Approach Considerations
The workup of patients with systemic light chain (AL) amyloidosis requires adequate technology and expertise, and is best performed at specialized centers. [4] The workup includes the following [3] :
-
Serum protein electrophoresis with immunofixation (SPEI), to assess for clonal immunoglobulin production
-
Urine protein electrophoresis with immunofixation (UPEI), to assess for clonal light chain production
-
Serum free light chain (FLC) kappa-to-lambda ratio, to detect low-level clonal light chain production
The combination of a normal SPIE and UPIE and a normal serum FLC ratio nearly rules out systemic AL amyloidosis. In patients with kidney disease, the FLC ratio is often mildly elevated, but if SPIE/UPIE are normal, a kappa:lambda ratio up to 2.5 can typically be considered normal. [3]
Assessment for organ involvement may involve the following laboratory tests [3] :
-
Heart – Troponins I and T, brain natriuretic peptide (BNP), N-terminal pro-brain natriuretic peptide (NT-proBNP)
-
Kidneys – Urine albumin:creatinine ratio
-
Liver – Alkaline phosphatase
Procedures for assessment of organ involvement may include the following, if clinically indicated [29] :
-
Cardiac involvement: Echocardiogram with strain assessment; cardiac MRI (in certain circumstances)
-
Liver and gastrointestinal (GI) tract involvement: Gastric emptying scan, if gastroparesis present; abdominal ultrasound or computed tomography scan to document hepatomegaly
-
Peripheral nervous system involvement: Electromyography (EMG)/nerve conduction studies
-
Lung invovlement: Pulmonary function tests, chest CT without contrast
Consensus criteria for organ involvement in amyloidosis, from the XII International Symposium on Amyloidosis, are as follows [30] :
-
Kidney: 24-h urine protein > 0.5 g/d, predominantly albumin
-
Heart: Echocardiograpy showing mean wall thickness > 12 mm, no other cardiac cause; or NT-proBNP > 332 ng/L in the absence of kidney failure or atrial fibrillation
-
Liver: Total liver span > 15 cm in the absence of heart failure, or alkaline phosphatase > 1.5 times upper limit of normal
-
Peripheral nerves: Symmetric lower-extremity sensorimotor neuropathy
-
Autonomic nervous system: gastric-emptying disorder, pseudo-obstruction, voiding dysfunction not related to direct organ infiltration
-
GI: Symptoms, verified by direct biopsy
-
Lungs: Symptoms, verified by direct biopsy; interstitial radiographic pattern
-
Soft tissue: Tongue enlargement, arthropathy, claudication (presumed vascular amyloid), skin lesions, myopathy by biopsy or pseudohypertrophy, lymphadenopathy (may be localized), carpal tunnel syndrome
Biopsy and subtyping
The presence of an abnormality in SPEI/UPEI or FLC ratio testing is insufficient for a diagnosis of AL amyloidosis; these abnormalities are also seen in monoclonal gammopathy of undetermined significance (MGUS) and multiple myeloma. To confirm the diagnosis, it is necessary to perform a biopsy, with Congo red and immunologic staining of the tissue sample to identify amyloid and it.
The abdominal fat pad has become the most commonly selected biopsy site, replacing the older choice of rectal tissue. Abdominal fat pad biopsies for amyloidosis have sensitivity of over 80%. [3] If the specimen is negative for amyloid, tissue should be obtained from an organ or area with suspected involvement, such as the heart, kidney, liver, or sural nerve, although these have the disadvantage of being invasive procedures. [31]
Imaging Studies
Echocardiography is valuable in the evaluation of amyloid heart disease. It usually reveals a concentrically thickened left ventricle and often a thickened right ventricle, with a normal-to-small cavity. Cardiovascular magnetic resonance imaging provides high-definition structural imaging and tissue characterization that are often incremental to information obtained on echocardiography. [32, 33]
Doppler studies are useful and may show abnormal relaxation early in the course of the disease. Advanced involvement is characterized by restrictive hemodynamics.
