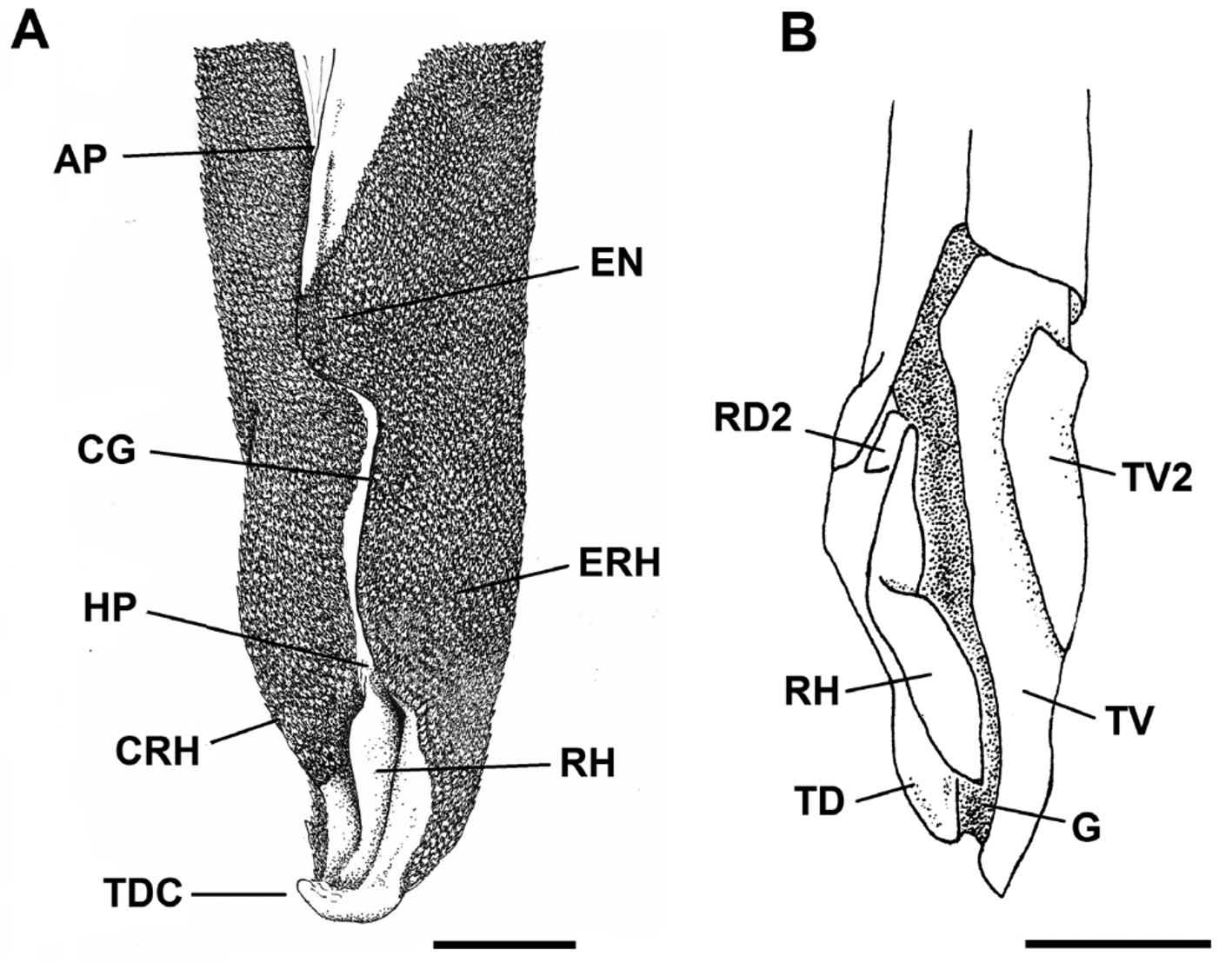
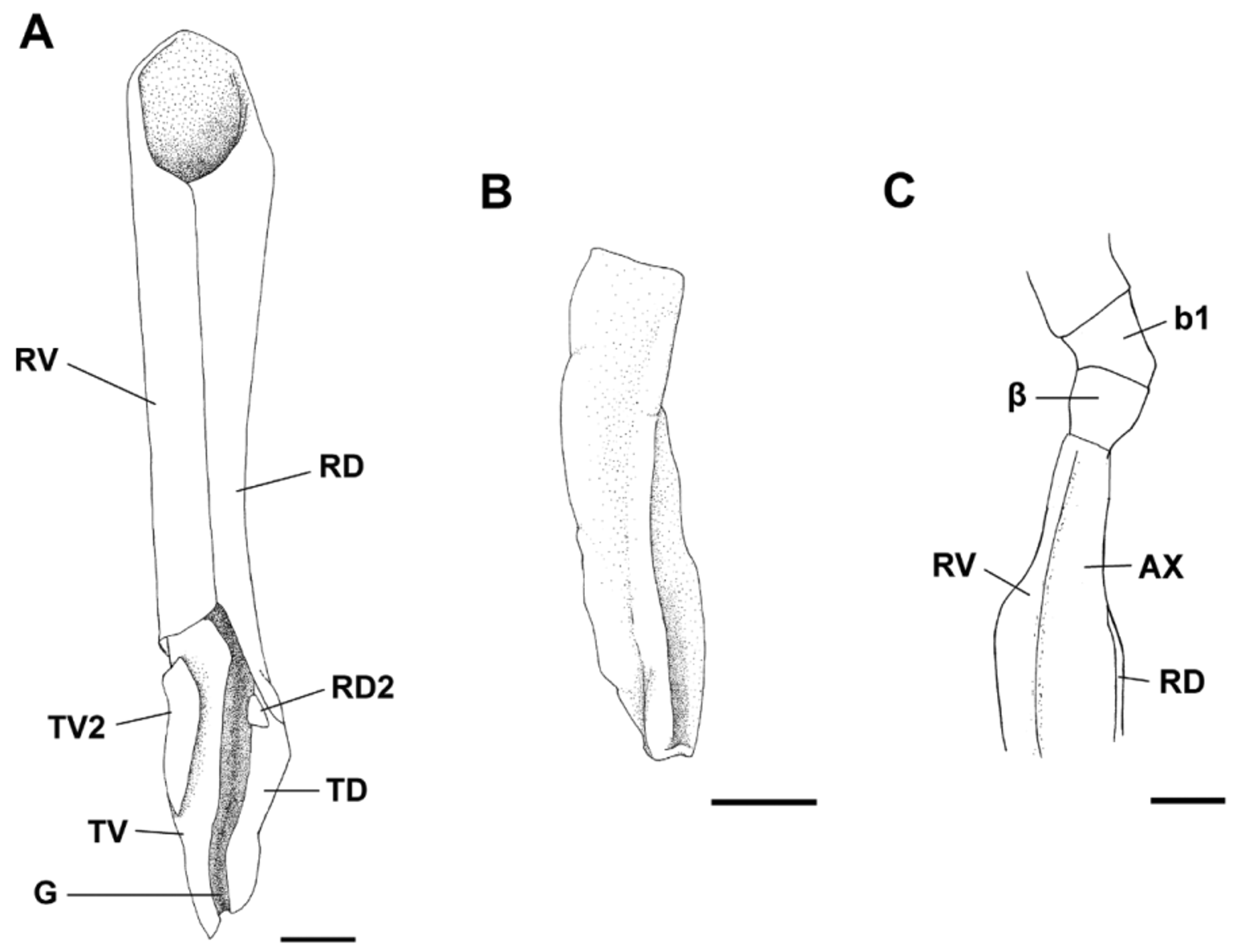
Claspers short, slender and cylindrical, sometimes extending from free rear tips of pelvic fins ( Fig. 14 View FIGURE 14 a); clasper inner length 1.1–1.4 times length of pelvic anterior margin ( Tab. 4 View TABLE 4 ). Most of clasper covered by dermal denticles with anteriorly directed cusps, except apopyle (AP), hypopyle (HP), dorsomedial surface of glans, rhipidion (RH) and terminal dermal cover (TDC). Exorhipidion (ERH) well developed corresponding to 13.5–14% of clasper inner length (CLI). Clasper hooks absent. Rhipidion well developed medially, covered by an anteriorly dilated cover rhipidion (CRH); insertion of rhipidion at anterior portion of accessory marginal dorsal cartilage (RD2) ( Fig. 14 View FIGURE 14 b). Cover rhipidion delimited anteriorly by a distinct envelope (EN), its length 10.9–11.2% CLI ( Tab. 4 View TABLE 4 ); in some specimens, cover rhipidion covers the medial portion of exorhipidion. Apopyle and hypopyle connected by a long clasper groove, with its dorsal margins fused over the clasper groove (CG). Pseudosiphon poorly developed and visualized only internally; pseudopera absent ( Fig. 15 View FIGURE 15 ). A terminal dermal cover present in the hindmost portion of clasper, covering one third of the ventral terminal cartilage and contacting exorhipidion and cover rhipidion.
