Introduction
Diabetes mellitus (DM) is an independent risk factor for heart failure and there exists a bidirectional relationship between DM and heart failure (HF). The prevalence of heart failure in patients with DM is 4 times higher than in the general population.1 According to the Framingham Heart Study, cardiovascular disease (CVD) attributable to DM has increased over the past 50 years. Amongst other risk factors, only DM demonstrated an increase in the population attributable risk (PAR) for heart failure over the 2 time periods (1952 to 1974 and 1975 to 1998).2 The pathophysiology of heart failure preserved ejection fraction (HFpEF) is closely related to DM and approximately 40% of HFpEF patients have DM.3 Heart failure with reduced ejection fraction (HFrEF) is often associated with DM progression. HFrEF has a strong association with type 1 diabetes mellitus (T1DM).4
DM can cause various structural and functional changes in the myocardium. These changes are characterized by abnormal cardiac structure and function in the absence of other cardiac risk factors and was first reported in a postmortem study from diabetic patients who developed heart failure symptoms without evidence of coronary artery or valve disease. In 2013, the American College of Cardiology Foundation, the American Heart Association (ACC/AHA), and the European Society of Cardiology (ESC) in collaboration with the European Association for the Study of Diabetes (EASD) defined diabetic cardiomyopathy as a clinical condition of ventricular dysfunction that occurs in the absence of coronary atherosclerosis and hypertension in patients with diabetes mellitus.5,6 In the early stages, some structural and functional changes occur, some of which are left ventricular (LV) hypertrophy, fibrosis, and cell signaling disruption.7 These changes evolve into HF and further into HFrEF. The goal of this review is to summarize current knowledge about diabetic cardiomyopathy, its current pathophysiology and novel treatments.
Methods
The research design of this study was a short narrative review. We conducted a literature search on diabetic cardiomyopathy and existing novel treatment from databases consisting of PubMed and Google Scholar. We found 465 literature search results with “diabetic cardiomyopathy” as a keyword and 36 literature search results with its “novel treatment”. We limited our research for literature written in the English language and for which the access to full text was available. The selection of the literature results reviewed in the manuscript was performed qualitatively by authors. Screening for duplicates was done automatically using citation manager software, Mendeley.
Results
Diabetes mellitus and Heart Failure
There is a strong association between DM and HF. There are 2 forms of heart failure described in DM: HFrEF (LVEF < 40%) and HFpEF (LVEF 41-49%). The prevalence of DM among patients with HFpEF is around 45%. A large cohort study of 1.9 million people with DM found that the most common CVD events were heart failure (14.1%) and peripheral arterial disease (PAD) (16.2%).8 Based on a population-based study in Reykjavik, impaired glucose regulation in diabetes mellitus is also associated with a risk of congestive heart failure. The prevalence of glucose abnormalities and heart failure increased with age. This study supports the suggestion that glucometabolic abnormalities confer risk for heart failure progression.9 While in T1DM, a cohort study found that a 1% increase in HbA1C (hemoglobin A1C) was associated with a 30% increased risk of developing HF, the risk of heart failure increased with several factors, such as age, duration of diabetes, and other factors.10
