Laboratory Studies
Complete blood count (CBC) and differential generally are obtained to assist with therapeutic decisions, although these test results are nonspecific.
-
A significant decrease in hematocrit (at least 4%) or hemoglobin (at least 2 g/dL) occurs in approximately one half of patients with bilateral adrenal hemorrhage.
-
Leukocytosis frequently occurs in patients with adrenal hemorrhage, and it may be associated with the underlying cause of adrenal hemorrhage. Eosinophilia is present in only a small percentage of patients with adrenal hemorrhage.
Serum electrolytes, blood urea nitrogen (BUN), creatinine, and plasma glucose may be of limited diagnostic use, but they also should be obtained to assist with patient management.
-
Hyponatremia, hyperkalemia, and prerenal azotemia are present in approximately 50% of patients with extensive, bilateral adrenal hemorrhage. Mild hypercalcemia may rarely occur. Although the combination of low serum sodium and high serum potassium is suggestive of adrenal insufficiency in the appropriate clinical setting, their absence never should exclude this diagnosis.
-
Hypoglycemia may occur in patients with adrenal hemorrhage and adrenal insufficiency, but it rarely is severe.
Serum cortisol, plasma ACTH, serum aldosterone, and plasma renin activity (PRA) always should be obtained in suspected adrenal hemorrhage cases, because they provide important information on adrenal function.
-
In an acutely ill patient, the combination of increased plasma ACTH and low, or even low-normal (ie, < 13 mcg/dL), serum cortisol is highly suggestive of glucocorticoid deficiency due to primary adrenal insufficiency. Conversely, a serum cortisol of over 25 mcg/dL in an acutely ill patient excludes glucocorticoid deficiency. The combination of low serum aldosterone and increased PRA suggests mineralocorticoid deficiency.
-
In order to provide useful diagnostic information, blood samples for these tests should be obtained before glucocorticoid administration, because several exogenous glucocorticoids (hydrocortisone and prednisone, but not dexamethasone) cross-react with endogenous cortisol in radioimmunoassay.
-
Because results are not available immediately, these tests are not helpful in the acute setting, but they provide retrospective diagnostic information.
The short cosyntropin (Cortrosyn) stimulation test confirms the diagnosis of adrenal insufficiency.
-
This test involves the bolus intravenous or intramuscular administration of the ACTH analog cosyntropin (Cortrosyn, 250 mcg) and serum collection 1 hour after cosyntropin administration for cortisol assay. A peak serum cortisol of over 20 mcg/dL (1 h after cosyntropin administration) indicates normal adrenal response in a nonstressed individual.
-
Although the normal adrenal response to cosyntropin has not been defined precisely in acutely ill patients, individuals with bilateral adrenal hemorrhage and clinical evidence of adrenal insufficiency have a markedly blunted adrenal response, according to the above criteria, in this test.
-
Measurement of the aldosterone response to cosyntropin (Cortrosyn) administration has been advocated in order to assess mineralocorticoid reserve. Serum samples are obtained immediately before and 30 minutes after the administration, as above, of intravenous cosyntropin. Either a peak serum aldosterone of over 16 ng/dL or a rise in serum aldosterone of at least 4 ng/dL indicates a normal adrenal response.
-
Although the short cosyntropin (Cortrosyn) stimulation test is the criterion standard for the diagnosis of adrenal insufficiency, its performance may not be practical in the acute setting, particularly in a hypotensive or otherwise unstable patient. In these cases, only basal cortisol and ACTH levels need to be obtained before urgent glucocorticoid administration, and the more definitive Cortrosyn stimulation test can be performed after the patient has been stabilized. Alternatively, because the presence of dexamethasone does not interfere with cortisol immunoassays, this medication can be administered to stabilize the condition of a patient with suspected adrenal insufficiency, before the short Cortrosyn stimulation test is performed.
-
Because the results of the short cosyntropin (Cortrosyn) stimulation test are not available immediately, this test is not helpful in the acute setting to guide management decisions, but it provides retrospective confirmation of adrenal insufficiency.
