Approach Considerations
Typical electroencephalographic (EEG) abnormalities are highly supportive of the clinical diagnosis of juvenile myoclonic epilepsy (JME). As for all IGEs, neuroimaging studies are usually normal in JME. Many clinicians believe that in the presence of an adequate supportive history, EEG abnormalities, normal intelligence, and normal neurologic findings, neuroimaging studies are unnecessary. However, the clinical scenario might not be as clear as the classical description would suggest.
Electroencephalography
The study of choice for confirming the clinical diagnosis of juvenile myoclonic epilepsy (JME) is sleep-deprived EEG with activation procedures (ie, hyperventilation, photic stimulation). A normal study does not rule out epilepsy or JME, as the sensitivity of a routine study is limited. Repeat routine EEG studies are reported to increase yield following a first non-diagnostic study. This can also be achieved with prolonged (eg, 3-day) EEG. Typical EEG abnormalities are highly supportive of the clinical diagnosis.
Interictal EEG
The typical interictal EEG abnormality consists of a generalized greater than or equal to 3- to 5.5-Hz spike or polyspike and slow-wave discharges lasting 1–20 seconds seen in both wakefulness and sleep (see the image below). Usually, 1–3 spikes precede each slow wave. Focal or multi-focal spikes and spike-wave discharges are observed in up to 20% of patients, and can lead to a wrong diagnosis of focal epilepsy, as can asymmetries. [68] When absence seizures are also present, 3-Hz spike-and-wave (SW) activity may be seen in addition to the polyspike-and-wave (PSW) pattern. Occasionally, isolated fragments of generalized spikes can also be seen.
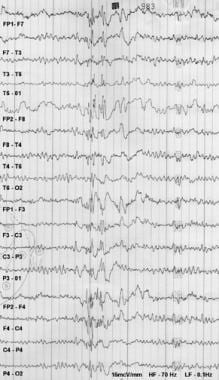 Findings in a man with a history of generalized tonic-clonic seizures (mostly nocturnal) and myoclonic jerks (mostly in the morning) since the age of 14 years. Carbamazepine exacerbated his myoclonic seizures. Sleep-deprived EEG was digitally recorded and then plotted by using an analog paper machine. The patient was getting drowsy when this burst of polyspike and slow wave was recorded.
Findings in a man with a history of generalized tonic-clonic seizures (mostly nocturnal) and myoclonic jerks (mostly in the morning) since the age of 14 years. Carbamazepine exacerbated his myoclonic seizures. Sleep-deprived EEG was digitally recorded and then plotted by using an analog paper machine. The patient was getting drowsy when this burst of polyspike and slow wave was recorded.
Treatment with medications clinically effective in JME might also reduce the frequency of interictal abnormalities. Levetiracetam adjunctive therapy in patients with JME increased the likelihood of a normal EEG from 8% to 53% after maintenance therapy was achieved. There was a decrease in frequency of interictal discharges and suppression of the paroxysms induced by photic stimulation. [25]
Ictal EEG
The ictal EEG associated with myoclonic jerks typically reveals 10- to 16-Hz polyspike discharges. These may be preceded by SW activity and are often followed by 1- to 3-Hz slow waves. The number of spikes is typically 5-20 and tends to be proportionately correlated with the clinical intensity of the seizure. These epileptic discharges may briefly persist, even after clinical activity has ceased. Seizures in patients with JME tend to be associated with polyspikes and disorganization of the paroxysm. [26]
Absence seizures of JME may be associated with ictal EEG patterns consisting of 3-Hz SW activity. Sometimes, these are preceded by 4- to 6-Hz PSW discharges, which slow to 3 Hz as the patient loses consciousness.
Background
Background activity of the EEG is normal in JME.
Activation
Hyperventilation and photic stimulation often facilitate the appearance of epileptiform discharges. Photic stimulation frequently precipitates SW patterns or a photo-paroxysmal response, which is seen in more than a third of patients and can be detected in up to 90% of untreated patients. [68]
SW patterns by photic stimulation occur in 30% of patients with JME, compared with 18% of patients with childhood absence epilepsy, 13% of patients with epileptic seizures on awakening, and 7.5% of patients with juvenile absence epilepsy.
Hyperventilation can provoke clinical absence seizures. [68]
Other EEG findings
In addition to generalized epileptiform discharges, focal abnormalities may be found in a subset of patients with JME, with reported rates ranging from 4% to 55%. These include focal slow waves, generalized discharges that evolve from a focal onset, and focal spikes or SW discharges. Focal spikes or SW discharge mostly occur in the frontal regions. [68] EEG discharges can also be asymmetric [72] and lead to a wrong diagnosis of focal epilepsy. One published series of idiopathic primary generalized epilepsy patients evaluated with video EEG reported focal interictal epileptiform discharges and semiologic features of focal seizure in 35% of patients. However, no seizures with focal electrographic onset were reported in that study. [28]
The etiology of these focal abnormalities is unclear. A possible explanation is structural changes in the cerebral cortex secondary to recurrent seizures or head injury; another is fluctuation in the refractoriness of the cortex to the influence of a spike/wave generator.
