Practice Essentials
Carotid body tumors (CBTs) are rare neoplasms, although they represent about 50-60% of head and neck paragangliomas. [1] These tumors develop within the adventitia of the medial aspect of the carotid bifurcation. Various imaging studies can be used to confirm the diagnosis of carotid body tumor (CBT), starting with simple ultrasonography with color Doppler. [2] The growths are treated with either surgery or radiotherapy.
The carotid body, a small, reddish-brown, oval structure that originates in the neural crest, is important in the body's acute adaptation to fluctuating concentrations of oxygen, carbon dioxide, and pH. The carotid body protects the organs from hypoxic damage by releasing neurotransmitters that increase the ventilatory rate when stimulated.
See the image below.
The following 3 different types of carotid body tumors (CBTs) have been described in the literature:
-
Familial
-
Sporadic
-
Hyperplastic
The sporadic form is the most common type, representing approximately 85% of carotid body tumors (CBTs). The familial type (10-50%) is more common in younger patients. The hyperplastic form is very common in patients with chronic hypoxia, which includes those patients living at a high altitude (> 5000 feet above sea level), like those patients living in New Mexico, Peru, and Colorado. [3] The hyperplastic form is also seen in patients who have chronic obstructive pulmonary disease (COPD) or cyanotic heart disease.
Workup in carotid body tumors
With regard to laboratory studies, check urinary catecholamines in patients who have any symptoms of a functional carotid body tumor (CBT).
Various imaging studies can be used to confirm the diagnosis of carotid body tumor (CBT), starting with simple ultrasonography with color Doppler, which can assess the vascularity of the neck mass and can sometimes reveal a possibility of a carotid body tumor (CBT), although it is not the best imaging modality to detect these tumors. [2]
Computed tomography (CT) scanning of the head and neck is also helpful and typically reveals a hypervascular tumor located between the external and internal carotid arteries.
Magnetic resonance imaging (MRI) is considered to be the criterion standard for carotid body tumors (CBTs), and the tumor has a characteristic salt and pepper appearance on T1-weighted images.
Magnetic resonance angiography (MRA) provides better insight into the vascularity of the tumor and its feeder vessels. [2]
Management of carotid body tumors
Carotid body tumors (CBTs) are treated with either surgery or radiotherapy. When choosing treatment, consider the following factors: the presence of other paragangliomas, the presence of bilateral carotid body tumors (CBTs), the age and the health of the patient, and the patient's preference. [4]
History of the Procedure
Descriptions of surgery for carotid body tumors have existed for over 100 years. The early reports described significant complications, particularly mortalities secondary to intraoperative bleeding. [5, 6, 7]
In the United States, the earliest successful carotid body tumor resection was performed by Scudder in 1903. [5]
Even into the middle of the 20th century, resecting these tumors remained a problem because of the complications; Hayes Martin, in his textbook of head and neck tumors, recommended against resection of any tumor that is now considered a Shamblin type III (see Staging). [8]
Modern imaging and current surgical and vascular techniques have significantly improved the safety and success of this operation.
Epidemiology
Frequency
Parasympathetic paragangliomas are rare, with a prevalence of 1-2 per 100,000 population. [9] Carotid body tumors (CBTs) constitute about 50-60% of head and neck paragangliomas.
Carotid body tumors (CBTs) can occur in children; however, carotid body tumors (CBTs) are considered to be a disease of middle age. The mean age of onset is reported to be 45 years. [10] Paragangliomas are inherited in 10-50% of cases. Age of onset in the hereditary group is typically younger, in the second to fourth decade. [11]
A retrospective study by Davila et al suggested that carotid body tumors also tend to appear at a younger age in patients with succinate dehydrogenase mutations (see Pathophysiology). In the study, of 183 patients with carotid body tumors, 18 patients underwent succinate dehydrogenase testing, with 17 found to be positive for mutations. The positive patients were diagnosed with tumors at a mean age of 38.0 years, compared with 50.3 years for patients without known mutations. [12]
About 5% of carotid body tumors (CBTs) are bilateral and 5-10% are malignant, but these rates are much higher in patients with inherited disease. [13, 14, 15] Familial tumors are found to be 5.8 times more common among patients who have carotid body tumors as compared with patients who have paragangliomas at other sites.
Interestingly, the male-to-female ratio differs in patients dwelling at high altitudes above 2,000 meters (1:8.3) than those patients dwelling at sea level (1:1.0-1.4). [16]
Etiology
The only known risk factors are the presence of chronic hypoxic stimulation and the genetic predisposition.
Pathophysiology
Carotid body tumors (CBTs) are classified into sporadic, familial, and hyperplastic forms. The familial paraganglioma form is a genetically heterogenous entity; currently, 4 genes are identified. The first 3 genes encode the subunits of the enzyme succinate dehydrogenase complex, which is part of the Kreb's cycle. Paraganglioma (PG) syndrome 1, 3, and 4 occur because of mutations of the corresponding genes of the subunits D, C, and B. [17] PG syndrome 2 gene mutations are yet to be identified.
