Overview
Acute myeloid leukemia (AML) with myelodysplasia-related changes (AML-MRC) includes those forms of AML that occur in patients with any of the following criteria:
-
A previous history of a myelodysplastic syndrome (MDS) or a myelodysplastic/myeloproliferative neoplasm (MDS/MPN)
-
An MDS-related cytogenetic abnormality
-
Multilineage dysplasia in at least 50% of cells of at least two cell lineages, in the absence of NPM1 or biallelic CEBPA mutations
The revised 4th edition (2016/2017 update) of the World Health Organization (WHO) Classification of Tumours of Haematopoietic and Lymphoid Tissues has focused increased attention on genetic abnormalities to characterize and subclassify AML, including AML-MRC. [1, 2]
AML-MRC encompasses the following:
-
AML arising in MDS or in MDS/MPN
-
AML with MDS-related cytogenetic abnormalities
-
AML with multilineage dysplasia
This category of AML primarily occurs in elderly patients; it is rare in children. AML-MRC represents 25-35% of AML cases. [3, 4, 5, 6]
Definition
By definition, the diagnosis of acute myeloid leukemia (AML) with myelodysplasia-related changes (AML-MRC) requires the presence of at least 20% blasts in the peripheral blood (PB) and/or bone marrow (BM), an absence of any of the AML-associated recurrent genetic abnormalities seen in AML (such as inv(3), t(6;9), or NPM1 mutation), and the absence of a history of cytotoxic chemotherapy or radiation therapy for an unrelated disease. [7]
In new cases of AML, careful review of the patient's history to identify a previous diagnosis of myelodysplastic syndrome (MDS) or myelodysplastic/myeloproliferative neoplasm (MDS/MPN) and to exclude prior exposure to cytotoxic treatments (chemotherapy and/or radiotherapy) is necessary for proper classification.
The list of AML-MRC-related cytogenetic abnormalities is shown in the section under "Molecular/Genetic Features and Methods."
Lastly, to classify a new diagnosis of AML as having myelodysplasia-related changes based on morphology, dysplasia must be present in at least 50% of the cells in at least two bone marrow cell lines. Additionally, cases of AML with an NPM1 or biallelic CEBPA mutation are classified separately, even if sufficient morphologic evidence of dysplasia is present. [8, 9]
Clinical and Morphologic Features
Clinical features
Patients with acute myeloid leukemia (AML) with myelodysplasia-related changes (AML-MRC) often present with severe pancytopenia. Cases arising in association with a prior myelodysplastic syndrome (MDS) or myelodysplastic/myeloproliferative neoplasm (MDS/MPN) as well as those arising in childhood may be slowly progressive.
Morphologic features
AML with multilineage dysplasia is established by demonstrating at least 20% blasts and by identifying dysplastic features in at least 50% of cells in at least two bone marrow (BM) cell lines (ie, dysgranulopoiesis, dyserythropoiesis, or dysmegakaryopoiesis). [7] Features of dysgranulopoiesis include hypogranularity, hyposegmentation of granulocytes (ie, pseudo-Pelger-Huët forms), or other abnormalities of nuclear lobation or cytoplasmic granularity (eg, pseudo-Chediak-Higashi granules) (see the images below). As such, cytochemical staining, especially with myeloperoxidase (MPO), may be aberrant, because patients may develop an acquired MPO deficiency as part of the dysplastic process. [10, 11, 12]
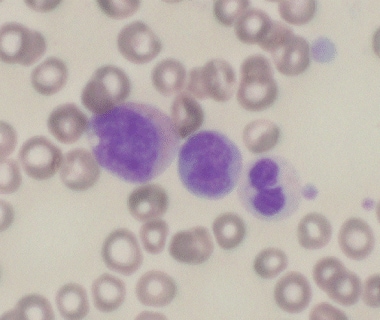 Pathology of Acute Myeloid Leukemia With Myelodysplasia-Related Changes (AML-MRC). This image reveals a dysplastic promyelocyte with a cytoplasmic vacuole (left), blast (center), and dysplastic neutrophil with pseudo-Pelger-Huet anomaly and hypogranularity (right).
Pathology of Acute Myeloid Leukemia With Myelodysplasia-Related Changes (AML-MRC). This image reveals a dysplastic promyelocyte with a cytoplasmic vacuole (left), blast (center), and dysplastic neutrophil with pseudo-Pelger-Huet anomaly and hypogranularity (right).
 Pathology of Acute Myeloid Leukemia With Myelodysplasia-Related Changes (AML-MRC). Dysgranulopoiesis demonstrating Chediak-Higashi-like granules (arrow) is shown.
