1.9: Heat and changes in physical states of matter
- Page ID
- 372556
Among the four physical states of matter, solid has the lowest thermal energy. Intermolecular forces in solids are strong and do not let the molecules slide past each other. The molecules and the bonds in them can still have vibrational motions that account for the thermal energy contents of the material.
The temperature reflects the thermal energy content of the material—the addition of heat increase the vibrational motions, and temperature increases. Ultimately, the solid changes to a liquid and the liquid changes to a gas phase as more heat is added, as illustrated in Figure 1.9.1.
Melting and freezing
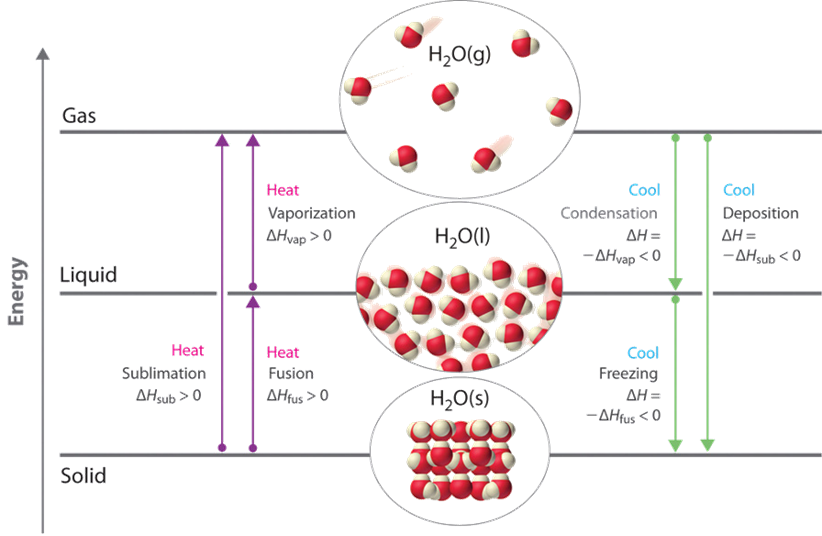
When the temperature reaches the melting point of the solid upon heating, the temperature does not increase further, but the sold changes gradually to the liquid phase. The heat added at the melting point is used to change the particles from a well-arranged form in the solid to an irregular arrangement in the liquid phase. This process is called the melting of solid.
The energy needed to melt a unit amount of the substance is the heat of fusion (∆Hfus).
The heat of fusion is usually expressed in the units of joules per gram (\(\frac{J}{g}\)) for the unit amount in grams or in joules per mole (\(\frac{J}{mol}\)) for the unit amount in moles.
If heat is removed from a substance at its melting point, the reverse of melting, i.e., freezing, happens, i.e., the liquid gradually changes from liquid to solid phase. The energy equal to the heat of fusion is released during the freezing process. Fig. 1.9.2 shows ice and water at 0 oC –an example of melting and freezing.

Vaporization and condensation
After melting, the heat addition causes an increase in the temperature of the liquid until the boiling point is reached. Some of the molecules in a liquid have high enough kinetic energy to cross the liquid-gas boundary and become gas phase. This process is called vaporization.
The energy needed to evaporate a unit amount of a liquid is called the heat of vaporization (∆Hvap).
The heat of vaporization is usually expressed in the units of joules per gram (\(\frac{J}{g}\)) for the unit amount in grams or joules per mole (\(\frac{J}{mol}\)) for the unit amount in moles.
The reverse of evaporation is called condensation, which releases heat equal to the heat of vaporization. Fig. 1.9.3 demonstrates the co-existence of liquid and gas-phase bromine at room temperate through the simultaneous evaporation and condensation processes.
When the temperature reaches the boiling point of the liquid, the temperature does not increase further, but the added heat is used to evaporate the liquid. Heating increases the temperature of the gas phase after all of the liquid has changed to the gas phase.

Sublimation and deposition
The solid can change directly to the gas phase without going through the liquid phase. This process is called sublimation.
The energy required in sublimation (∆Hsub) is the addition of the heat of fusion and the heat of vaporization, i.e.,:
\[\Delta H_{\text {sub }}=\Delta H_{\text {fus}}+\Delta H_{\text {vap }}\nonumber\]
The reverse of the sublimation is called deposition, i.e., the gas phase changes directly to the solid phase. Fig. 1.8.4 shows the sublimation of iodine crystals on a hot plate and deposition of iodine gas on an ice-cold watch glass.

The sublimation is responsible for drying clothes below 0 oC conditions in cold areas. Sublimation is also used in freeze-drying vegetables and other foods. Bacteria can not grow on dried foods because they need some moisture to grow. Fig. 1.9.5 shows the terminologies related to the phase changes described in the previous paragraphs.