Imaging Studies
CT scanning
Computed tomography (CT) scanning of the adrenals (thin slice) is the study of choice for demonstrating adrenal hemorrhage in the acute setting. [4] However, it is practical only in hemodynamically stable patients.
CT scanning of patients with adrenal hemorrhage shows adrenal enlargement that may be asymmetrical in cases of bilateral adrenal hemorrhage. The glands become rounded or oval shaped and have high attenuation (50-90 Hounsfield units) without contrast enhancement in the acute setting.
In cases of unilateral, traumatic adrenal hemorrhage, a streaky appearance of the perirenal fat frequently is observed posterior to the gland. [24] This finding is not specific to traumatic adrenal hemorrhage, because it also has been observed in patients with adrenal hemorrhage and metastatic tumors to the adrenals. Extension of the hemorrhage into the perirenal space, with perinephric hematoma formation, has been observed in patients with metastatic disease.
Associated findings may include a mixed-density, inhomogeneous mass in patients with primary or metastatic cancer. Significant contrast enhancement further suggests the presence of an associated lesion (such as pheochromocytoma or metastatic tumor).
Several weeks after the acute adrenal hemorrhage, CT scanning shows a gradual decrease in adrenal size and attenuation. In addition, the adrenals may have a cystic appearance.
Several months after the acute event, CT scanning of the adrenals shows progressive atrophy, with the variable appearance of calcifications. The presence of adrenal calcifications does not invariably indicate a previous episode of adrenal hemorrhage, because this finding also is associated with adrenal cysts, adrenocortical adenomas and carcinomas, pheochromocytomas, neuroblastomas (only in children), metastatic tumors, and granulomatous diseases, including tuberculosis and histoplasmosis.
A study by Tan and Sutherland suggested that signs of adrenal congestion (adrenal gland thickening, periadrenal fat stranding) on CT scanning can predict subsequent nontraumatic adrenal hemorrhaging. The study involved four patients with nontraumatic adrenal hemorrhage, who were compared with 12 randomly selected intensive care patients. [25]
Recognizing that adrenal hematomas with a masslike configuration can be mistaken for adrenal neoplasms, leading to surgical resection, Rowe et al described the CT-scan characteristics of five adrenal hematomas. The lesions were well-defined, with an ovoid morphology, an average maximum diameter of 8.9 cm, and greatly varying attenuation on noncontrast CT scan. Peripheral enhancement in four of the hematomas was either thin and somewhat uniform or heterogeneous and irregular. The five lesions did not invade periadrenal fat or adjacent organs. [26]
CT scans are shown below.
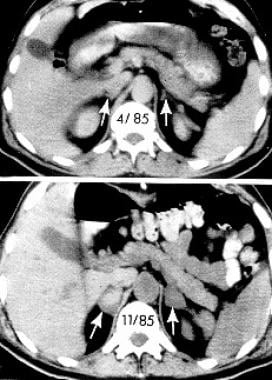 Computed tomographic (CT) scans of the abdomen show normal adrenal glands several months before the onset of hemorrhage (upper panel) and enlarged adrenals 2 weeks after an acute episode of bilateral adrenal hemorrhage (lower panel). The attenuation of the adrenal glands, indicated by arrows, is increased after the acute event. Reproduced from Rao RH, Vagnucci AH, Amico JA: Bilateral massive adrenal hemorrhage: early recognition and treatment. Ann Intern Med. Feb 1 1989;110(3):227-35 with permission from the journal.
Computed tomographic (CT) scans of the abdomen show normal adrenal glands several months before the onset of hemorrhage (upper panel) and enlarged adrenals 2 weeks after an acute episode of bilateral adrenal hemorrhage (lower panel). The attenuation of the adrenal glands, indicated by arrows, is increased after the acute event. Reproduced from Rao RH, Vagnucci AH, Amico JA: Bilateral massive adrenal hemorrhage: early recognition and treatment. Ann Intern Med. Feb 1 1989;110(3):227-35 with permission from the journal.
MRI
Magnetic resonance imaging (MRI) may be used to help exclude the presence of malignant tumors or pheochromocytomas, and it may provide an estimate of the age of the hematoma. [5, 6] Less experience with the use of MRI in adrenal hemorrhage exists in comparison with CT scanning.