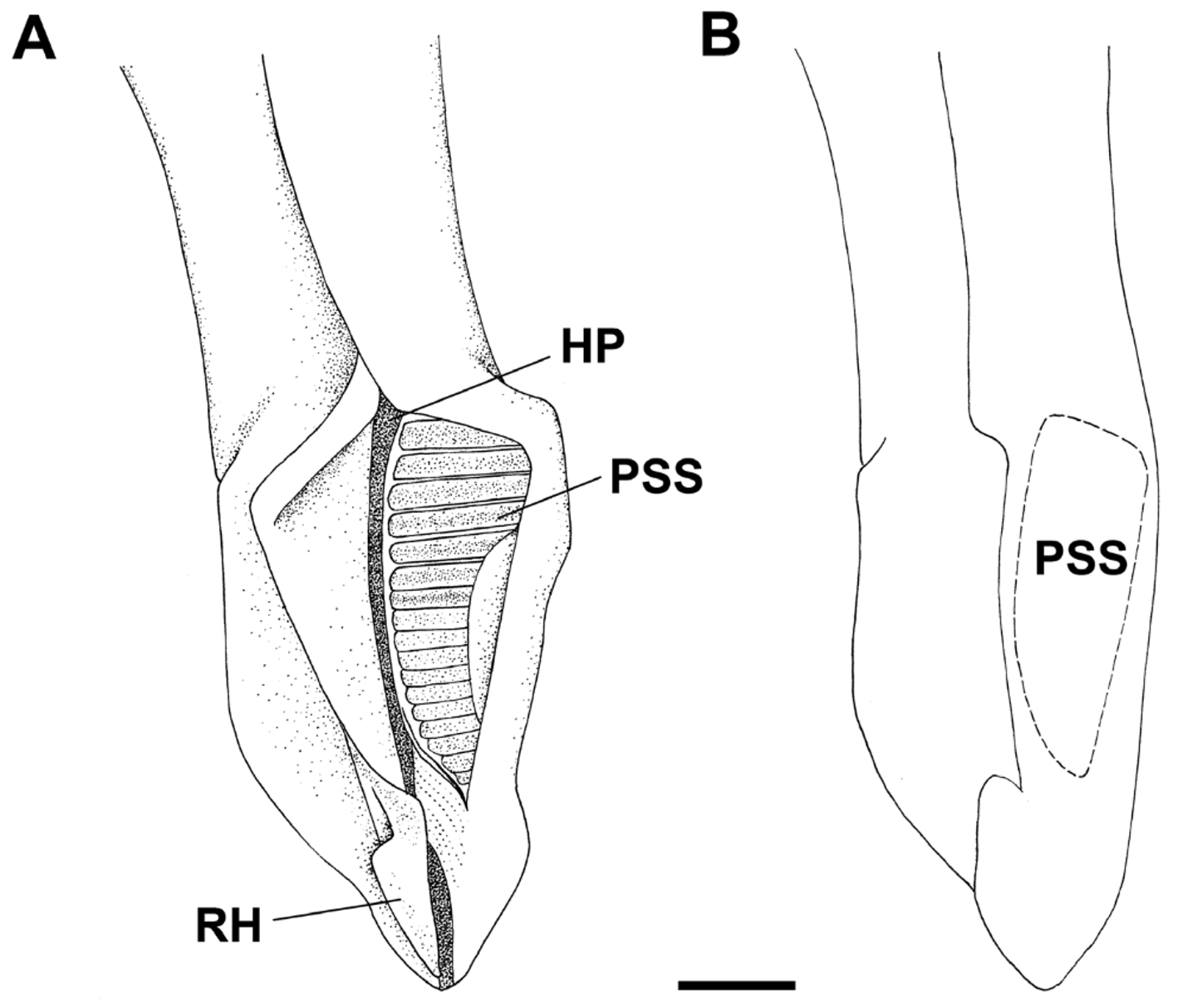
Measurements Scyliorhinus haeckelii Clasper skeleton relatively simple ( Fig. 16 View FIGURE 16 a). Ventral marginal cartilage 25.6–27.2% CLI; dorsal marginal cartilage 31.3–32% CLI ( Tab. 4 View TABLE 4 ). A poorly developed accessory dorsal marginal cartilage present between the terminal portion of the dorsal marginal cartilage and anterior portion of the dorsal terminal (TD). Terminal portion of the clasper resembling a ‘knife’; dorsal terminal cartilage slightly shorter than ventral terminal (TV). Accessory terminal cartilage and dorsal terminal 2 cartilage absent. Ventral terminal 2 cartilage (TV2) slender, positioned over the ventral terminal cartilage. Groove (GR) on terminal portion of ventral terminal cartilage absent or poorly developed ( Fig. 16 View FIGURE 16 b). End-style (G) elongated and extending between terminal cartilages.
First dorsal fin triangular or squared tipped, with a rounded apex and angular free rear tip ( Figs. 9 View FIGURE 9 , 10 View FIGURE 10 ). Anterior margin of first dorsal fin 1.3–1.5 times its base; first dorsal fin height 0.8 times its base. First dorsal fin origin positioned above insertions of pelvic fins. Total first dorsal radial count 12 (11–14) ( Tab. 2).
Second dorsal fin low, triangular or squared tipped, with bluntly rounded apex, narrowly rounded free rear tip, and straight inner margin ( Figs. 9 View FIGURE 9 , 10 View FIGURE 10 ). Anterior margin of second dorsal fin 1.0–1.4 times its base; second dorsal fin base 1.4 times its height and equal to dorsal-caudal space. Second dorsal fin origin slightly behind anal fin midbase. Total second dorsal fin radial count 10 (9–12) ( Tab. 2). First dorsal fin 1.4–1.6 times larger than second dorsal ( Tab. 1 View TABLE 1 ).