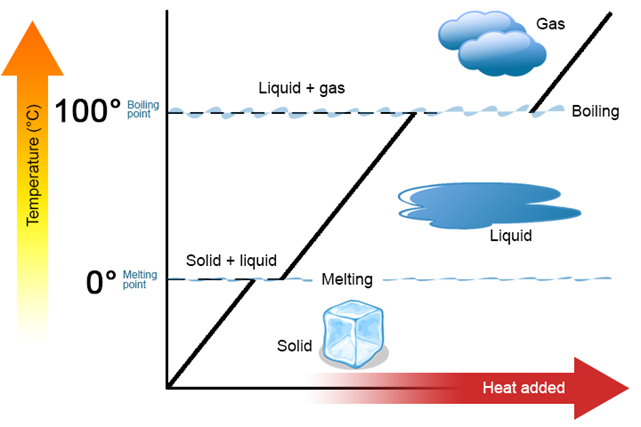
Heating curve
A graphical presentation of the relationship of heat added versus the temperature change and phase changes of a matter is called a heating curve.
Fig. 1.9.6 shows the heating curve of water. The curve shows the heating of ice initially, followed by co-existing of solid and liquid at the freeing point, then hating of liquid water, then co-existing of liquid and gas phases at the boiling point, and finally the heating of steam –the gas phase of water. The reverse of the heating curve is called the cooling curve.
Heat calculations on heating or cooling curves
The heat required or released can be calculated by using the specific heat of the substance's solid, liquid, and gas phases. The heat of fusion is needed at the freezing point, and the heat of evaporation is needed at the substance's boiling point. The heat calculation is explained in the following example.
Calculate the energy required to heat 10.0 g of ice from -20.0 oC to steam (water vapor) at 110 oC?
Solution
1st step –heating the ice from -20.0 oC to the melting point of the ice, i.e., 0.00 oC:
m = 10.0 g, Cs of ice = 2.06 \(\frac{J}{g \cdot{ }^{\circ} \mathrm{C}}\), ∆T = 0.00 oC – (-20.0 oC) = 20.0 oC
\[\mathrm{q}_{1}=\mathrm{C}_{\mathrm{s}} \mathrm{m} \Delta \mathrm{T}=2.06 \frac{J}{g \cdot{ }^{\circ} \mathrm{C}}\times 10.0 \mathrm{~g} \times 20.0^{\circ} \mathrm{C}=412 \mathrm{~J}\nonumber\]
2nd step – melting of ice, multiply the heat of fusion with the amout of substance:
m = 10.0 g, ∆Hfus = 334 \(\frac{J}{g}\).
\[\mathrm{q}_{2}=\Delta \mathrm{H}_{\text {fus }} \times \mathrm{m}=334\frac{J}{g}\ \times 10.0 \mathrm{~g}=3340 \mathrm{~J}\nonumber\]
3rd step –hating of the water from 0.00 oC to the boiling point of water, i.e., 100 oC:
m = 10.0 g, Cs of liquid water = 4.184 \(\frac{J}{g \cdot{ }^{\circ} \mathrm{C}}\), ∆T = 100 oC – 0.00 oC = 100 oC
\[\mathrm{q}_{3}=\mathrm{C}_{\mathrm{s}} \mathrm{m} \Delta \mathrm{T}=4.184 \frac{J}{g \cdot{ }^{\circ} \mathrm{C}}\times 10.0 \mathrm{~g} \times 100^{\circ} \mathrm{C}=4180 \mathrm{~J}\nonumber\]
4th step – boiling of liquid water, multiply the heat of vaporization with the amount of the substance:
m = 10.0 g, ∆Hvap = 2260 \(\frac{J}{g}\)
\[\mathrm{q}_{4}=\Delta \mathrm{H}_{\text {vap }} \times \mathrm{m}=2260 \frac{J}{g}\ \times 10.0 \mathrm{~g}=22600 \mathrm{~J}\nonumber\]
5th step –hating of the stem from 100 oC to 110 oC:
m = 10.0 g, Cs of steam = 2.00 \(\frac{J}{g \cdot{ }^{\circ} \mathrm{C}}\), ∆T = 110 oC – 100 oC = 10.0 oC
\[\mathrm{q}_{5}=\mathrm{C}_{\mathrm{s}} \mathrm{m} \Delta \mathrm{T}=2.00\frac{J}{g \cdot{ }^{\circ} \mathrm{C}} \times 10.0 \mathrm{~g} \times 10.0^{\circ} \mathrm{C}=200 \mathrm{~J}\nonumber\]
Total heat needed = \(q_{1}+q_{2}+q_{3}+q_{4}+q_{5}=412 \mathrm{~J}+3340 \mathrm{~J}+4180 \mathrm{~J}+22600 \mathrm{~J}+200 \mathrm{~J}=30700 \mathrm{~J}\)
\[30700 \mathrm{~J} \times \frac{1 \mathrm{~kJ}}{1000 \mathrm{~J}}=30.7 \mathrm{~kJ}\nonumber\]
The most significant portion of the heat is consumed in boiling the water to steam, i.e., 22.6 kJ out of 30.7 kJ total. The same amount of heat is released when the steam condenses. That is why the steam burn is much more severe than the burn by hot water.


