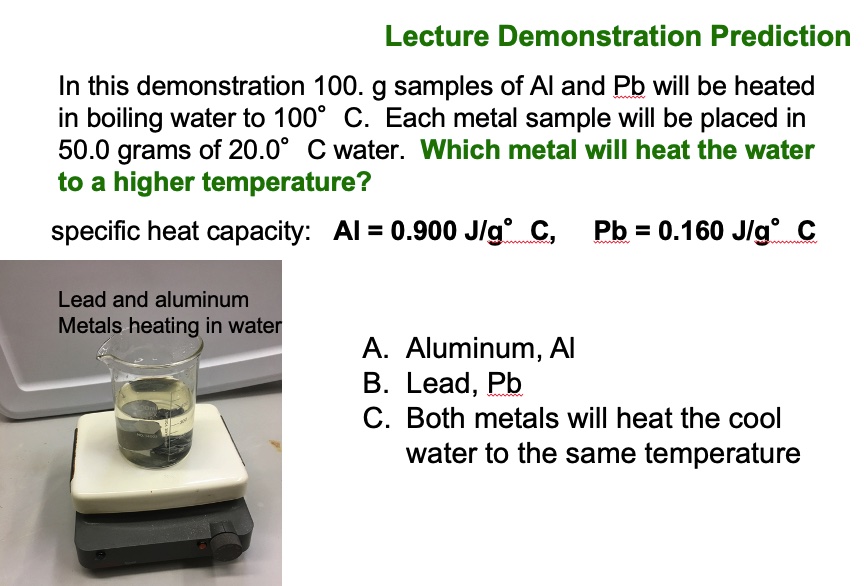
This demonstration uses a calorimeter to run quick experiments by placing two (or three) samples of hot metals in cool water and determining which metal raises the temperature of water the most. A qualitative comparison is made of three metals: copper, lead, and aluminum.
Target concept: Given the specific heat of each metal and the same mass of each metal, ask students which metal will transfer the most energy to 80.0 grams of water in order to achieve a the highest temperature? Then, ask students to explain their reasoning.
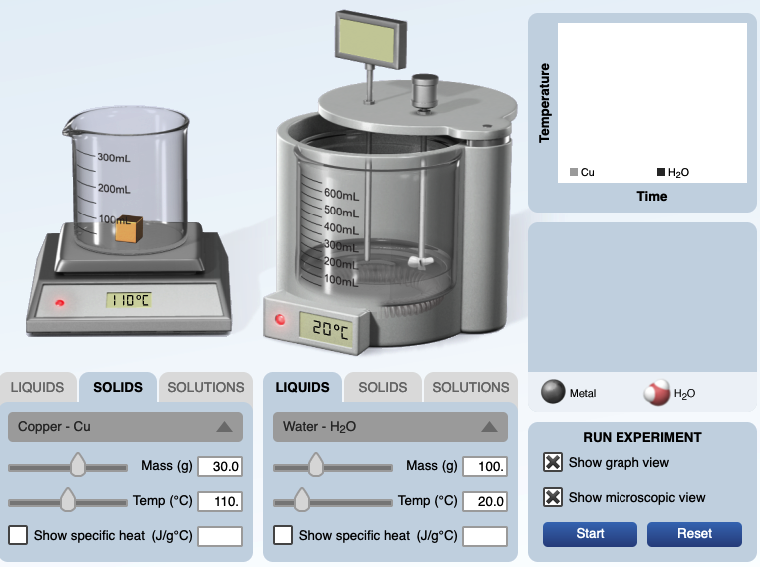
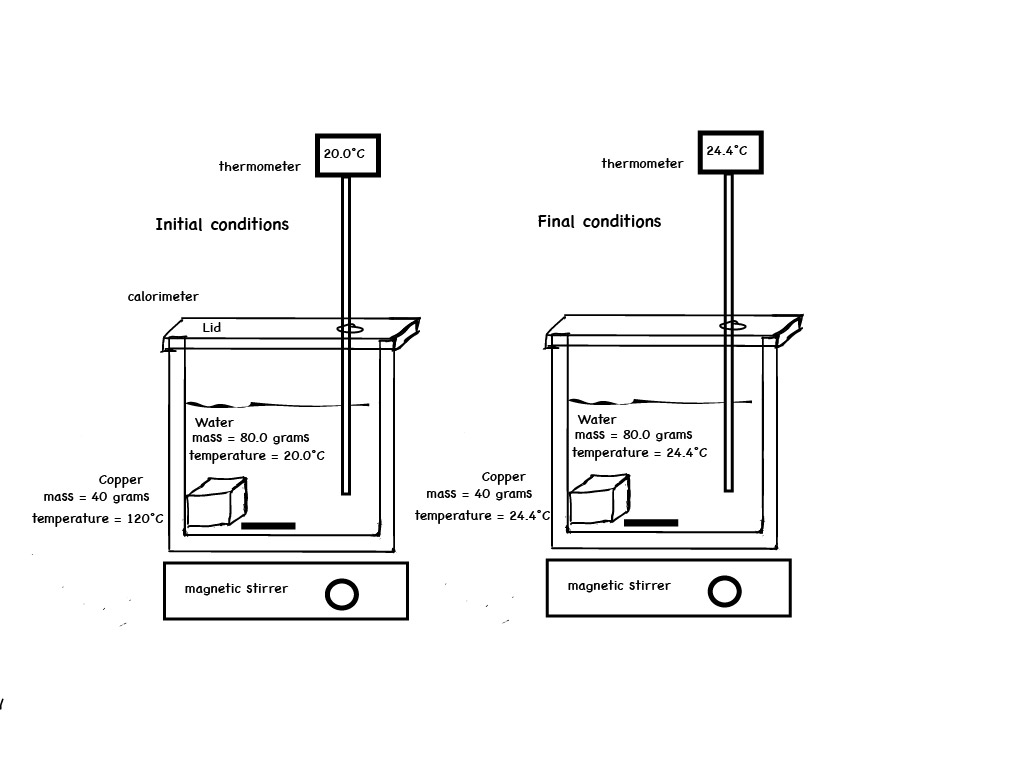
A Calorimetry Computer Simulation is also used to visualize the energy exchanged by the interaction of particles. In the calorimeter experiment below, 30.0 grams of copper metal at 110.°C is ready to be placed in 100. grams of water at 20.0°C. After the copper is placed in water, at the end of the experiment, the copper and water are in thermal equilibrium, each has the same temperature of 22.42°C. A temperature versus time graph shows the hot copper metal decreased in temperature and the cool water increased in temperature.