Defective succinate dehydrogenase has been postulated to cause an increase in the intracellular concentration of molecular hypoxia mediators and the vascular endothelial growth factor (VEGF) thus resulting in hyperplasia, angiogenesis, and neoplasia. [9]
In patients who lack a positive family history, germline mutations in the paraganglioma susceptibility genes are still possible via multiple causes, including genomic imprinting, reduced penetrance, or de novo mutations in the genes of the parental gametes. [17] (Genetic testing is recommended in patients under age 40 years with a carotid body tumor (CBT) and in individuals with bilateral or multiple CBTs. [18] )
Chronic hypoxic conditions, such as patients living at high altitudes or those who have chronic obstructive pulmonary disease (COPD) or cyanotic heart problems, can overburden the carotid bodies and subsequently lead to hypertrophy, hyperplasia, and neoplasia of the chief cells. [17] This condition is seen in the hyperplastic type of carotid body tumors (CBTs). However, the mechanism by which reduced oxygen concentrations can lead to CB hyperplasia is unclear.
Carotid body tumors (CBTs) can be occasionally coupled with nonparaganglonic tumors in syndromes, including MEN type II, von Hippel-Lindau syndrome, and neurofibromatosis type 1.
A study by de Franciscis et al suggested that carotid body tumors have a neuroendocrine effect on arterial blood pressure. The study, on 17 patients with benign or malignant carotid body tumors, found that at admission, individuals with malignant carotid body tumors had higher blood pressure than did controls and that those with malignant tumors had higher blood pressure than did those with benign neoplasms. Moreover, the investigators determined that 10 days following resection of the tumors, all 17 patients showed significant reductions in blood pressure and in the level of matrix metalloproteinases. [19]
Presentation
Carotid body tumors (CBTs) present most commonly as an asymptomatic palpable neck mass in the anterior triangle of the neck. They are slow-growing tumors that can remain asymptomatic for many years. The doubling time (TD) of carotid body tumors (CBTs), as estimated by Jansen et al using sequential imaging, was 7.13 years with a median growth rate of 0.83 mm/year. [20]
On examination, the mass is typically vertically fixed because of its attachment to the bifurcation of the common carotid (Fontaine sign). A bruit can be felt; however, the absence of a bruit does not rule out a carotid body tumor (CBT). Vagal body tumors are more cranially located and sometimes project into the lateral pharynx as a pulsatile mass.
Approximately 10% of the cases present with cranial nerve palsy with paralysis of the hypoglossal, glossopharyngeal, recurrent laryngeal, or spinal accessory nerve, or involvement of the sympathetic chain. [21] Carotid body tumors (CBTs) may, therefore, be associated with pain, hoarseness, dysphagia, Horner syndrome, or shoulder drop.
As the tumor enlarges and compresses the carotid artery and the surrounding nerves, other symptoms may also be present, such as pain, tongue paresis, hoarseness, Horner syndrome, and dysphagia.
Fever is an uncommon sign of carotid body tumor (CBT), although the literature has reported it as one of the causes of fever of unknown origin. [22] In cases of functional carotid body tumors (CBTs), symptoms similar to those of pheochromocytoma, such as paroxysmal hypertension, palpitations, and diaphoresis, are seen.
Relevant Anatomy
The carotid body is a small, reddish-brown, oval structure, located in the posteromedial aspect of the carotid artery bifurcation. The healthy gland measures 3-5 mm in diameter and weighs less than 15 mg on average. [23] The vast majority of the literature states that the gland is located in the adventitia near the carotid artery bifurcation. However, according to Maxwell et al, most surgeons experienced with carotid body dissection maintain that it is more peripherally located, within periadventitial tissue. This distinction is critical, as dissections in the deeper planes of the carotid artery are associated with higher risk for complications from vessel injury. [24]
The gland is highly vascular and receives its blood supply from feeder vessels running through the Mayer ligaments, primarily from the external carotid artery, typically the ascending pharyngeal artery. It is innervated by the Hering nerve, originating from the glossopharyngeal nerve about 1.5 cm distal to the jugular foramen. [25]
-
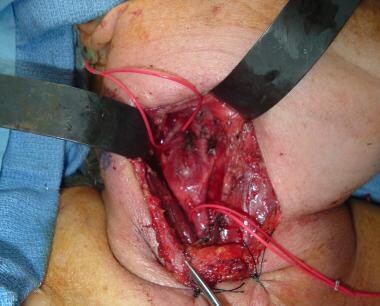
Proximal and distal control of the carotids with vessel loops.
-
Complete subadventitial removal of the tumor with intact carotids.
-
Carotid body tumor after total excision.
-
CT scan with IV contrast demonstrating a large left enhancing carotid body tumor extending into the parapharyngeal space to the oropharynx.
-
Preauricular excision extended from the neck in patients who have large tumors.
-
Proximal and distal control of the carotids is extremely important and can be difficult in larger tumors.
-
Postoperative picture after removal of the tumor.
-
Four-vessel angiography of a 57-year-old patient with bilateral carotid body tumors.