Anal fin low, apically narrow, not falcate and somewhat larger than second dorsal fin ( Figs. 9 View FIGURE 9 , 10 View FIGURE 10 ); anal fin base 1.5 times second dorsal fin base. Anal anterior margin nearly straight, apex narrowly rounded, free rear tip acutely pointed, and inner margin straight. Anal fin base without preanal ridges, anal fin origin and anal fin base length posterior to pelvic insertions. Anal fin base 0.6–0.8 times the interdorsal space and 1.3 times the dorsalcaudal space. Anal fin anterior margin 1.4–1.7 times posterior anal margin; anal fin height 0.3–0.6 times anal base. Total anal fin radial count 20 (17–22) ( Tab. 2).
Caudal fin narrow-lobed and asymmetrical, with well-developed large terminal lobe ( Figs. 9 View FIGURE 9 , 10 View FIGURE 10 ). Caudal fin corresponding to 24% of TL. Dorsal caudal lobe 2.2–2.7 times larger than the ventral lobe; subterminal caudal margin 0.5–1.1 times the terminal caudal margin. Dorsal caudal margin slightly convex, without lateral undulations. Denticles on caudal fin margins lacking crests.
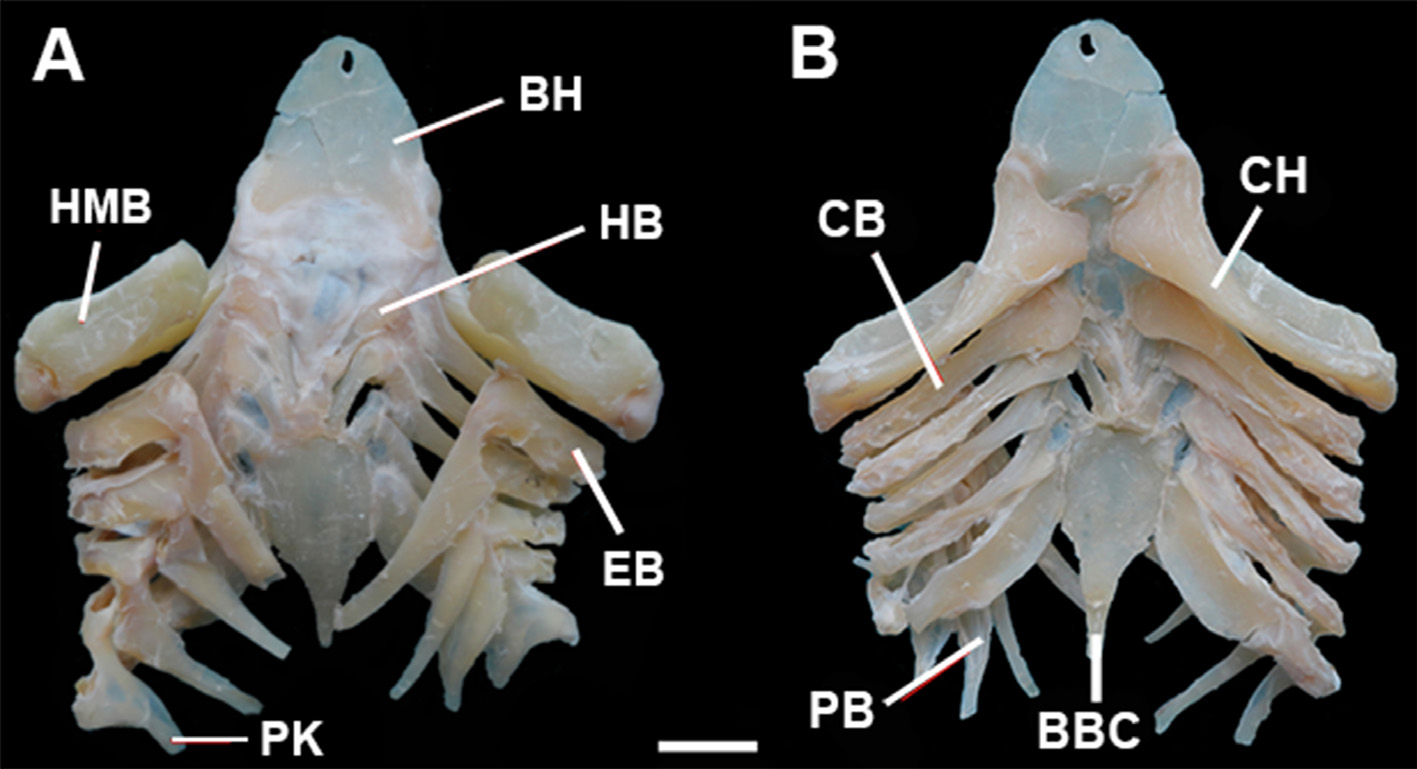
Total vertebral counts 124 (117–127), monospondylous precaudal (MP) centra 38 (36–40). Transition between MP and diplospondylous (DP) centra posterior to pelvic bases and over clasper shafts. Last MP centrum before MP–DP transition smaller than the anterior MP centrum and larger than the first DP centrum, not forming a 'stutter zone' of alternating long and short centra.