 | 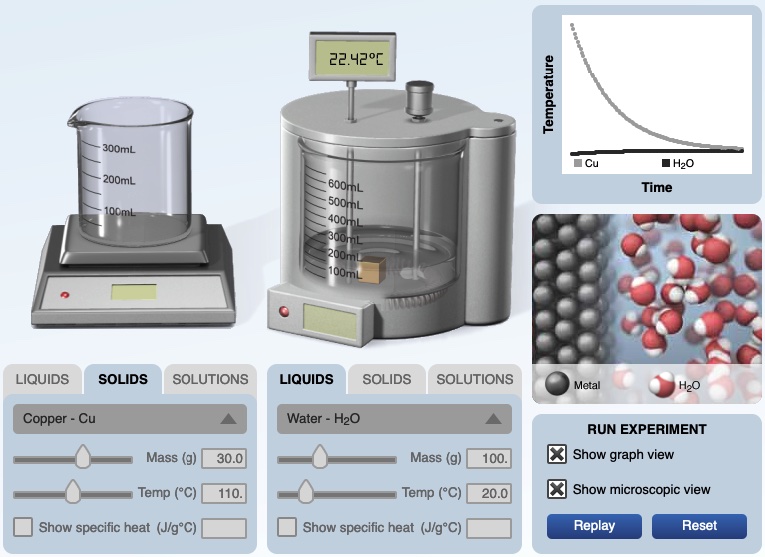 |
When the hot copper metal is added to the cool water, an animation representing the copper atoms and water molecules provides a visualization of how energy is transferred at the particle level. When the cool water molecules collide with the hot copper atoms at the surface of the metal, this collision transfers energy. The hot copper atoms decrease in energy (less vigorous vibrations) and the cool water molecules increase in energy (an increase in motion). The more vibrant colored water molecules move faster compared to the original movement of water molecules (dull color) prior to the placement of the copper metal in the water.
 |
The html5 calorimeter simulation is available on this web site. If you use the simulation please include a citation in your write-up and in your list of references.
https://media.pearsoncmg.com/bc/bc_0media_chem/chem_sim/calorimetry/Calor.php
©2016 Greenbowe, Abraham, Gelder University of Oregon, Oklahoma State University, University of Oklahoma, Pearson: Hoboken, NJ.
