1. Wolfgang Pauli’s hypothesis
Before explaining how Pauli’s came to state his famous hypothesis, let’s do a quick recap on notation and beta decays.
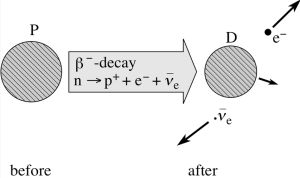
The notation we will use is the following: the decay of a parent nucleus, P, to a daughter nucleus, D, generally involves the emission of other decay products:
P -> D + other products + Q
The quantity Q represents the energy required to balance this process. Its value can be determined by applying the conservation of (relativistic) energy to the process. That is, Q can be found by requiring that the total energy immediately before the decay is equal to the total energy immediately after the decay, where the term TOTAL energy is taken to include the energy equivalent of any rest masses involved (as given by Einstein’s mass-energy equation, E = mc2) as well as the relativistic kinetic energy of the particles. The energy represented by Q appears as the kinetic energy shared between the daughter nucleus, D, and other products. Beta decays, like other radioactive decays not discussed here, result in positive Q values.
In a two-body process, the lighter emitted particle, here, the electron (also called a BETA particle in the case of a beta decay), would be expected to carry away most of the released energy, which would have a unique value. Why?
Because the masses of the electron and the daughter nucleus are fixed, the available energy (or Q-value) would split in a definite proportion between the two and only one definite kinetic energy for the electron would be possible. ONLY if there is an additional third particle involved can the energy be split between the three in an infinite number of ways, limited only by the total energy available. All kinetic energies for the electron, up to some maximum (total energy available), become possible.
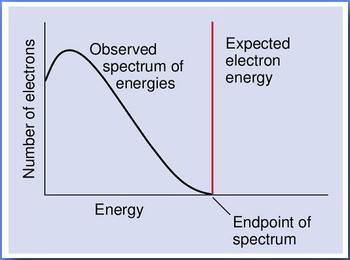
However, experiments show that the electrons are emitted with a continuous spectrum of energies. In fact, the observed differential distribution in the number of emitted electrons as a function of their energy has the shape given by this picture:
The endpoint corresponds to the maximum energy of any emitted electron. That is, the experiment shows that the electrons have a spectrum of energies, with most values lying well below that predicted by energy conservation in two-body decays.
When this was first observed, it appeared to be in contradiction with the conservation of energy and as we will see, the conservation of momentum too!
The questions that are at the origin of Pauli’s hypothesis are the following:
First, why beta particles have a range of energies? There must be some process which randomly selected energies. Why did it choose one energy and not another?
Second, when a nucleus emitted a beta particle it didn’t recoil backwards in a straight line but normally at an angle. This seemed to violate the principle of conservation of momentum. Why an angle? Is there a third particle possibly carrying some of the momentum?
Third, there is an apparent violation of the law of conservation of angular momentum. The total spin angular momentum of the initial particle before beta decay occurs is not equal the total angular momentum of all particles produced afterwards.
The beta decays experiment in the 1930s could not see the third particle but it looked like there was one “ghost” particle. This “ghost” particle was needed in order to satisfy the conservation of mass and energy but it had no charge and interacted very weakly with ordinary matter. The Italian nuclear physicist Enrico Fermi took up Pauli’s idea and on its basis developed a theory of beta decay. Fermi also coined the term “neutrino”, after Pauli had spoken of “neutron”, but the latter designation was reserved for the heavy component of the atomic nucleus discovered in 1932. Pauli’s hypothesis was presented in 1933. It then took a further 23 years (until 1956) before the experimental proof of the existence of the neutrino succeeded.
Wolfgang Pauli proposed in 1933 that the third particle, one that was difficult to detect, was emitted in beta-decay. Conservation of electric charge required this particle to be electrically neutral, just like the neutron and the photon. In fact, this would explain why it was so hard to detect this particle. We know now that this neutral particle, the neutrino, does not interact readily with matter, and this is the main reason why it is so difficult to observe. Because the maximum energies for electrons emitted in beta-decay corresponded to the disintegration energy of the nucleus, it meant that this new particle had
to be essentially massless. Furthermore, if the postulated neutrino were to restore the conservation of angular momentum, then it would have to be a fermion with spin angular momentum 1/2.
2. Discovery: The Cowan and Reines experiment of 1956
In short, the famous Cowan and Reines experiment is the first experiment which detects neutrinos indirectly by observing the effects of neutrino collisions with matter. After a collision, there is creation of an antiparticle, the annihilation of this antiparticle creates high energy photons or gamma rays, which are amplified by a liquid scintillator, which gives of flashes of visible light in response to invisible gamma rays. These flashes of visible light have to be detected by photomultiplier-tubes some microseconds after the gamma rays were emitted. The problem with this method is it’s sensibility to neutrinos created by cosmic rays and by the sun which are considered background noise for this experiment.
The experiment used a nuclear reactor as the source of a neutrino flux of 5×10^13 neutrinos per second per square centimeter. It was far higher than any attainable flux from other radioactive sources. Negative beta decay in the nuclear reactor, which is only one type of radioactive decay, produces an electron antineutrino, the electron antineutrinos interacted with protons in two tanks of water, creating neutrons and positrons:
(1)
The positron quickly finds an electron, and they annihilate each other, so that each positron created a pair of gamma rays when it annihilated. The gamma rays were detected by sandwiching the water tanks between tanks filled with liquid scintillator. The scintillator material gives off flashes of light in response to the gamma rays, and these light flashes are detected by photomultiplier tubes.
This experiment was not conclusive enough, so they devised a second layer of certainty. They detected the neutrons by placing cadmium chloride in the tank. Cadmium is a highly effective neutron absorber and gives off a gamma ray when it absorbs a neutron. The gamma ray from the cadmium capturing one neutron produced by reaction (1) would be detected 5 microseconds after the gamma ray from the positron annihilation, if it were truly produced by a neutrino.
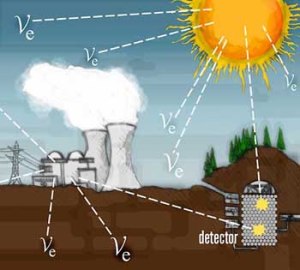
The experiment was moved several times in order to decrease the background noise, and get better results by shielding the experiment against cosmic rays. Because cosmic rays are also producing neutrinos in the upper atmosphere. This shielded location was far away from the reactor and several meters underground, as depicted on this picture:
After months of data collection, they had accumulated data on about three neutrinos per hour in their detector! To be absolutely sure that they were seeing neutrino events from the detection scheme described above, they shutdown the reactor to show that there was a difference in the number of detected events.
Sources: Wikipedia and Das & Ferbel, Introduction to Nuclear and Particle Physics (2003)