Overview
Spitz nevus is named after Dr. Sophie Spitz, who in 1948 reported a case series of "melanomas of childhood." [1, 2] Nearly all of Spitz’s original series of 13 cases are now regarded as representing Spitz nevi, only one case having proved to be a melanoma resulting in metastasis and death.
Despite its benign nature, Spitz nevus has also been described under the headings of "juvenile melanoma," "benign juvenile melanoma," and "prepubertal melanoma." These terms are potentially confusing as Spitz nevus is benign, but as these synonyms imply, the differential diagnosis of Spitz nevus and spitzoid melanoma can be among the most challenging in dermatopathology. Elucidation of many of the molecular genetic underpinnings of Spitz nevi over the past several years have revealed a variety of mutations that support a biologic spectrum of Spitz tumors ranging from Spitz nevi to spitzoid melanoma, with an intermediate group designated "atypical Spitz tumors" (AST) representing tumors partly transformed towards potential melanoma and reflected by ambiguous, intermediate, or indeterminate histopathologic appearances and intermediate malignant potential. [3]
Spitz nevus may still be regarded as a clinically and histologically distinct variant of melanocytic nevus composed of spindled and/or epithelioid melanocytes (in the configuration of a benign tumor). Synonyms include Spitz’s nevus, Spitz tumor, and spindle and epithelioid cell nevus.
The spectrum of Spitz nevi includes junctional, compound (most common), and intradermal variants. Most desmoplastic Spitz nevi are intradermal or predominantly intradermal. Pigmented spindle cell nevus (Reed nevus) is regarded by most authorities as a clinically and histologically distinct variant of Spitz nevus. [4] Many other clinical and histologic variants have been described.
Spitz nevus has been characterized as relatively uncommon: it is estimated to account for only about 1% of surgically removed nevi. [5] Spitz nevus affects males and females, and the majority arise before the age 20 years. [6]
See Spitz Nevus and Melanocytic Nevi for more information.
Pathophysiology and Etiology
Spitz nevus usually represents a solitary, acquired nevus. Rare congenital Spitz nevi have been documented.
There is evidence that cell proliferation in Spitz nevi, as in other types of nevi and in melanoma, is primarily mediated by activating mutations affecting genes in the RAS-RAF-MEK-ERK-MAP kinase pathway.
A minority of Spitz nevi (approximately 15%) exhibit HRAS mutations, reflected by an isolated gain of chromosome 11p as a characteristic DNA copy number alteration. HRAS -positive Spitz nevi exhibit increased immunohistochemical expression of p16 and cyclinD1. [7, 8] To date, HRAS mutations have not been identified in spitzoid melanoma. [9] HRAS mutations have also been identified in a minority of deep penetrating nevi (DPN), suggesting that DPN are more closely related to Spitz nevi than blue nevi. [10]
Lazova et al performed exome sequencing of 77 melanocytic specimens composed of Spitz nevi (n=29), spitzoid melanomas (n=27), and benign melanocytic nevi (n=21), and they compared the results with published melanoma sequencing data. With the exception of activating HRAS variants, they found few additional mutations in Spitz nevi, and few copy number changes other than 11p amplification and chromosome 9 deletions. The data showed significantly lower somatic mutation burden in Spitz nevi and conventional nevi, relative to their malignant counterparts. [11]
Spitzoid nevi with epithelioid melanocytes and immunohistochemical loss of BAP1 expression (so-called BAPoma or Wiesner nevus) define a distinct form of tumor that in some cases may be associated with a heritable melanoma syndrome characterized by increased risk for ocular melanoma. [12, 13, 14] However, patients diagnosed with this tumor many not manifest other clinical features of the syndrome.
Spitz nevi (as well as as atypical Spitz tumors and spitzoid melanomas) frequently harbor kinase fusions (ROS1, NTRK1, ALK, BRAF, RET, MET) in mutually exclusive pattern. These kinase fusions represent partial transformation towards potential melanoma as well as potential therapeutic targets for metastatic melanoma harboring these kinase fusions. [15, 16, 17]
Clinical Features
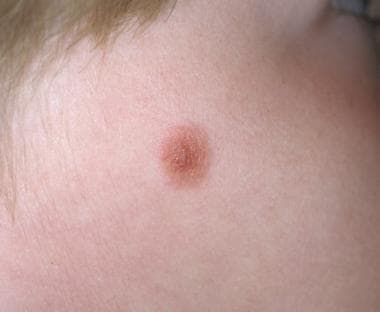
Spitz nevus most commonly arises on the skin of the head and neck or the extremities (more commonly on the lower extremities than on the upper). Involvement of the central face (eg, nose, cheek) is classic (see the image below). However, it is prudent generally to be skeptical about a diagnosis of Spitz nevi on skin with severe solar elastosis or in elderly individuals.