Intestinal valve of conicospiral type, with 6 (6–8) turns.
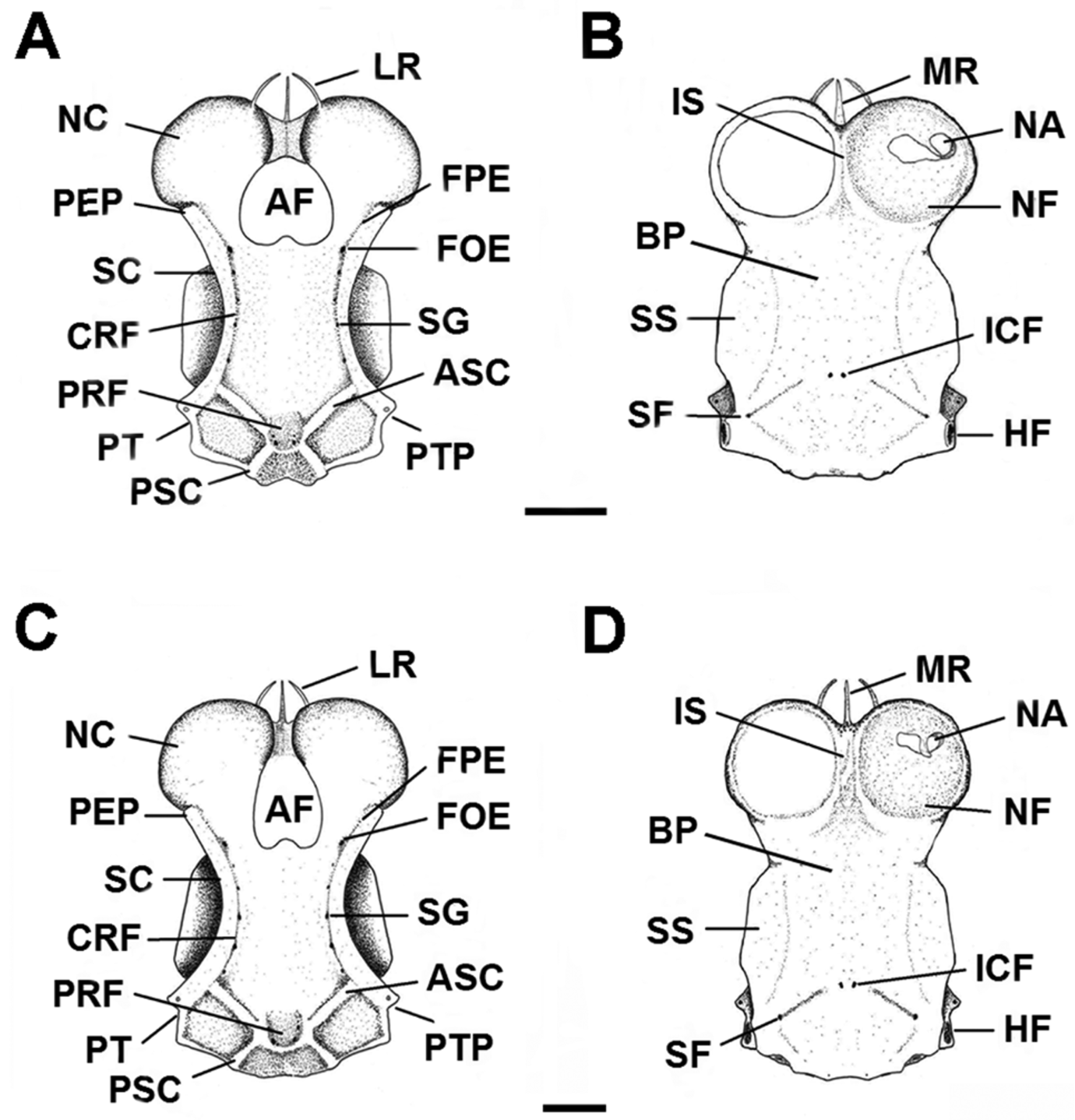
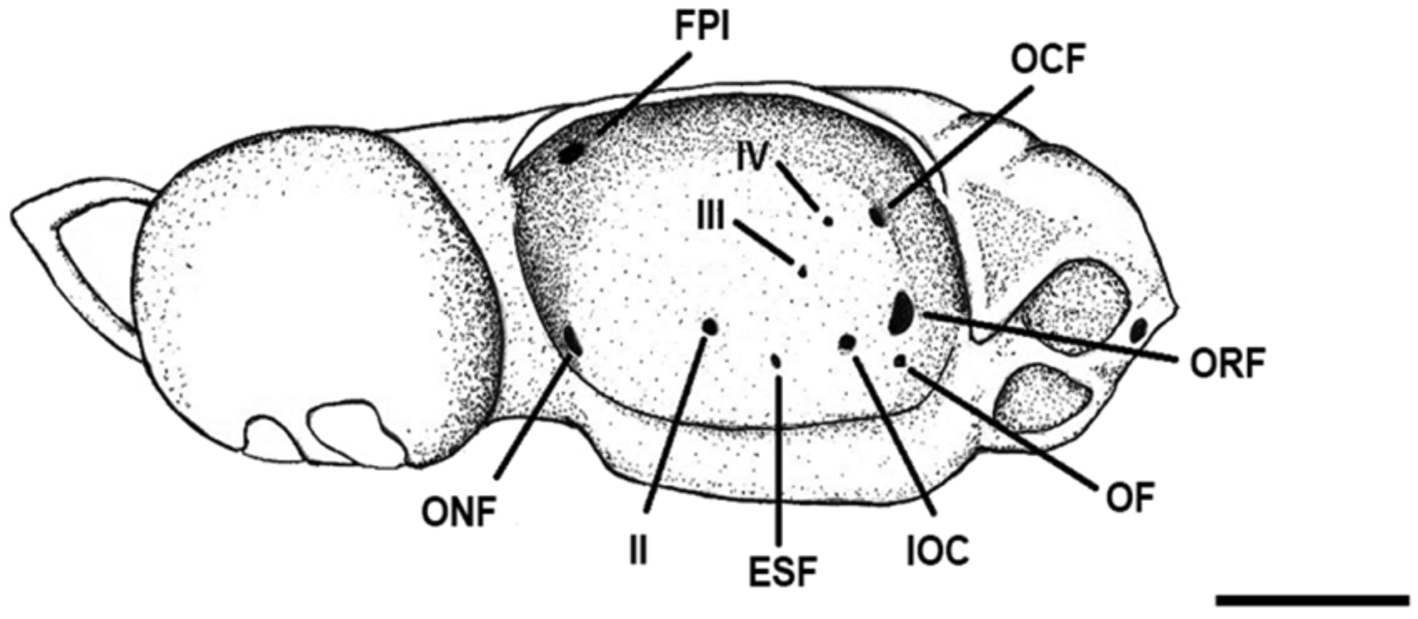
Neurocranium broad and somewhat flattened, corresponding to 10% TL ( Fig. 17 View FIGURE 17 , Tab. 6 View TABLE 6 ). Rostral cartilages short and slender; rostral length 22.5–30% of nasobasal length (NL). Distance between lateral rostral cartilages 12.7–18.3% NL. Nasal capsules (NC) large, oval and expanded laterally; their width about equal to their length. Width across nasal capsules proportionately larger in males (73–79.1% NL) compared to females (70–73.4% NL). Nasal apertures (NA) broadly circular and laterally positioned on nasal capsules, 2/3 of their area covered by nasal fontanelle (NF). Width of nasal openings 22.2–27.2% NL. Distance between rostral base and anterior edge of anterior fontanelle (AF) increasing with growth, reaching 45.8% NL. Anterior fontanelle broad and subrectangular in males, and more heart-shaped in females, its width proportionally larger in females (22.5–24.5% NL) compared to males (17.7–20.8% NL). Basal plate (BP) flat and without keels, its width corresponding to 33.3–40.8% NL. Orbits oval to subrectangular, their length 2.2 times in nasobasal length. Width across postorbital processes 1.2 times preorbital processes (PEP). Optic capsules short, their length 4.6 times in nasobasal length; their width 2.2– 2.5 times optic capsules length.