Morning EEG
A morning EEG has been proposed as a superior strategy to detect generalized epileptiform discharges in patients with JME. In this particular study, a morning awake EEG detected interictal epileptiform discharges in 69% of patients, whereas an afternoon awake EEG in the same patients demonstrated epileptiform discharges in fewer than 20% of patients. [29]
Video EEG
Video EEG monitoring in patients with atypical features of JME might be needed. In a one study, most people with JME only required no more than 2 days of stay to demonstrate diagnostic abnormalities in the EEG. [30]
A combined magnetoencephalography and EEG study demonstrated interictal spikes with localizations mainly in the central and premotor regions in patients with JME as compared with other absence syndromes. [31]
Magnetic Resonance Imaging
As in all idiopathic (genetic) generalized epilepsies (IGEs), magnetic resonance imaging (MRI) of the brain usually yields unremarkable results. This observation reflects the fact that juvenile myoclonic epilepsy (JME) is an idiopathic generalized epilepsy and is not caused by conditions leading to focal cortical brain pathology such as brain tumors or encephalitis. However, quantitative morphometric studies using a voxel-based technique have shown some differences among patients with JME.
For example, decreased gray matter volume was found in thalami, insula cortices, and cerebellar hemispheres bilaterally in patients with JME. An increase in gray matter volume was observed in the right superior frontal, orbitofrontal, and medial frontal gyri of patients with JME as compared with age-matched controls. Patients with JME who are photosensitive had decreased gray matter volume in the visual cortex as compared with a control group; this was not found in patients with JME who were not photosensitive. [32, 33]
Some patients with brain MRIs, particularly if the MRIs are high-definition (or high-Tesla) studies, have shown minor abnormalities of cortical development. Tae et al reported reduction in the cortical thicknesses of frontal and temporal gyri in patients with JME. [34] However, these observations were not confirmed in the study by Roebling et al. [35]
Progressive thalamic atrophy was also reported in patients with JME by the same group. [36] The decreased thalamic volume has been confirmed by several other groups and might be related to executive function impairment. [37, 38, 39] Furthermore, studies using diffusion tension imaging (DTI) have also confirmed that abnormalities in the degree of thalamocortical fiber orientation and tissue anisotropy correlate with the frequency of generalized tonic-clonic seizures (GTCSs). [40]
Magnetic resonance spectroscopy (MRS) has also confirmed abnormalities in the thalamus and thalamocortical system of patients with JME. [41, 42] Functional MRI (fMRI) studies have not shown significant abnormalities in patients with JME. [35] However, it has been noted that during working memory tasks, the motor cortex co-activated with the supplementary motor cortex in addition to areas of higher cognitive function including the frontal and parietal lobes; interestingly, this co-activation is decreased with increased valproic acid dosages. [67]
Positron emission tomography (PET) imaging has revealed neurotransmitter and metabolic changes in the dorsolateral prefrontal cortex, which is in keeping with JME patients’ previously noted psychiatric comorbidities. [67] H-magnetic resonance spectroscopy reveals progressive thalamic dysfunction, which can extend to the occipital lobe in those with photosensitivity. [65] Based on studies of neurotransmitters using PET imaging, the serotonin system may also be affected in JME. The dopaminergic system also appeared to be affected with impaired signaling in the substantia nigra and midbrain, but was normal in the caudate and putamen. [67]
Using proton magnetic resonance spectroscopy, studies have found that patients with JME have lower N-acetyl aspartate (NAA) both in the thalamus and prefrontal areas. [67]
Overall, with more sophisticated imaging there have been some noted abnormalities in JME, most significantly in the thalami, prefrontal cortex, and motor cortex with co-activation with the supplementary motor cortex and the frontal and parietal lobes. In addition, neurotransmitters such as dopamine, serotonin, and NAA may play a role in JME.
Despite these minor quantitative differences, the guidelines of the International League Against Epilepsy (ILAE) do not recommend routine neuroimaging studies in patients with JME. [43]
Transcranial Magnetic Stimulation
Although not standard of care at this time, transcranial magnetic stimulation (TMS) has also been used in this setting. Studies using TMS show abnormalities in cortical excitability in patients with juvenile myoclonic epilepsy (JME). [44]
-
Findings in a man with a history of generalized tonic-clonic seizures (mostly nocturnal) and myoclonic jerks (mostly in the morning) since the age of 14 years. Carbamazepine exacerbated his myoclonic seizures. Sleep-deprived EEG was digitally recorded and then plotted by using an analog paper machine. The patient was getting drowsy when this burst of polyspike and slow wave was recorded.