Patients with HF can also have an increased risk for new-onset DM. In a cohort study, HF severity was associated with a greater likelihood of developing DM.11 Another study reported that patients with a history of HF have a 2-fold increased risk of developing diabetes mellitus within 3-4 years independent of age, gender, and other comorbidities (e.g. hypertension). They suggest that HF may cause further worsening of DM status.12
The correlation between HF and DM is unclear, but there are possible explanations. Patients with HF have decreased cardiac output hence oxygen, insulin, and glucose distribution to peripheral tissue are also decreased. Due to impaired blood flow, adrenaline and noradrenaline levels are increased. The increased adrenaline and noradrenaline are suggested to increase insulin resistance and decrease insulin production in the pancreas.13 Cortisol and catecholamine hormones are also increased thus increasing the blood glucose level. Activation of the sympathetic systems stimulates gluconeogenesis and glycogenolysis. The increasing level of catecholamines can also cause insulin resistance.14
Pathophysiology of Diabetic Cardiomyopathy
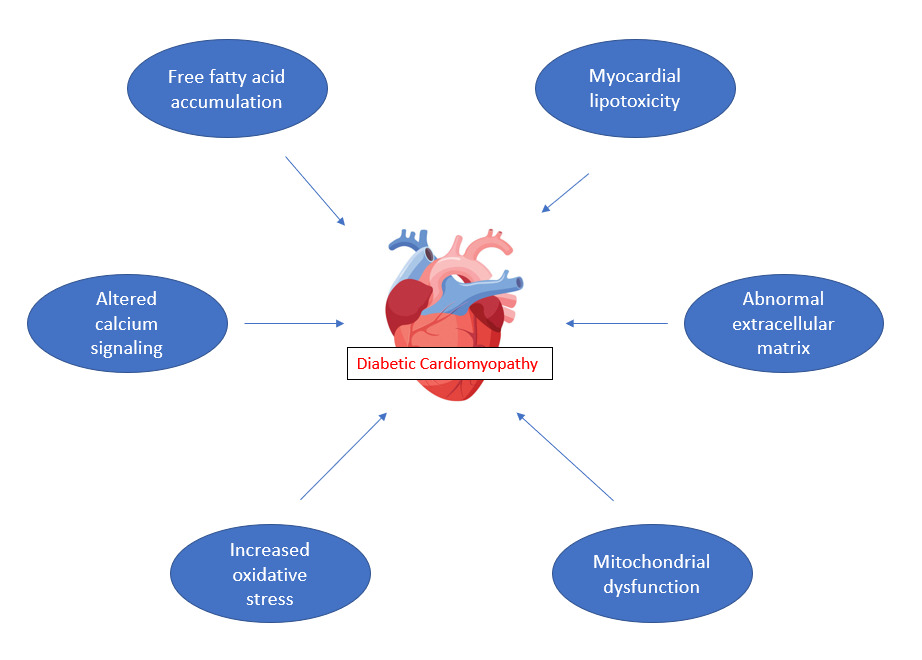
Various mechanisms are thought to be responsible for heart failure associated with diabetes mellitus and it is not limited to diabetic cardiomyopathy. Abnormal extracellular matrix, lipotoxicity to the myocardium, increase in oxidative stress and inflammation, and mitochondrial dysfunction are some of the mechanisms causing heart failure. Increased levels of glucose residues and metabolites upregulate the production of advanced glycation end products (AGEs), which can affect cardiomyocytes and endothelial cells.15 Figure 1 outlines some of the mechanisms thought to contribute to diabetic cardiomyopathy.
Free Fatty Acid Accumulation
Free fatty acids are increased due to diabetes mellitus and obesity accumulating in the adipose tissue mainly as triglycerides. Fatty acid intake and β-oxidation are increased to maintain sufficient levels of ATP production but overtime β-oxidation cannot adequately metabolize all incoming fatty acids resulting in the accumulation of free fatty acid (FFA).16 Ectopic fat that accumulates in organs other than the adipocytes of visceral fat and subcutaneous fat causes the dysfunction of cells and organs, such as the liver, pancreatic β cells, the skeletal muscle, and myocardium, through the deterioration of mitochondrial function. This condition is called lipotoxicity.17
Fat accumulation is present in the heart and in the myocardium. Pericardial fat is divided into two types, pericardial fat located on the outside and epicardial fat located on the inside. High epicardial fat mass has been reported to be an independent predictor of the development of coronary artery disease.17 The myocardial FFA build-up leads to decreased myocardial energy production, reduced myocyte contractility, and lipoapoptosis.18
Altered Calcium Signaling
Calcium (Ca2+) has a vital role in myocardial contraction. During an action potential, membrane depolarization-induced an initial Ca2+ signal so there is a Ca2+ influx to activate the Ca2+ channel and finally activate myofibrils to contract. In type 1 diabetes, there is a reduced Ca2+ influx due to reduced expression of sarcolemmal L type Ca2+ channels (LTCC) where Ca2+ ions pass through.19 The intracellular [Ca2+] is decreased as well as the systolic rate of [Ca2+] rise and decay.