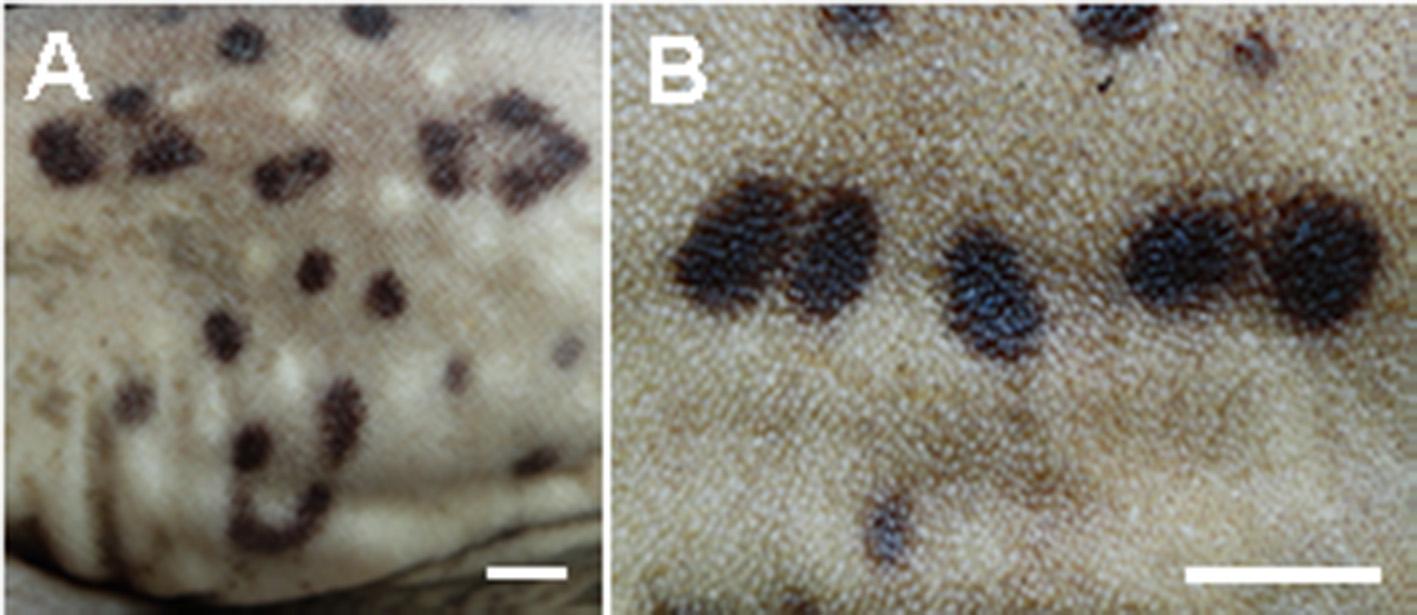
Coloration. Color pattern with saddles darker than background color, not delineated by light or dark spots, and without sharp median projections; light and dark spots large and small, arranged in approximate bilateral symmetry, sometimes in intersaddle areas. Spots double, lunate or clear-centered, present on the back and sides. Lighter general color on ventral region. In most of cases, males more pigmented than females ( Fig. 18 View FIGURE 18 ). Adult males with white spots as of the post pectoral saddle ( Fig. 19 View FIGURE 19 a), while in adult females, when present, as of the first dorsal fin saddle. Lunate spots larger and more frequent in males than in females; double spots more frequent in females ( Fig. 19 View FIGURE 19 b). Juveniles smaller than 250 mm TL with little or no sexual dimorphism in color pattern.
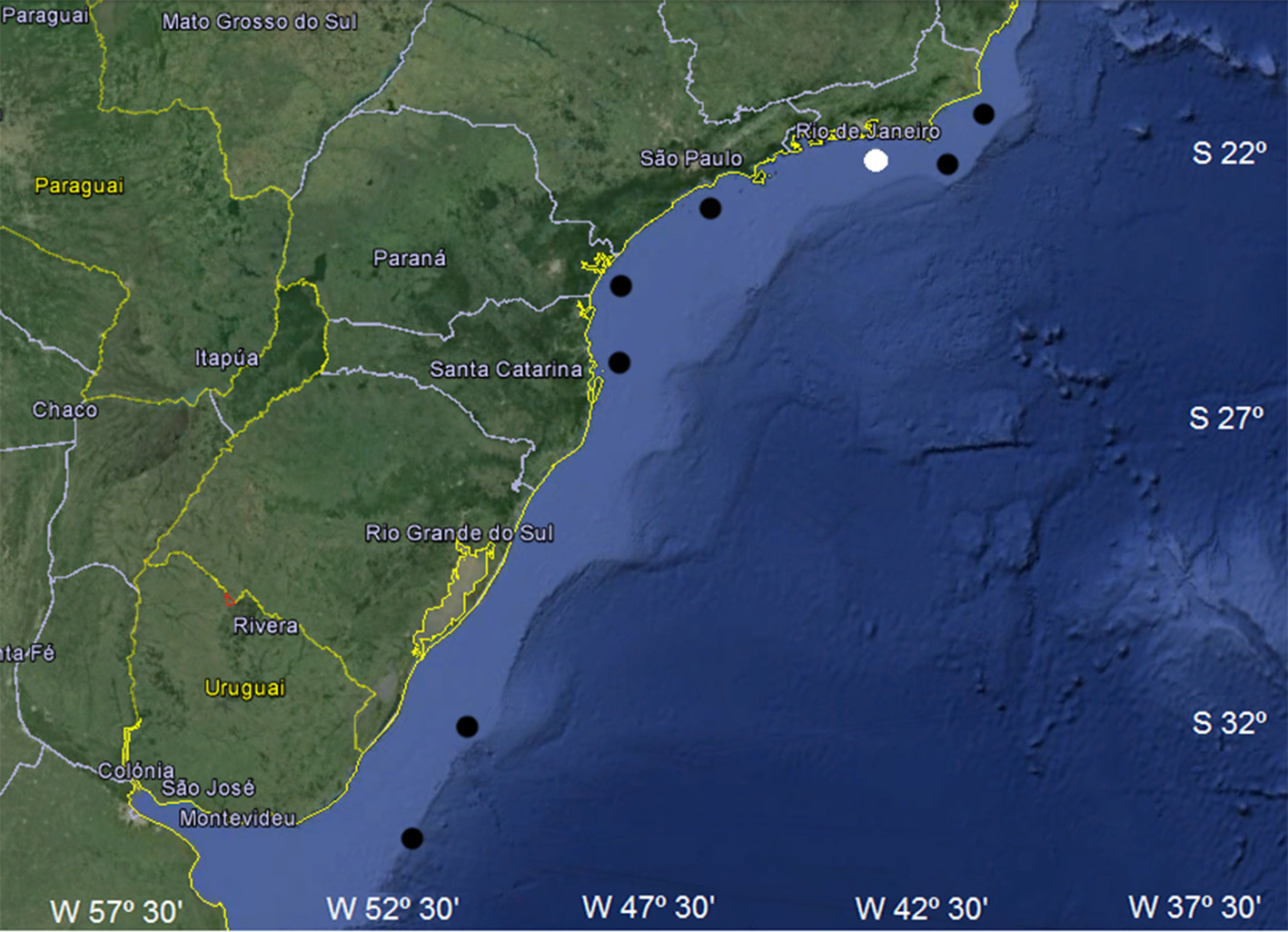
Distribution and biological data. This species is recorded from the coast of Venezuela to Amapá state ( Brazil), but confirmation is needed, and from northern Rio de Janeiro state to Argentina ( Springer, 1979; Compagno et al., 2005; Figueroa, 2011); records of its occurrence in northeastern Brazil are unknown (specimens analyzed in this study were from the southwestern Atlantic; Fig. 20 View FIGURE 20 ). Most recorded specimens occur between 37– 402 m in depth, associated with coral or calcareous algal formations where it possibly lays it egg-capsules ( Bigelow & Schroeder, 1948; Springer, 1966, 1979). Stomach contents include squid beaks and skeletal elements of bony fishes. Maximum total length is about 570 mm TL. Males range in size between 147–566 mm TL (n = 58), with well-developed claspers at about 353 mm TL; females are known from 165–509 mm TL (n = 67), adults from 415 mm TL ( Gadig, 2001). Egg-capsules are light amber to yellowish, without longitudinal grooves and measure about 60 mm in length and 25 mm in width (Gomes & de Carvalho, 1995).
