Advertisement
Advertisement
November 30, 2023
Medtronic’s PulseSelect PFA System and Nitron CryoConsole Approved in Europe
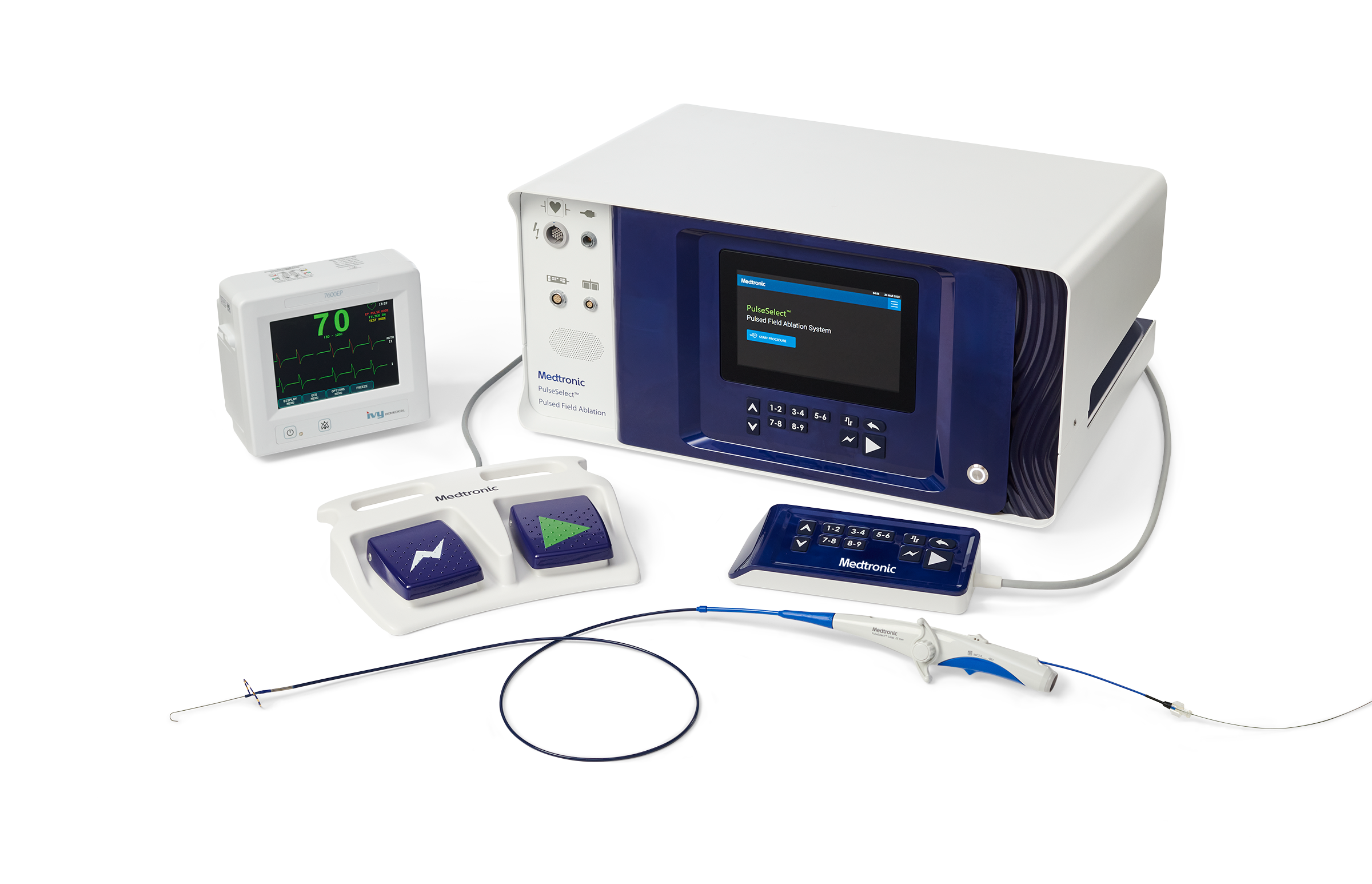 November 30, 2023—Medtronic recently announced European CE Mark approval for the PulseSelect pulsed field ablation (PFA) system and the Nitron CryoConsole. Both PulseSelect and Nitron will be commercially available in select European countries early next calendar year.
November 30, 2023—Medtronic recently announced European CE Mark approval for the PulseSelect pulsed field ablation (PFA) system and the Nitron CryoConsole. Both PulseSelect and Nitron will be commercially available in select European countries early next calendar year.
The PulseSelect system, which is designed to treat atrial fibrillation (AFib) effectively, efficiently, and safely, features a new ablation modality that uses pulsed electric fields to isolate the pulmonary veins.
The next-generation Nitron CryoConsole supports the commercially available Arctic Front and Freezor family of cardiac cryoablation catheters.
According to the company, PulseSelect’s nonthermal approach to preferentially target the pulmonary veins for ablation, avoids injury to surrounding structures, which is a risk of current ablation technologies (ie, thermal energy sources). The catheter used the company’s biphasic waveform, a built-in over-the-wire design, and 20º forward tilt to support procedural maneuverability, reliability, and safety.
CE Mark approval of PulseSelect comes after the PULSED AF global study met its safety and effectiveness endpoint. The study also demonstrated the device’s efficient pulmonary vein isolation with an average of 30 seconds of total energy delivery time to isolate all veins.
“The electrophysiology community is eagerly awaiting new innovation to enhance the safety and efficiency of AFib ablation,” commented cardiologist Lucas V.A. Boersma, MD, in Medtronic’s press release. “PulseSelect is specifically engineered to deliver pulsed field energy safely and maneuvers well, with great overall control of the energy applications. The safety profile shown by the PulseSelect system in its global pivotal study is one of the best compared to today’s standard of care for catheter ablation technologies.”
Professor Boersma is from the Department of Cardiology at St. Antonius Hospital in Nieuwegein, The Netherlands, and Professor of Cardiology at Amsterdam University Medical Centers, University of Amsterdam in Amsterdam, The Netherlands.
In March 2023, the PULSED AF data were presented by Atul Verma, MD, at ACC.23/WCC, the ACC’s annual scientific session together with the World Congress of Cardiology held in New Orleans, Louisiana, and simultaneously published by Dr. Verma et al online in Circulation (2023;147:1422-1432). The study was funded by Medtronic.
Medtronic noted that PULSED AF studied two patient populations—paroxysmal and persistent. PulseSelect exceeded its safety performance goal with an adverse event rate of 0.7%. There were no esophageal events, instances of pulmonary vein stenosis, or phrenic nerve injury. PULSED AF exceeded the threshold for its efficacy performance goal. Further, clinical success, freedom from recurrence of any symptomatic atrial arrhythmias, was at least 80% for both paroxysmal and persistent patient cohorts.
Medtronic advised that this addition to the Medtronic PFA portfolio with the European approval of the Affera mapping and ablation system in March 2023 provides the company with both single shot and focal PFA options to meet different patient and clinician needs.
Additionally, the Nitron console builds upon the legacy of Medtronic’s Cryo franchise, elevating and optimizing the workflow for cryoballoon ablation.
Advertisement
Advertisement