Pteropods (or ‘sea butterflies’) are snails that are part of the zooplankton. As part of my PhD I am especially interested in shelled pteropods, the thecosomes (euthecosomes and pseudothecosomes; here I focus on euthecosomes). Also shell-less pteropods exist: gymnosomes. Pteropods have modified parts of their soft tissue to resemble paired swimming wings, using them to migrate vertically in the water column, coming towards the ocean surface at night. This is why we sample overnight. Most genera are epipelagic and are represented in our samples during the cruise, but a minority of species lives in deeper waters.

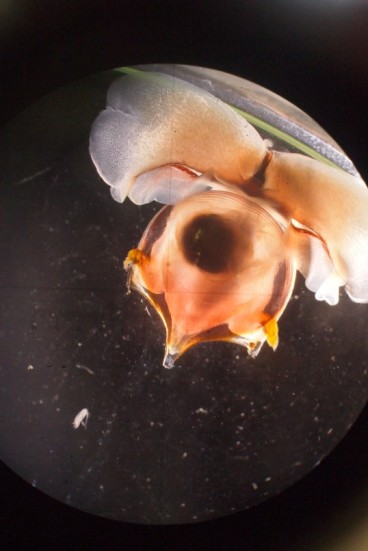
Cuvierina cancapae (photographed by Alice Burridge)
Euthecosomes have amazingly diverse shell morphologies and ontogenies (development of an organism). Limacinidae (Limacina, Heliconoides, Thielea) have characteristic shells that coil sinistrally (anti-clockwise direction) and are generally small in size (0.5 – 4 mm), one exception is the deep water species Thielea helicoides (up to 15 mm). Shells of Cavoliniidae have all kinds of shapes and are generally bigger than Limacinidae shells. Styliola, Creseis and Hyalocylis have pointy shells throughout their lives, with Hyalocylis striata having remarkable ribs on its fragile shell. Also Clio species have pointy shells, but they have a much broader aperture. Cuvierina species have large bottle-shaped shells (up to 10 mm). Cavolinia, Diacavolinia and Diacria have roundish, complex shell morphologies, with some Diacria species having long spines (such as Diacria trispinosa). Diacria danae and Cuvierina species shed their pointy juvenile shells before reaching their adult morphologies.
Apart from sampling, counting and preserving pteropods, I have also tried to run some experiments with them, but they are difficult to keep alive for very long. During the incubation the water had to be cleared from mucus regularly. Pteropods feed by building mucus webs in which they entangle food. These webs can be several cm wide, much bigger than the animals themselves. Although they were hard to culture, it was fascinating to observe the different ways they swim through the water column. For example, Creseis virgula swims with its curved tip pointing in its horizontal swimming direction, while the bulky and roundish Cavolinia uncinata and Diacavolinia clumsily and seemingly less efficiently try to swim along. Cuvierina cancapae is, when unharmed from the sampling procedure, a very active swimmer, its bottle-shaped shell tilting back and forth. Their active swimming does not seem to be the most energy efficient in the universe of zooplankton.
My most interesting catch during this cruise was a beautiful and big Cavolinia tridentata, probably a one-time catch. It took a while, but when it came alive in its jar it was too active to image using the microscope camera, and too big anyway (15 mm), so I tried to image it directly through the lens of the microscope. Quite some other scientists came over to gaze at this amazing creature. You could even hear it swim.
It only took us a few days to get through the tropics and now we are in the subtropics, which we are about to leave by October 23rd already. Still, every time the net comes up it is a surprise what’s in there. The transition to a new oceanographic province is noticeable in the changing species composition. This shows that biogeographic barriers in the ocean do exist but they are not hard boundaries. As such, the tropical zone is a barrier for some species, but a niche for others. Some pteropod species have returned in our samples after having been absent in the tropical zone, occurring in both the northern and the southern subtropics. I wonder what I will find even further down south, apart from more turbulent waters.


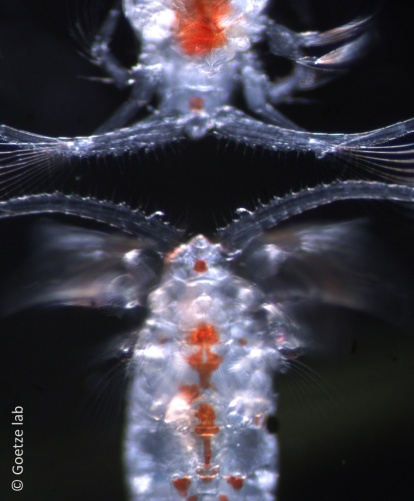 This beautiful Pleuromamma copepod is distinct from both P. abdominalis and P. quadrungulata, with which it co-occurs here in the equatorial region. It has long been noted that there is variation in the presence and degree of development of spines on the antennules of Pleuromamma copepods. In the photo, you can see two animals, oriented head to head, with the top animal lacking the strong spines on the antennules that are very visible in the animal below. It turns out that this variation is informative, and can be used to distinguish good species. Stay tuned for an upcoming publication from Junya Hirai (University of Tokyo) on the genetic lineages within the ubiquitous species P. abdominalis. I hope to find the time to establish a name for this organism, along with the many other undescribed lineages within this nominal species. Establishing a new name for a species is an opportunity to honor your predecessors and acknowledge the heroes that inspired your scientific journey. I hope to name at least one of these species P. frosti, in honor of Bruce Frost.
This beautiful Pleuromamma copepod is distinct from both P. abdominalis and P. quadrungulata, with which it co-occurs here in the equatorial region. It has long been noted that there is variation in the presence and degree of development of spines on the antennules of Pleuromamma copepods. In the photo, you can see two animals, oriented head to head, with the top animal lacking the strong spines on the antennules that are very visible in the animal below. It turns out that this variation is informative, and can be used to distinguish good species. Stay tuned for an upcoming publication from Junya Hirai (University of Tokyo) on the genetic lineages within the ubiquitous species P. abdominalis. I hope to find the time to establish a name for this organism, along with the many other undescribed lineages within this nominal species. Establishing a new name for a species is an opportunity to honor your predecessors and acknowledge the heroes that inspired your scientific journey. I hope to name at least one of these species P. frosti, in honor of Bruce Frost.