Cardiac scintigraphy with 99mtechnetium-labeled bone-tracers has proved sensitive and specific for initial diagnosis. [34] 18F-Fluorodeoxyglucose positron-emission tomography has also been used in primary systemic amyloidosis evaluation. [35]
Histologic Findings
The best way to identify amyloid is to stain paraffin-embedded sections with alkaline Congo red and to examine them with polarized light to elicit a green fluorescence. Routine hematoxylin-eosin staining may show a homogeneous, faintly eosinophilic mass if enough amyloid is present. See the images below.
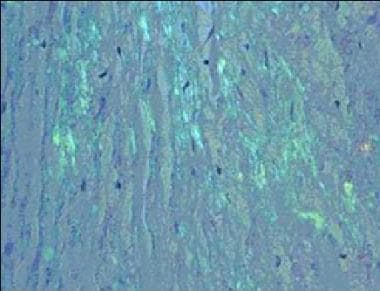 Amyloidosis. Congo red staining of a cardiac biopsy specimen containing amyloid, viewed under polarized light
Amyloidosis. Congo red staining of a cardiac biopsy specimen containing amyloid, viewed under polarized light
Analysis of a skin biopsy specimen of a papule reveals an amorphous or fissured eosinophilic mass in the papillary dermis with associated thinning or obliteration of the rete ridges. Nodules and plaques may demonstrate diffuse amyloid deposition in the reticular dermis or subcutis. Amyloid depositions are usually not associated with an inflammatory infiltrate.
The appearance of amyloid infiltration of the blood vessel walls, pilosebaceous units, arrector pili muscles, and lamina propria of sweat glands and infiltration around individual fat cells in the subcutis (known as amyloid rings) are characteristic findings. Amyloid may be deposited in the nail bed of dystrophic nails.
The finding of light chain–restricted plasma cells with amyloid may be a manifestation of myeloma or a marginal cell lymphoma. Clinical correlation is required. [36]
Staging
Prognostic models used for risk stratification in AL amyloidosis include the following [32] :
-
Mayo AL amyloidosis 2004 model [37]
-
Mayo AL amyloidosis 2012 model [16]
-
European 2016 model, which is a modification of the Mayo model [38]
-
Boston University 2019 prognostic model [39]
The Mayo AL amyloidosis 2012 model classifies AL amyloidosis into four stages, based on the presence of risk factors. Each of the following risk factors is assigned 1 point:
-
N-terminal pro–B-type natriuretic peptide (NT-proBNP) ≥1800 ng/L
-
Cardiac troponin T (cTnT) ≥0.025 µ/L (or cardiac troponin I [cTnI] ≥0.1 µ/L)
-
Difference between involved and uninvolved serum free light chains ≥18 mg/dL
Patients are then staged as follows:
-
Stage I – 0 points
-
Stage II – 1 point
-
Stage III – 2 points
-
Stage IV – 3 points
The European model divides stage III into stage IIIA and stage IIIB, based on a NT-proBNP level of 18000-8500 ng/L or > 8500 ng/L, respectively. [38]
The Boston University model replaces NT-proBNP with brain natriuretic peptide (BNP), to accommodate centers that do not have access to NT-proBNP. [39] Stages are as follows [40, 41] :
-
Stage I: Neither BNP ≥81 pg/mL nor TnI ≥0.1 ng/mL
-
Stage II: Either BNP ≥81 pg/mL or TnI ≥0.1 ng/mL (but not both)
-
Stage III: Both BNP ≥81 pg/mL and TnI ≥0.1 ng/mL
-
Stage IIIb: BNP ≥700 pg/mL and TnI ≥0.1 ng/mL
-
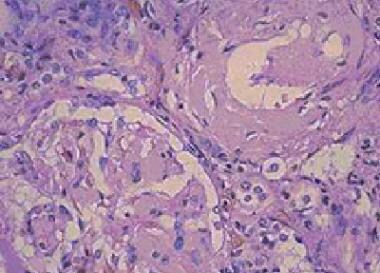
Amyloidosis. Amorphous eosinophilic interstitial amyloid observed on a kidney biopsy specimen.
-
Amyloidosis. Congo red staining of a cardiac biopsy specimen containing amyloid, viewed under polarized light