Pathology of Acute Myeloid Leukemia With Myelodysplasia-Related Changes (AML-MRC). Dysgranulopoiesis demonstrating Chediak-Higashi-like granules (arrow) is shown.
Features of dyserythropoiesis include megaloblastoid forms, nuclear budding, irregular nuclear contours, nuclear fragmentation, multinucleation, karyorrhexis, ring sideroblasts, and cytoplasmic vacuolization (see the following images).
 Pathology of Acute Myeloid Leukemia With Myelodysplasia-Related Changes (AML-MRC). Numerous dysplastic erythroid cells are seen, demonstrating nuclear budding, irregular nuclear contours, and multinucleation.
Pathology of Acute Myeloid Leukemia With Myelodysplasia-Related Changes (AML-MRC). Numerous dysplastic erythroid cells are seen, demonstrating nuclear budding, irregular nuclear contours, and multinucleation.
 Pathology of Acute Myeloid Leukemia With Myelodysplasia-Related Changes (AML-MRC). The bone marrow aspirate shows a binucleate erythroid precursor.
Pathology of Acute Myeloid Leukemia With Myelodysplasia-Related Changes (AML-MRC). The bone marrow aspirate shows a binucleate erythroid precursor.
Features of dysmegakaryopoiesis include small size, nuclear hypolobation, nuclear hypersegmentation, and separated nuclear lobes (see the image below).
 Pathology of Acute Myeloid Leukemia With Myelodysplasia-Related Changes (AML-MRC). The bone marrow core biopsy evaluation reveals dysmegakaryopoiesis with numerous small hypolobated megakaryocytes.
Pathology of Acute Myeloid Leukemia With Myelodysplasia-Related Changes (AML-MRC). The bone marrow core biopsy evaluation reveals dysmegakaryopoiesis with numerous small hypolobated megakaryocytes.
 Pathology of Acute Myeloid Leukemia With Myelodysplasia-Related Changes (AML-MRC). A cluster of dysplastic megakaryocytes with small hypolobated nuclei is seen on a bone marrow aspirate smear preparation.
Pathology of Acute Myeloid Leukemia With Myelodysplasia-Related Changes (AML-MRC). A cluster of dysplastic megakaryocytes with small hypolobated nuclei is seen on a bone marrow aspirate smear preparation.
Rare cases of AML-MRC demonstrating basophilic differentiation have been reported. [13]
Immunophenotypic Features and Methods
By flow cytometry, the blasts in acute myeloid leukemia (AML) with myelodysplasia-related changes (AML-MRC) show a myeloblast phenotype, with expression of blast markers (CD34, CD117) and myeloid markers such as CD13, CD33, and/or myeloperoxidase (MPO); aberrant expression of CD5, CD7, or CD56 may also be seen. The background granulocytes may also aberrantly overexpress CD33 and underexpress CD45, CD11b, and CD15 by flow cytometry. Similarly, lower expression levels of CD14, CD56, and CD45 may be seen on background monocytic populations in AML-MRC. [14] Rare cases of AML-MRC with mixed-lineage phenotype have been reported. [15]
Immunohistochemical staining of the bone marrow biopsy specimen may be especially helpful in cases with increased fibrosis and an inadequate aspirate specimen. In AML-MRC, CD34 and/or CD117 immunostains reveal numerous (>20%) blasts, which often form large clusters and sheets. In cases emerging from a preexisting myelodysplastic syndrome (MDS), these stains may also highlight increased blasts located away from the bony trabeculae (atypical localization of immature precursors [ALIP]) (see the image below).
A 2017 study indicated that when molecular genetic modalities are not available, immunohistochemistry for p53 expression may be useful for identifying cases of AML-MRC that are TP53 mutated, have complex karyotype, and have poor prognosis (see the following image). [16]
 Pathology of Acute Myeloid Leukemia With Myelodysplasia-Related Changes (AML-MRC). A posttreatment bone marrow evaluation (left) shows a hypercellullar marrow (hematoxylin and eosin) with small dysplastic megakaryocytes containing hypolobated nuclei (arrows), as well as clusters of medium to large blasts (circles) with dispersed chromatin, and prominent nucleoli located away from the bony trabeculae (ie, atypical localization of immature precursors [ALIP]). Immunohistochemistry shows CD34+ blasts (upper right) and strong nuclear staining of scattered marrow elements for p53 (lower right), each comprising about 10% of overall cellularity. These findings are consistent with persistent [posttherapy AML-MRC with high p53 expression by immunohistochemistry. A TP53 mutation was confirmed on molecular genetic analysis performed concurrently.
