Learning Outcomes
- Identify enzymes and their role in chemical reactions
Enzymes are proteins that have the ability to bind substrate in their active site and then chemically modify the bound substrate, converting it to a different molecule — the product of the reaction. Substrates bind to enzymes just like ligands bind to proteins. However, when substrates bind to enzymes, they undergo an enzyme-induced chemical change, and are converted to products.
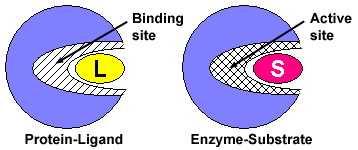
Figure 1. Compare the protein-ligand interaction to the enzyme-substrate interaction. Notice that both binding proteins and enzymes have binding sites for their ligands (L) and substrates (S), respectively. This area of the enzyme is called the active site because it also contains amino acids that are important for the conversion of substrate to product.
The substrate binds to the enzyme by interacting with amino acids in the binding site. The binding site on enzymes is often referred to as the active site because it contains amino acids that both bind the substrate and aid in its conversion to product.
You can often recognize that a protein is an enzyme by its name. Many enzyme names end with –ase. For example, the enzyme lactase is used to break down the sugar lactose, found in mammalian milk. Other enzymes are known by a common name, such as pepsin, which is an enzyme that aids in the digestion of proteins in your stomach by breaking the peptide bonds in the proteins.
Enzymes are catalysts, meaning that they make a reaction go faster, but the enzymes themselves are not altered by the overall reaction. Examine this image to see how enzymes work.
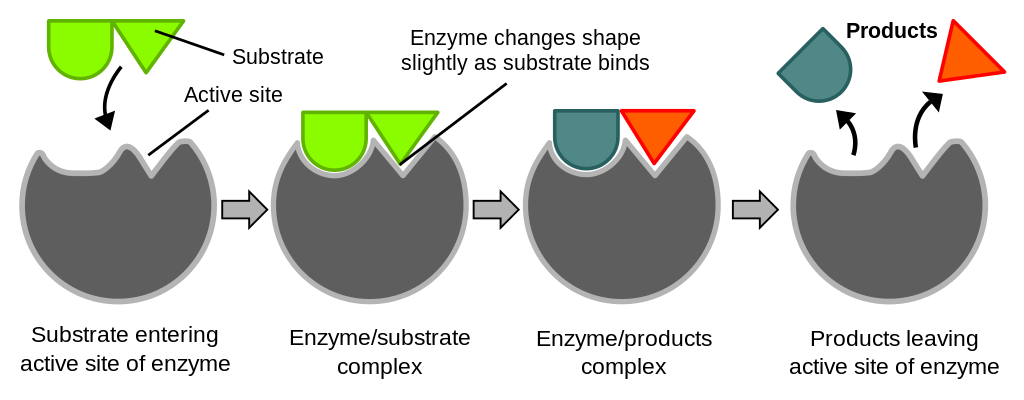
Figure 2. Simplified enzymatic reaction. The substrate reversibly binds to the active site of the enzyme, forming the enzyme-substrate (ES) complex. The bound substrate is converted to product by catalytic groups in the active site, forming the enzyme-product complex (EP). The bound products are released, returning the enzyme to its unbound form, ready to catalyze another round of converting substrate to product.
The amino acids in the active site of enzymes play two roles, and sometimes those roles overlap. Some of the amino acids in the active site are responsible for binding of the substrate and others are responsible for facilitating the chemical reaction. Enzymes are generally quite specific for their substrates. Although lactase and pepsin both catalyze the same type of reaction, breaking a bond using water (hydrolysis: “hydro” means “water” and “lysis” means “to break”), lactase only functions when lactose is its substrate and pepsin can only break peptide bonds.
Practice Question
Two substrates—lactose and a short protein—are shown on the left. Two enzymes are shown on the right, labeled A and B. Which of the two enzymes is lactase?

How Enzymes Work
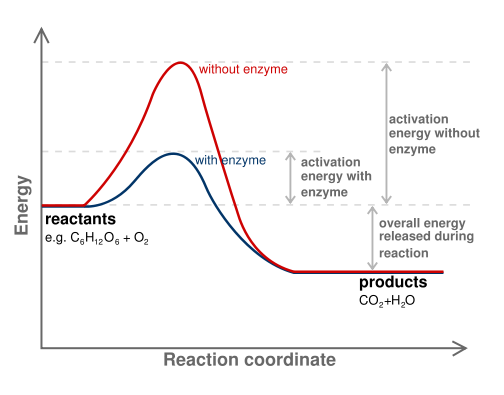
Figure 3. Diagram of a catalytic reaction showing difference in activation energy in uncatalysed and catalysed reaction. The enzyme reduces the energy barrier required to activate the substrate, allowing more substrates to become activated, which increases the rate of product formation. Note that the energy difference between the substrate and the product is not changed by the enzyme.
In all chemical reactions, there is an initial input of energy that is required before the reaction can occur. If this initial energy requirement (called the activation energy or energy barrier) is small, then the reaction will happen quickly and easily. If the activation energy is large, then the reaction will take longer to occur. Enzymes function to reduce the activation energy required for a chemical reaction to occur.
First, the enzyme binds to the substrate and slightly distorts its shape. The change in shape activates the substrate molecule and decreases the total activation energy required for the substrate to be turned into product. As the number of activated substrate molecules increases, so does the conversion of substrate to product. An analogy for this effect is a ski hill, with skiers at the bottom of one side of the hill representing substrates, skiers on the top of the hill representing activated substrates, and the products being the number of skiers that ski down the other side. If the height of the hill is lowered (due to the presence of the enzyme), then more skiers can make it to the top, increasing the number that ski down to become products.
Practice Questions
Fill in the blank: When an enzyme catalyzes a reaction, ________.
- it raises the activation energy of the reaction.
- it is used once and discarded.
- it becomes a product.
- it acts as a reactant.
- it lowers the activation energy of the reaction.
What will happen to the rate at which a chemical reaction proceeds if the activation energy is increased?
- The reaction will happen faster (at a higher rate).
- The reaction will happen slower (at a lower rate).
- The reaction rate will not change.
In Summary: Enzymes
Enzymes are proteins that speed up reactions by reducing the activation energy. Each enzyme typically binds only one substrate. Enzymes are not consumed during a reaction; instead they are available to bind new substrates and catalyze the same reaction repeatedly.
Try It