In type 2 diabetes mellitus (T2DM), similar to T1DM, there is also a diminished LTCC density and Ca2+ current (ICa) density.20–23 Some studies also reported a depressed ryanodine receptor (RyR), a Ca2+ release channel.24–26 RyR activity can also be regulated during acute hyperglycemia. Hyperglycemia leads to O-Glc-NAcylation of proteins such as CaMKII which plays a key role in the regulation of excitation-contraction coupling. A recent study showed that a sudden increase of glucose or O-linked N-acetylglucosamine is directly responsible for CaMKII-dependent diastolic sarcoplasmic reticulum (SR) Ca2+ leak from the RyRs leading to consequent SR Ca2+ load depletion which is consistent with the increase of SR Ca2+ leak observed in different early stage of diabetes.27
Increased Oxidative stress
Chronic hyperglycemia leads to the generation of oxidative stress in pancreatic β-cells.28 Hyperglycemia promotes the overproduction of reactive oxygen species by the mitochondrial electron transport chain and exacerbates the formation of AGE.29,30 High glucose levels are metabolized into sorbitol through the polyol pathway with NADPH (nicotinamide adenine dinucleotide phosphate) and NAD+ (nicotinamide adenine dinucleotide). Increased activity of polyol pathway causing an elevation in NADH/NAD+ ratio that leads to overproduction of reactive oxygen species (ROS).31 AGEs have a dominant presence in the diabetic heart, and it is possible that AGE also has a role in the pathogenesis of diabetic cardiomyopathy. AGE receptor (RAGE) is a member of the immunoglobulin superfamily of cell surface molecules and the binding of ligands to RAGE stimulates various signaling pathways.32 The AGE-RAGE interaction stimulates NADPH oxidase-1 which contributes to reactive oxygen species production in diabetes.33 All this leads to cardiac fibrosis and hypertrophy.34 Hyperglycemia, oxidative stress, and the hexosamine biosynthetic pathway that provide substrate for proteoglycan synthesis and for O-linked glycosylation of certain proteins are associated with cardiomyocyte apoptosis.35
Mitochondrial Dysfunction
The heart is an organ that greatly depends on mitochondria as this organelle makes up to 1/3 of cardiac volume and produces adenosine triphosphate (ATP) from the oxidation of fatty acid and glucose.36 In a diabetic state where the insulin production or action is reduced, the mitochondria will use fatty acid as a source to make ATP instead of glucose which can also increase ROS.37 Dysfunctional calcium handling, where there is an excessive calcium influx or reduced calcium efflux can trigger the opening of mitochondrial permeability transition pore (mPTP), leading to mitochondrial dysfunction.38
Increased oxidative stress and mitochondrial dysfunction can cause cells, protein, and nucleic acid destructions that lead to cell apoptosis. The heart consumes large amounts of ATP therefore it has a rather low ATP reserve. In the pathological condition, however, fatty acids only provide 50-70% energy needed by the human heart.39 Mitochondria can switch the source of ATP production depending on the availability of the nutrients. Insulin also plays a role in this selection of energy sources.40 High consumption of ATP depletes the ATP reservoir, and low ATP production may lead to decreased cardiac function.41
Structural changes
Being in a chronic hyperglycemic state may alter the structure and function in the myocardium. In the patient with DM, there seems to be an increase in LV mass, and based on a study, a 1% rise in HbA1C level contributes to a 3.0 gr increase in LV mass, although further studies need to be done to assess the duration of elevated HbA1C that may contribute to the increased of LV mass.42 LV hypertrophy in patients with DM is mainly eccentric although both forms of hypertrophy can be present.43 As the disease progresses, remodeling can also shift from eccentric to concentric.44 Another hallmark of diabetic cardiomyopathy is left ventricular diastolic dysfunction.45,46 The initial characteristic of diastolic dysfunction in patients with DM are prolonged and delayed LV filling and LV relaxation.47
On a cellular level, an extracellular matrix (ECM) remodeling leads to myocardial fibrosis, usually in the later stage of the disease. In the early stage, myocytes appear to be hypertrophic rather than fibrotic.48 Collagen deposits can also be seen as a result of apoptotic myocyte death and impaired collagen degradation from glycosylation of lysine residues on collagen.49
Diagnosis
There are two stages of diabetic cardiomyopathy; the early stage is characterized by left ventricular concentric hypertrophy, increased myocardial stiffness, increase in atrial filling pressure, and impaired diastolic function; while the late stage is characterized by an increase in cardiac fibrosis, further impairment in diastolic function, and appearance of systolic dysfunction. There are no distinct criteria nor biochemical markers or physical characteristics for diagnosing diabetic cardiomyopathy. The pathological changes during the disease progress are often asymptomatic, so the only way to detect any changes regarding the disease is through further examination. Tissue doppler imaging and strain rate imaging may be used to assess LV dysfunction during stress testing. The ratio of the medial mitral annulus (e′) with early passive transmitral inflow velocity (E) has been shown to be a reliable index of left ventricular filling pressure and is a useful prognostic biomarker in diabetic patients.50