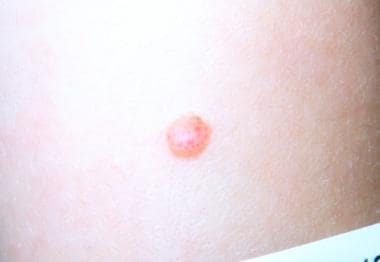
Spitz nevus typically presents as a solitary, soft, well-demarcated, nonulcerated, dome-shaped papule that is usually less than 6-10 mm in diameter (rarely up to 3 cm) and contains a fairly even degree of pigmentation anywhere within the pink-red-tan–light brown–dark brown color spectrum (see the image below). Spitz nevi harboring ALK fusions may be amelanotic or darkly pigmented. [18, 19]
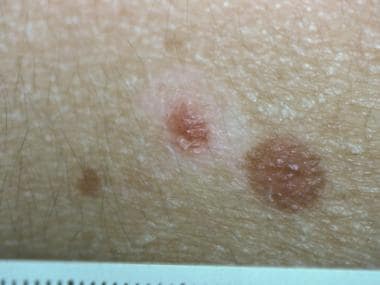
A self-limited period of rapid growth over weeks to months, followed by stability in size, is often noted; however, pigmentary changes may continue to occur over time. Halo, congenital, hyperpigmented, agminated (grouped) or disseminated Spitz nevi represent uncommon clinical variants (see the image below).
Dermoscopic (dermatoscopic) evaluation may reveal distinctive features of the nevi.
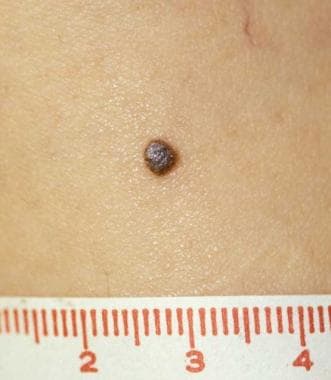
Pigmented spindle cell nevus (Reed nevus) is a uniformly heavily pigmented (dark brown-black), well-defined, round macule or minimally elevated papule, usually less than 6 mm in diameter (see the image below). Involvement of the proximal extremity of a woman is classic. Head and neck involvement is very unusual and should raise concerns about possible melanoma, [20] especially in adults.
Because this lesion is a variant of melanocytic nevus, there is no staging classification for a correctly diagnosed Spitz nevus.
Differential Diagnosis
The differential diagnosis includes the following:
Other conditions to consider include the following:
-
Hemangioma
-
Melanoma arising in association with melanocytic nevus
-
Nonmelanocytic tumors (eg, epithelioid histiocytoma, reticulohistiocytoma)
Gross and Microscopic Findings
Gross inspection of a formalin-fixed specimen containing a Spitz nevus parallels its clinical and dermoscopic appearance and does not yield diagnostically conclusive information.
As of early 2016, short of evaluation of biologic behavior (eg, long-term follow-up, metastasis), histologic assessment remains the practical gold standard for diagnosis of all melanocytic tumors, including Spitz nevi. However, selected immunohistochemical tests may facilitate the interpretation of challenging cases, and increasing availability, validation, and ultimately reliance on ancillary molecular diagnostic testing (fluorescence in situ hybridization [FISH], array comparative genomic hybridization (aCGH), next-generation sequencing) is expected to play a more prominent role in the assessment of the most challenging subset of cases. (See Immunohistochemistry.)
The recognition of atypical Spitz tumors (AST) reflects the fact that some melanocytic proliferations exhibit microscopic features (and biologic behavior) intermediate between classic Spitz nevus and melanoma. [21] Historically, the original series of AST was designated "malignant Spitz nevus," [26] reflecting the fact that AST are frequently associated with regional lymph node involvement—usually without widespread metastasis or death after a reasonable follow-up period. [25, 23] {ref22 Comprehensive discussion of AST is beyond the scope of this article. Practically speaking, its recognition and acceptance highlights the long-standing challenge in the interpretation of spitzoid melanocytic neoplasms as one of the most difficult areas in all of dermatopathology. Diagnoses should be rendered by experienced dermatopathologists with additional consultation and ancillary diagnostic testing in difficult cases in order to avoid misdiagnosis. [4] Each "atypical" case must be judged on its own attributes, in the context of the degree of diagnostic confidence and competence.