Web page author: T. Greenbowe, University of Oregon. This page is under construction.
This computer simulation allows one to select the mass and initial temperature of various substance, put the substances in a calorimeter, and record the final temperature.
In the "solids" mode: placing metals in water placing metals in ethanol
In the "liquids" mode: mixing hot and cold water mixing hot and cold ethanol
Curriculum Notes
Target concepts associated with this demonstration:
- Specific heat is defined as the amount of thermal energy required to increase the temperature of one gram of a substance by one degree Celsius. The metal with the highest specific heat capacity will release more energy per gram when placed in cool water, causing the temperature of the water to increase the most.
- When two objects (or systems) are in contact with one another in a calorimeter (isolated system), thermal energy will transfer from the object at higher temperature to the one at lower temperature until they both reach the same temperature.
- Two objects at different temperature when placed in contact will achieve the same temperature. This process is called thermal equilibrium. If two systems or objects, A and B, are in thermal equilibrium with each another, and B is in thermal equilibrium with a third system or object, C, then A is also in thermal equilibrium with C. All three objects in contact will have the same temperature. This is the Zeroth Law of Thermodynamics.
- The Law of Conservation of Energy (part of the First Law of Thermodynamics) dictates that in a calorimetry experiment, the energy released by the hot metal is equal to the energy gained by the cool water, given perfect conditions.
- Thermal energy is energy of a system due to the motion or vibrations of atoms, molecules and or ions.
- Differentiate the terms heat, thermal energy, and temperature.
- Thermal interaction - the process whereby energy is transferred from a hotter object to a cooler object by atomic-level collisions (particle collisions). [Energy can also be transferred to cause a phase change of a pure substance with no change in temperature of the substance while it undergoes a phase change.]
- Visualization of the process of energy transfer at the particle level. When the cool water molecules collide with the hot copper atoms at the surface of the metal, this collision transfers energy. The hot copper atoms transfer energy to the cool water molecules. The copper atoms involved in the collision decrease in energy (less vigorous vibrations) and the cool water molecules increases in energy.
Materials
Metals: Al and Pb, (and Zn) 100 g each
Digital thermometers, LapTop/PC with digital thermometer display
Hot plate
Balance, centigram (0.01-g precision) Insulated coffee cups, 6
Beaker, 600-mL Beakers, 400-mL,
Stirring rods, 3 Ring stand and clamp
Boiling stones Beaker tongs
1.0 L of Deionized Water ; Graduated cylinder, 100-mL
The Law of Conservation of Energy is the "big idea" governing this experiment. A natural transfer of thermal energy from a region of higher temperature to a region of lower temperature until an equilibrium temperature is reached. In this demonstration, thermal energy is transferred from a hot metal sample to a cool sample of water: qlost + qgain = 0
Each different type of metal causes the temperature of the water to increase to a different final temperature. This indicates that each metal has a different ability to absorb thermal energy and to transfer thermal energy. The thermal energy exchanged between a sample of hot metal and cool water is a physical processes.
The ability of a substance to contain or absorb thermal energy is called its heat capacity. Heat capacity is an extensive property—it depends on the amount or mass of the sample. Specific heat is a measure of the heat capacity of a substance. Specific heat is defined as the amount of heat required to increase the temperature of one gram of a substance by one degree Celsius.
specific heat values: Al 0.903 J/g°C Pb 0.160 J/g°C
Procedure Two different metals, aluminum and lead, of equal mass are heated to the same temperature in a boiling water bath. The specific heat capacities of each metal is displayed to students: Specific heat: Al 0.903 J/g°C Cu 0.385 J/g°C Pb 0.160 J/g°C Note: Students realize the two logical choices are aluminum or lead.