Pathology of Acute Myeloid Leukemia With Myelodysplasia-Related Changes (AML-MRC). A posttreatment bone marrow evaluation (left) shows a hypercellullar marrow (hematoxylin and eosin) with small dysplastic megakaryocytes containing hypolobated nuclei (arrows), as well as clusters of medium to large blasts (circles) with dispersed chromatin, and prominent nucleoli located away from the bony trabeculae (ie, atypical localization of immature precursors [ALIP]). Immunohistochemistry shows CD34+ blasts (upper right) and strong nuclear staining of scattered marrow elements for p53 (lower right), each comprising about 10% of overall cellularity. These findings are consistent with persistent [posttherapy AML-MRC with high p53 expression by immunohistochemistry. A TP53 mutation was confirmed on molecular genetic analysis performed concurrently.
Molecular/Genetic Features and Methods
The cytogenetic abnormalities that are myelodysplastic syndrome (MDS) related and are sufficient to categorize acute myeloid leukemia (AML) with myelodysplasia-related changes (AML-MRC) (even without morphologic evidence of dysplasia) are as follows:
-
Complex karyotype: Three or more unrelated abnormalities, none of which include the recurrent cytogenetic abnormalities encountered in AML [7]
-
Unbalanced abnormalities: -7/del(7q), -5/del(5q), i(17q)/t(17p), -13/del(13q), del(11q), del(12p)/t(12p), and idic(X)(q13) [1]
-
Balanced abnormalities: t(11;16)(q23;p13.3), t(3;21)(q26.2;q21.2), t(1;3)(p36.3;q21.1), t(2;11)(p21;q23), t(5;12)(q33p13.2t(5;7)(q33q11.2), t(5;17)(q33p13.2 t(5;10)(q33q21), and t(3;5)(q25;q34) [1]
Cases of AML with NPM1 or biallelic CEBPA mutations as the only genetic alterations are classified separately, even if morphologic evidence of dysplasia is present. [1, 8, 9]
A variety of MDS-associated mutations, including those involving U2AF1, ASXL1, and TP53, have been reported to be more common in AML-MRC than in AML not otherwise specified (AML-NOS). [2] TP53 mutations are frequently associated with a complex karyotype. [16] PIGN gene expression aberration appears to be associated with genomic instability and leukemic progression in AML-MRC. [17] This gene may play an essential role in regulating mitotic integrity to maintain chromosomal stability and preventing leukemic transformation/progression.
Prognosis and Predictive Factors
Patients with acute myeloid leukemia (AML) with myelodysplasia-related changes (AML-MRC) generally have a poor prognosis. [18] The percentage of patients who achieve complete remission is lower with this form of AML than with other AML types. [19] As mentioned previously, cases arising in association with an myelodysplastic syndrome (MDS) or myelodysplastic/myeloproliferative neoplasm (MDS/MPN) may show slower progression and thus may be less clinically aggressive. The prognosis is also affected by associated cytogenetic abnormalities: high-risk cytogenetic abnormalities include -5, -7, del(5q), abn(3q), and complex karyotype. [20]
The presence of MYC oncoprotein expression appears to be an independent poor prognostic factor in patients with AML-MRC. [21] AML-MRC subtype, patient age, and serum lactate dehydrogenase levels also appear to be independent predictors of outcome, with better overall survival in patients with AML-MRC with multilineage dysplasia and AML-MRC with history of prior MDS or MDS/myeloproliferative neoplasm (MDS/MPN), whereas worse outcomes are associated with AML-MRC with cytogenetic abnomalities or AML-MRC with a history of previously treated MDS or MDS/MPN. [22]
The clinical and prognostic significance of AML‐MRC as defined by morphologic criteria alone has been debated. [23] In a study of de novo AML lacking MDS-related cytogenetic abnormalities, patients who fulfilled criteria for AML-MRC based solely on morphologic criteria did not show significantly different event-free survival compared to patients with AML not otherwise specified (AML-NOS) who lacked multilineage dysplasia. [24] The same study also ananlyzed individual dysplastic features and found the presence of more than 50% micromegakaryocytes and/or over 50% hypogranulated myeloid cells to be associated with adverse event‐free survival, suggesting that a more restrictive definition of morphologic dysplasia may have greater relevance in terms of risk stratification in patients lacking cytogenetic abnormalities. [24] Overall, the finding of multilineage dysplasia still appears to confer an adverse prognosis, albeit not as poor as that in cases with high-risk cytogenetic abnormalities. [2]
Finally, the increased use of myeloid-related gene mutation panels has identified mutations of potential adverse prognostic signfiicance in AML-MRC, including TP53 and ASXL1. [25] AML patients with normal karyotypes who have isolated NPM1 mutation without accompanying FLT3 mutations, or those with biallelic CEBPA mutations are considered to have favorable prognosis. [2, 23] The lack of adverse prognostic significance of multilineage dysplasia in AML with mutated NPM1 or biallelic CEBPA mutations [1, 8, 9] supports the guidance of the revised 4th edition of the World Health Organization (WHO) classification of not including these entities within the spectrum of AML-MRC, even if morphologic dysplasia is present. [2]
-
Pathology of Acute Myeloid Leukemia With Myelodysplasia-Related Changes (AML-MRC). This image depicts dysgranulopoiesis: hypogranulosis.