TABLE 6. Measurements of neurocrania in mm and as percentages of nasobasal length (% NL) for males and females of S. haeckelii. Abbreviation: n, number of specimens.
| Nasobasal length (NL) | Female (n = 6) Mean % NL 43.3 100 | Male (n = 6) Mean % NL 51.5 100 |
|---|---|---|
| Rostral length Width across lateral rostral cartilages Width across nasal capsules | 10.3 22.5–26.5 7.4 15.0–18.3 32.2 70.0–73.4 | 13.5 25.4–27.0 7.6 12.7–14.5 39.5 74.5–79.1 |
| Nasal capsule width Nasal capsule length Nasal aperture width | 17 37.5–38.8 16.6 36.5–37.5 11.3 24.5–25.0 | 21.5 40.0–43.7 20.1 38.1–39.5 13.5 25.0–27.2 |
| Distance between nasal apertures Distance between rostral base and posterior edge of anterior fontanelle Anterior fontanelle width Basal plate width | 5.3 10.0–14.3 17.2 38.7–40.0 10.5 22.5–24.5 16.3 35.0–40.8 | 7.5 12.5–14.5 22.5 41.8–45.8 11.5 20.0–22.9 20.3 37.5–40.0 |
| Orbit length Preorbital process length Postorbital process length | 19.1 42.8–45.0 3.2 5.0–8.1 3.5 7.5–8.1 | 23.1 41.8–47.9 3.0 5.4–6.2 4.5 8.3–9.0 |
| Width across orbital notches Suborbital shelf width Otic capsules width | 9.3 20.4–22.5 24.5 55.0 21.0 47.0–47.5 | 10.5 20.0–20.8 29.5 56.3–58.3 26.5 49.0–54.1 |
| Width across preorbital processes Width across postorbital processes | 24.5 52.5–57.1 30.5 69.3–72.5 | 31.2 58.1–62.5 37.5 70.9–75 |
| MNRJ |
Museu Nacional/Universidade Federal de Rio de Janeiro |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Scyliorhinus haeckelii
| Soares, Karla D. A., Gomes, Ulisses L. & De Carvalho, Marcelo R. 2016 |
Scyliorhinus haeckeli:
| Compagno 1984 |
Scyliorhinus besnardi:
| Springer 1979 |
Scyliorhinus retifer:
| Figueiredo 1977 |
Scyliorhinus retifer haeckelii:
| Springer & Sadowsky 1970 |
Scyliorhinus retifer besnardi
| Springer & Sadowsky 1970 |
Scyliorhinus haeckelii:
| Springer 1966 |
Scyliorhinus fernandezi
| Weibezahn 1953 |
Scyliorhinus boa:
| Bigelow & Schroeder 1948 |