Although it is often said that patients with diabetic cardiomyopathy usually have diastolic dysfunction, examination using strain imaging and cardiac magnetic resonance (CMR) has detected a subtle presence of systolic dysfunction and reduced longitudinal contractility without discrete diastolic dysfunction.51 Magnetic resonance (MR) spectroscopy is a novel diagnostic tool that can identify myocardial metabolic changes, such as quantifying myocardial triglyceride content. Assessment of interstitial fibrosis and steatosis by using delayed gadolinium enhancement cardiac MRI is possible but it is still undergoing investigation.52
Novel glucose-lowering drugs for Heart Failure
As mentioned above, DM is associated with poor prognosis and longer hospitalization for HF. Thus, lowering the glycemic index has become a goal in heart failure treatment. New classes of antihyperglycemic drugs such as glucagon-like peptide-1 (GLP-1) analog and sodium-glucose cotransporter 2 inhibitors (SGLT2i) have been shown to reduce cardiovascular mortality and improve glycemic control.53,54 However, the treatment of T2DM patients with HF using GLP-1 analogs remains controversial. Several studies in DM patients have found that GLP-1 analogs did not affect any major adverse cardiovascular event (MACE).55,56 Other trials showed that GLP-1 analogs have a significantly lower cardiovascular mortality rate, nonfatal myocardial infarction, or nonfatal stroke, improve lipotoxicity, and also protects cardiac function in T2DM patients.57–59 On the other hand, there are also studies that concluded the liraglutide (a GLP-1 analog) worsened the cardiac outcomes and significantly increase MACE.60,61
The EMPA-REG OUTCOME trial (The Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes mellitus Patients–Removing Excess Glucose) showed that T2DM patients who received empagliflozin, a selective SGLT2i, have a lower rate cardiovascular mortality, hospitalization for heart failure, nonfatal myocardial infarction, or nonfatal stroke.62 Canagliflozin, another SGLT2i drug, also showed a significantly reduced risk of mortality due to cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke but had a greater risk of amputation.63 SGLT2i also have blood pressure (BP) lowering properties but are not as effective as other antihypertensive drugs such as angiotensin-converting enzyme (ACE) inhibitors.64 It is found that empagliflozin was associated with reduced systolic and diastolic blood pressure compared with placebo (who received an additional glucose-lowering medicine and also antihypertensive medicine, including diuretics).62 On the other hand, there was no significant difference in systolic and diastolic blood pressure in the use of Canagliflozin compared to placebo.63 SGLT2i worked proportionally with the ambient glucose concentration, hence it may have a greater effect on individuals with poor glycemic control. However, the effect of SGLT2i on blood pressure doesn’t seem to be consistent with the blood glucose level, lowering systolic 4-6 mmHg and diastolic 1-2mmHg.64
The incretin-based drugs such as dipeptidyl peptidase-4 (DPP-4) inhibitors have no beneficial effect on HF but were shown to reduce the occurrence of hepatic steatosis.65,66 Other trials have shown that DPP-4 inhibitor was not superior to placebo.67–69 The ESC-EASD 2019 guideline only recommends DPP-4 inhibitors when HbA1C targets are not reached after using SGLT2i, metformin, and/or GLP-1 receptor agonists.70 The DPP-4 inhibitor that is not recommended for patients with or with risk of HF is saxagliptin as it can increase the risk for hospitalization for HF (HHF) and also increase the HF incidence in T2DM patients.71
Novel Targeted therapies for diabetic cardiomyopathy
MicroRNA (miRNA) is reported to have a role in the pathophysiology of diabetic cardiomyopathy, such as increase ROS production and promote cardiomyocyte apoptosis.72–75 Anti-miRNA and miRNA mimics are actively studied and developed to treat cardiomyopathy.76–78 Antioxidant therapies can be used for prevention and intervention for diabetic cardiomyopathy.79–85 Phenolic acids are beneficial for mitochondrial dysfunction as the protective agent of the heart against mitochondrial dysfunction and are obtained from plants such as nuts and fruits and thus can be added to the diets.86 Bile Acids are synthesized by cholesterol, bind and activate Farnesoid X Receptor (FXR) that leads to reduction of inflammation and have a regulatory effect on autophagy and mitochondrial function and can also suppress oxidative stress that showing a potential therapeutic effect.87 However further studies are still ongoing.
Conclusion
There is a strong association between diabetes mellitus and heart failure incidence. Patients with heart failure have increased risk for new-onset DM. The proposed mechanisms underlying the pathophysiology of diabetic cardiomyopathy include lipotoxicity related to free fattty acid accumulation, altered calcium signaling, increased oxidative stress due to chronic hyperglycemia leading to mitochondrial dysfunction and alteration of structure and function in the myocardium. SGLT2i and novel targeted therapies for diabetic cardiomyopathy are promising treatments but require further investigation. It is important for clinicians to be aware of diabetic cardiomyopathy in order to improve cardiovascular outcomes in diabetes mellitus.
Conflicts of interest
There are no conflicts of interest among any of the authors and received no specific support for this work.
Corresponding author
Sidhi Laksono, MD
Faculty of Medicine, Universitas Muhammadiyah Prof. Dr. Hamka
Jl. Limau II No.2, RT.3/RW.3, Kramat Pela, Kec. Kby. Baru, Kota Jakarta Selatan, Daerah Khusus Ibukota Jakarta 12130, Indonesia
E-mail: sidhilaksono@uhamka.ac.id