-
Pathology of Acute Myeloid Leukemia With Myelodysplasia-Related Changes (AML-MRC). This image displays dysgranulopoiesis: vacuolization. Image courtesy of Rector and Visitors of the University of Virginia.
-
Pathology of Acute Myeloid Leukemia With Myelodysplasia-Related Changes (AML-MRC). Dysgranulopoiesis demonstrating Chediak-Higashi-like granules (arrow) is shown.
-
Pathology of Acute Myeloid Leukemia With Myelodysplasia-Related Changes (AML-MRC). Dyserythropoiesis is seen.
-
Pathology of Acute Myeloid Leukemia With Myelodysplasia-Related Changes (AML-MRC). The bone marrow core biopsy evaluation reveals dysmegakaryopoiesis with numerous small hypolobated megakaryocytes.
-
Pathology of Acute Myeloid Leukemia With Myelodysplasia-Related Changes (AML-MRC). Dysmegakaryopoiesis in the multinucleated form is noted.
-
Pathology of Acute Myeloid Leukemia With Myelodysplasia-Related Changes (AML-MRC). This image reveals a dysplastic promyelocyte with a cytoplasmic vacuole (left), blast (center), and dysplastic neutrophil with pseudo-Pelger-Huet anomaly and hypogranularity (right).
-
Pathology of Acute Myeloid Leukemia With Myelodysplasia-Related Changes (AML-MRC). Note the dysplastic neutrophil with large pseudo-Chediak-Higashi granules.
-
Pathology of Acute Myeloid Leukemia With Myelodysplasia-Related Changes (AML-MRC). A dysplastic red blood cell with budding nuclei (left) is seen with a monocyte (center), and a blast (right).
-
Pathology of Acute Myeloid Leukemia With Myelodysplasia-Related Changes (AML-MRC). Numerous dysplastic erythroid cells are seen, demonstrating nuclear budding, irregular nuclear contours, and multinucleation.
-
Pathology of Acute Myeloid Leukemia With Myelodysplasia-Related Changes (AML-MRC). A cluster of dysplastic megakaryocytes with small hypolobated nuclei is seen on a bone marrow aspirate smear preparation.
-
Pathology of Acute Myeloid Leukemia With Myelodysplasia-Related Changes (AML-MRC). Note the dysplastic neutrophil with large pseudo-Chediak-Higashi granules.
-
Pathology of Acute Myeloid Leukemia With Myelodysplasia-Related Changes (AML-MRC). A posttreatment bone marrow evaluation (left) shows a hypercellullar marrow (hematoxylin and eosin) with small dysplastic megakaryocytes containing hypolobated nuclei (arrows), as well as clusters of medium to large blasts (circles) with dispersed chromatin, and prominent nucleoli located away from the bony trabeculae (ie, atypical localization of immature precursors [ALIP]). Immunohistochemistry shows CD34+ blasts (upper right) and strong nuclear staining of scattered marrow elements for p53 (lower right), each comprising about 10% of overall cellularity. These findings are consistent with persistent [posttherapy AML-MRC with high p53 expression by immunohistochemistry. A TP53 mutation was confirmed on molecular genetic analysis performed concurrently.
-
Pathology of Acute Myeloid Leukemia With Myelodysplasia-Related Changes (AML-MRC). Dysgranulopoiesis demonstrating Chediak-Higashi-like granules (arrow) is shown.
-
Pathology of Acute Myeloid Leukemia With Myelodysplasia-Related Changes (AML-MRC). The bone marrow aspirate shows a binucleate erythroid precursor.