Spitz nevi are composed of spindled and/or epithelioid melanocytes; the proportion of spindled versus epithelioid cells varies from case to case. [27] The melanocytes in Spitz nevi are generally larger than those in other forms of nevi. Cytologic details of the melanocytes may also vary somewhat, but the melanocytes of Spitz nevi generally display uniform features within each tumor. The melanocyte/nevocyte nuclei are generally round, vesicular, and centrally situated. Although a minority or markedly enlarged or pleomorphic cells may be seen, nuclear pleomorphism, hyperchromasia, and prominent nucleoli are fairly uniform through the tumor, with evidence of maturation (detailed below). Cytoplasm is abundant, eosinophilic or amphophilic, and often two-toned.
Multinucleation may occur, nuclear pseudoinclusions may be prominent, and the nucleoli may be enlarged and eosinophilic. However, eosinophilic nucleoli represent a common feature of Spitz nevus and melanoma. Cytoplasm is abundant and ranges from eosinophilic (most common) to slightly basophilic.
As a variant of melanocytic nevus, Spitz nevus fulfills all major histologic criteria for the diagnosis of melanocytic nevus, with one or more additional features that distinguish it as Spitz nevus. The least common denominators for the diagnosis of melanocytic nevus include the following:
-
Symmetrical profile at scanning magnification (including the lymphocytic host response, if present)
-
Circumscription: Sharp lateral demarcation, usually ending as a nest rather than single melanocytes
-
"Orderly" distribution of single and nested melanocytes exhibiting fairly uniform cytologic features
-
Evidence of dermal zonation/maturation: A gradual transition from predominantly larger nests of melanocytes in the superficial dermal component to smaller melanocytes disposed within smaller nests, dispersed aggregates (often including single file strands), and single units within the deep dermal component, often aggregating within and around adnexal structures (hair follicles, sweat ducts; also blood vessels, nerve fibers, arrector pili).
Architecturally, Spitz nevi exhibit a domed, flat-bottomed profile at scanning magnification (see the image below). With the exception of Spitz nevi undergoing halo reactions, the lymphocytic host response is typically sparse, although occasionally the host response may be more prominent, particularly in desmoplastic Spitz nevi. [28]
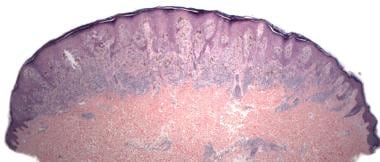 Pathology of Spitz nevi. Spitz nevus, compound type (hematoxylin-eosin stain). Symmetrical, circumscribed, dome-shaped, flat-bottomed profile at scanning magnification.
Pathology of Spitz nevi. Spitz nevus, compound type (hematoxylin-eosin stain). Symmetrical, circumscribed, dome-shaped, flat-bottomed profile at scanning magnification.
Guida et al found that reflectance confocal microscopy (RCM) could differentiate Spitz nevi and melanomas with globular and starburst patterns on dermoscopy. Striking cell pleomorphism within the epidermis, widespread atypical cells at the dermoepidermal junction, and marked pleomorphism within nests were significantly associated with the diagnosis of melanoma. By contrast, spindled cells and peripheral clefting were found exclusively with Spitz nevi and were pathognomonic of Spitz nevi. [29]
Junctional or compound Spitz nevus
Junctional or compound Spitz nevi exhibit distinctive epidermal changes, including epidermal hyperplasia. The junctional nests are typically vertically oriented, as are the spindled melanocytes within them, which resemble banana clusters (see the image below). Retraction artifacts or clefts typically surround the junctional nests.
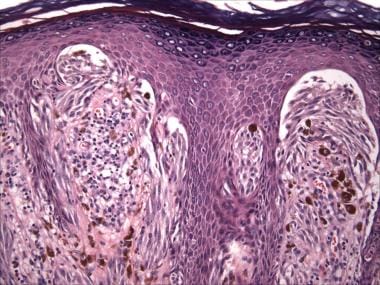 Pathology of Spitz nevi. Spitz nevus, compound type (hematoxylin-eosin stain). Epidermal hyperplasia with vertically oriented spindle cells within vertically oriented junctional nests ("banana clusters"). Clefting/retraction around junctional nests.
Pathology of Spitz nevi. Spitz nevus, compound type (hematoxylin-eosin stain). Epidermal hyperplasia with vertically oriented spindle cells within vertically oriented junctional nests ("banana clusters"). Clefting/retraction around junctional nests.