Acutely (ie, 24-72 h after onset), the adrenals are enlarged. Adrenal hemorrhage appears isointense with normal liver and muscle on T1-weighted images and appears hyperintense to the liver on T2-weighted images. Stranding of the perirenal and even subcutaneous fat has been observed in T2-weighted images in traumatic adrenal hemorrhage cases.
Subacutely (ie, approximately 3-7 d after onset), adrenal hemorrhage shows intermediate intensity on T1- and T2-weighted images. This appearance has been associated with the presence of deoxyhemoglobin in the adrenal hemorrhagic area. In addition, a hyperintense ring frequently is observed outlining the adrenals (on T1- and T2-weighted images). This hyperintense ring gradually may fill in centrally, and it appears to be secondary to the presence of free methemoglobin.
At a more chronic stage (ie, several wk after onset), MRI shows a decrease in adrenal size. In addition, adrenal hemorrhagic areas become hyperintense with a hypointense rim on T1- and T2-weighted images. The centrally located, high signal intensity may be secondary to the presence of free methemoglobin, and the hypointense rim has been associated with the presence of hemosiderin-laden macrophages in the fibrous capsule. Furthermore, necrotic areas in the adrenal hemorrhagic area may create a more heterogeneous appearance, with areas of low signal intensity on T1-weighted images.
In a suspected adrenal hemorrhage case, the lack of enhancement after gadolinium–diethylenetriamine penta-acetic acid (Gd-DTPA) administration and the above-outlined evolution of the appearance of the adrenals confirm the diagnosis of adrenal hemorrhage and help to exclude the presence of tumor.
Ultrasonography
Ultrasonographic examination of the adrenals (including Doppler ultrasonography) is quite helpful in neonatal adrenal hemorrhage cases, and it may reveal the presence of adrenal hemorrhage in utero.
In older children or adults, ultrasonographic examination may be employed at the bedside, although it is operator dependent and may be limited by large body habitus.
Ultrasonographic imaging of adrenal hemorrhage reveals hyperechoic masses that contain a central echogenic area in the adrenal glands. Several weeks after the acute event, the central echogenicity associated with adrenal hemorrhage decreases as the hematomas become cystic.
A study by Piskunowicz et al indicated that neonatal adrenal hemorrhages can be distinguished from malignant adrenal lesions via contrast-enhanced ultrasonography (CEUS). For example, by revealing contrast signals within even the smallest vessels, CEUS can aid in differentiating a vascular mass from a hemorrhage. Moreover, the modality can be useful in blood flow assessment, owing to its high temporal and spatial resolution. [27]
Procedures
Percutaneous biopsy is helpful in establishing the presence of metastatic disease in cases of adrenal hemorrhage in which suggestive features appear on CT scans.
Histologic Findings
Examination of the adrenals in adrenal hemorrhage cases typically reveals extensive hemorrhagic necrosis involving all 3 adrenal cortical cell layers, in addition to adrenal medullary cell necrosis. The hemorrhage may extend into the perirenal fat and the perirenal space.
Other common findings include adrenal vein thrombosis and the retrograde migration of medullary cells into the zona fasciculata. In contrast, vasculitis rarely has been observed in cases of adrenal hemorrhage, suggesting that it has a limited role in the pathogenesis of adrenal hemorrhage.
At a more chronic stage, the hematoma becomes organized as a fibrous capsule that forms around the adrenal hemorrhagic area. Hemosiderin-laden macrophages are present in the capsule and digest cell debris. In the months following acute adrenal hemorrhage, fibrous tissue gradually replaces the hemorrhagic areas.
-
Computed tomographic (CT) scans of the abdomen show normal adrenal glands several months before the onset of hemorrhage (upper panel) and enlarged adrenals 2 weeks after an acute episode of bilateral adrenal hemorrhage (lower panel). The attenuation of the adrenal glands, indicated by arrows, is increased after the acute event. Reproduced from Rao RH, Vagnucci AH, Amico JA: Bilateral massive adrenal hemorrhage: early recognition and treatment. Ann Intern Med. Feb 1 1989;110(3):227-35 with permission from the journal.