The metals are added to two insulated cups or calorimeters, each containing the same amount of water initially at room temperature. Students are asked to predict what will happen to the temperature of water and the temperature of the metals. The temperature of the water changes by different amounts for each of the two metals. This demonstration assess students' conceptual understanding of specific heat capacities of metals. When the accompanying computer animation is displayed, students can gain a conceptual understanding of heat transfer between a hot sample of metal and the cool water at the particle level (atom level). Compare the heat gained by the cool water to the heat released by the hot metal. Compare the heat gained by the water in Experiment 1 to the heat gained by the water in Experiment 2. This demonstration is not a laboratory experiment. The emphasis is on developing concepts and scientific reasoning.
Because the density of aluminum is much lower than that of lead and zinc, an equal mass of Al occupies a much larger volume than Pb or Zn. Choose a large enough beaker such that both the aluminum metal and lead metal will be submerged in the boiling water bath. Heat the metals for about 6 minutes in boiling water. Place 50 mL of water in a calorimeter. Measure and record the temperature of the water in the calorimeter. Have students predict what will happen to the temperature of the water in the two calorimeters when hot lead is added to one and hot aluminum is added to the other. See the attached clicker question. After students have answered the question, use the tongs and grab the hot lead metal and place it in 50 mL of room temperature water. Stir it up (Bob Marley). Record the temperature of the water. Use the tongs and grab the hot aluminum metal and place it in the second calorimeter containing 50 mL of room temperature water. Stir it up. Record the temperature of the water. Compare the final temperature of the water in the two calorimeters. Compare the heat gained by the cool water to the heat released by the hot metal. Compare the heat gained by the water in Experiment 1 to the heat gained by the water in experiment 2.
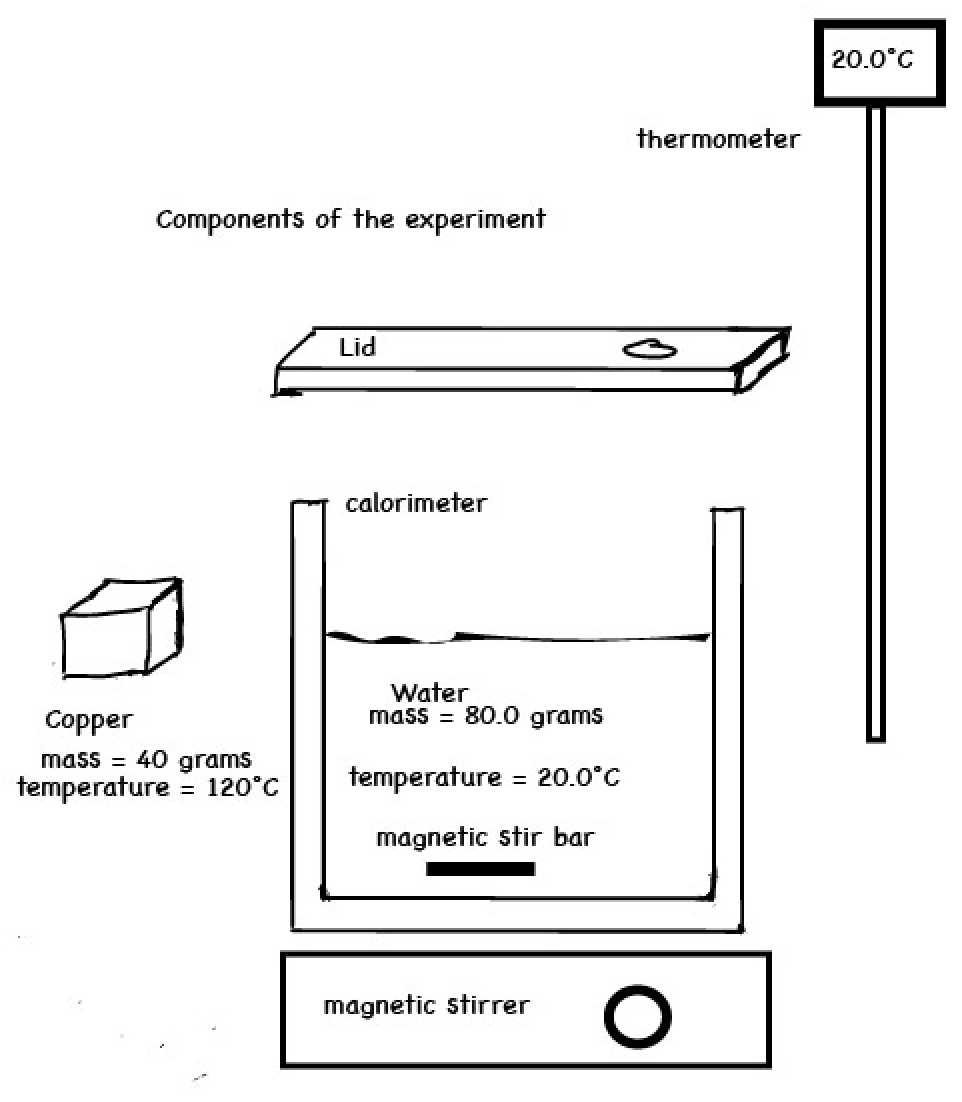
Sample Data and Sample Calculations
| Mass | Initial Temp . | Final Temp. | Temp. Change | |
| water | 50.0 g | 19.2°C | 24.9°C | +5.7°C |
| lead | 100.0 g | 100.0°C | 24.9°C | -75.1°C |
q = m x c x ∆t
q lost Pb = 100. g x 0.160 J/g °C x (-70.0°C) = -1201 J
q gained water = 50.0 g x 4.18 J/g °C x (5.7°C) = +1191 J
| Mass | Initial Temp . | Final Temp. | Temp. Change |
| water | 50.0 g | 19.2°C | 43.5°C | +24.3°C |
| aluminum | 100.0 g | 100.0°C | 43.5°C | -24.3°C |
q gained water = 50.0 g x 4.18 J/g °C x (24.3°C) = +5078 J
q lost Al = 100.0 g x 0.900 J/g °C x (-56.5°C) = +5085 J
Learning Objectives
1. Use experimental data to develop a conceptual understanding of the Law of Conservation of Energy and how to apply it to calorimeter experiments:
q lost + q gain = 0 The transfer of energy from a hot object (metal) to a cool object (water).
2. Ask a research question and design a series of experiments to provide data to answer the research question.
3. Use experimental data to develop a relationship among the variables: heat, thermal energy, mass, specific heat, and change in temperature.
4. Identify what gains thermal energy and what loses thermal energy in a calorimetry experiment.
5. For a physical process explain how thermal energy is transferred, released or absorbed, at the molecular level.
6. Calculate the thermal energy released by a sample of hot metal, qloss, involved in a given calorimetry experiment: mass of the metal, specific heat of the metal, change in temperature of the metal: qloss = m c ∆T
7. Calculate the thermal energy gained by a sample of cool water, qgain, involved in a given calorimetry experiment: mass of the metal, specific heat of the water, change in temperature of the water: qgain = m c ∆T
8. Calculate the specific heat of a sample of metal, given: mass of the metal, change in temperature of the metal, mass of the water, specific heat of the water, change in temperature of the water: qloss = m c ∆T qgain = m c ∆T
AP Chem Learning Objectives
Learning objective 5.7 The student is able to design and/or interpret the results of an experiment in which calorimetry is used to determine the change in enthalpy of a chemical process (heating/cooling, phase transition
The second law of thermodynamics states that if a physical process is irreversible, the combined entropy of the system and the environment must increase. The final entropy, S, must be greater than the initial entropy for an irreversible process: S final > S initial ( for an irreversible process)
An example of an irreversible process is: A hot object is put in contact with a cold object ina calorimeter. Eventually, both objects achieve the same equilibrium temperature. If the objects are separated, the objects remain at the equilibrium temperature and do not naturally return to their initial temperatures. The process of bringing the objects to the same temperature is irreversible.
References
Specific Heat A Chemistry Demonstration. Flinn Scientific, Batavia, Illinois. 2016. https://www.flinnsci.com
Johnstone, A. H. 1993. The development of chemistry teaching: A changing response to changing demand. Journal of Chemical Education, 70(9), p. 701-705.
Harrington, D.G. 2011. The Heat is on: An inquiry-based investigation for specific heat. Journal of Chemical Education, 88,1558-1561.
*********
Calorimetry: Hot Metal in Cold Water Heat Exchange Computer Simulation Old Flashed-Based
http://pages.uoregon.edu/tgreenbo/heat_metal.html
©2009 Greenbowe Chemistry Education Instructional Resources
University of Oregon, Department of Chemistry & Biochemistry, Eugene, Oregon 97403 USA
