Interorganellar Communication Through Membrane Contact Sites in Toxoplasma gondii
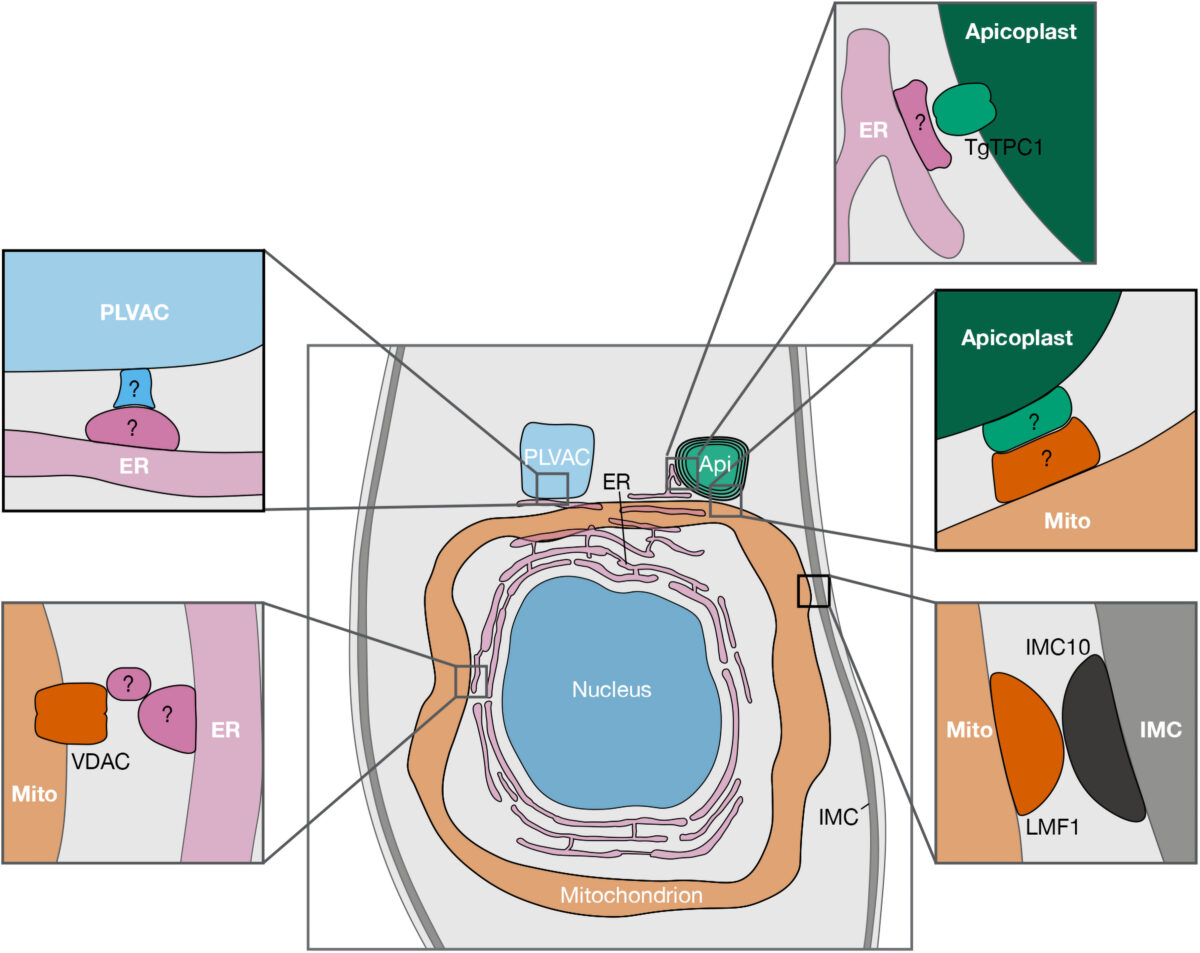
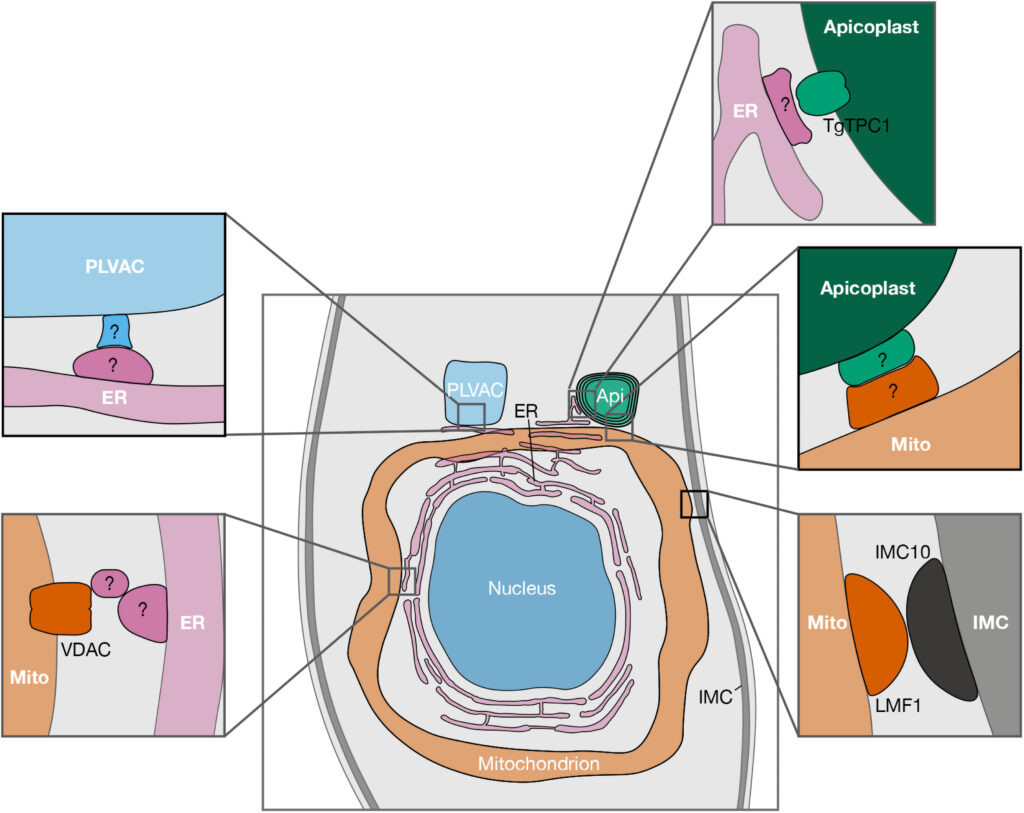
Apicomplexan parasites are a group of protists that cause disease in humans and include pathogens like Plasmodium spp., the causative agent of malaria, and Toxoplasma gondii, the etiological agent of toxoplasmosis and one of the most ubiquitous human parasites in the world. Membrane contact sites (MCSs) are widespread structures within eukaryotic cells but their characterization in apicomplexan parasites is only in its very beginnings. Basic biological features of the T. gondii parasitic cycle support numerous organellar interactions, including the transfer of Ca2+ and metabolites between different compartments. In T. gondii, Ca2+ signals precede a series of interrelated molecular processes occurring in a coordinated manner that culminate in the stimulation of key steps of the parasite life cycle. Calcium transfer from the endoplasmic reticulum to other organelles via MCSs would explain the precision, speed, and efficiency that is needed during the lytic cycle of T. gondii. In this short review, we discuss the implications of these structures in cellular signaling, with an emphasis on their potential role in Ca2+ signaling.
Diego Huet, Silvia N J Moreno. Contact (Thousand Oaks). 2023 Aug 6;6:25152564231189064. doi: 10.1177/25152564231189064. eCollection 2023 Jan-Dec.