Pale eosinophilic globules known as Kamino bodies (after Dr. Hideko Kamino, the lead author of the group that first described them [30] ) are usually present at the basement membrane zone (dermal-epidermal junction) of Spitz nevi (see the images below). Kamino bodies contain periodic acid-Schiff/periodic acid-Schiff, digested stain (PAS/PASD)-positive basement membrane constituents, including laminin and type IV collagen.
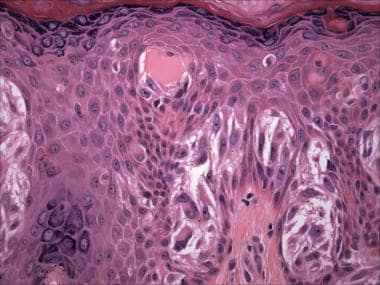 Pathology of Spitz nevi. Spitz nevus (hematoxylin-eosin stain). Vertically oriented spindled junctional melanocytes and Kamino body.
Pathology of Spitz nevi. Spitz nevus (hematoxylin-eosin stain). Vertically oriented spindled junctional melanocytes and Kamino body.
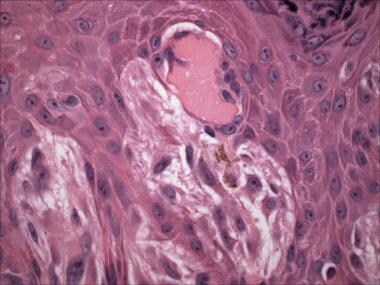 Pathology of Spitz nevi. Spitz nevus (hematoxylin-eosin stain). Spindled melanocytes and coalescent Kamino bodies.
Pathology of Spitz nevi. Spitz nevus (hematoxylin-eosin stain). Spindled melanocytes and coalescent Kamino bodies.
Kamino bodies are fairly specific for Spitz nevi but may rarely be seen in melanomas. When present in Spitz nevi, Kamino bodies are classically large, coalescent, and numerous. When present in melanomas, Kamino bodies are usually small and few in number and are often PAS negative; such characteristics suggest that these structures actually represent apoptotic cells.
Spitz tumors associated with ALK fusions exhibit strong and homogenous immunopositivity for ALK and are often amelanotic, compound, predominantly intradermal nevi exhibiting a plexiform pattern ("raining down") of growth composed of intersecting fascicles of fusiform melanocytes, often extending into the subcutis in wedge-shaped, bulbous fashion, and sometimes infiltrative growth at the periphery of the tumor. Elongated nests with radial orientation and "consumption" of the epidermis (a feature typically associated with melanoma) may also be seen. The tumor cells exhibit prominent nucleoli and fibrillar cytoplasm. [18, 19]
Intradermal Spitz nevus
Intradermal Spitz nevi often lack distinctive epidermal changes but often display a fibrotic/desmoplastic stromal reaction and may then be classified as a desmoplastic Spitz nevus or desmoplastic nevus (see the images below). [31, 32]
 Pathology of Spitz nevi. Spitz nevus, intradermal/desmoplastic type (hematoxylin-eosin stain). High-domed, symmetrical, flat-bottomed profile at scanning magnification. This nevus may be further classified as polypoid.
Pathology of Spitz nevi. Spitz nevus, intradermal/desmoplastic type (hematoxylin-eosin stain). High-domed, symmetrical, flat-bottomed profile at scanning magnification. This nevus may be further classified as polypoid.
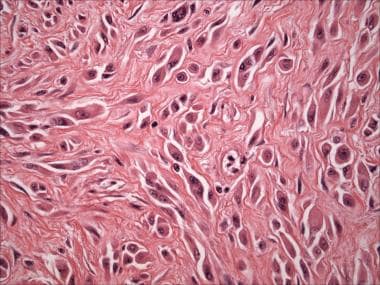 Pathology of Spitz nevi. Spitz nevus, intradermal/desmoplastic type (hematoxylin-eosin stain). Spindled and epithelioid melanocytes with prominent eosinophilic nucleoli, associated with fibrotic/desmoplastic stroma.
Pathology of Spitz nevi. Spitz nevus, intradermal/desmoplastic type (hematoxylin-eosin stain). Spindled and epithelioid melanocytes with prominent eosinophilic nucleoli, associated with fibrotic/desmoplastic stroma.
Other histologic variants include polypoid, plexiform, angiomatoid, hyperpigmented, combined, halo Spitz nevus, and recurrent Spitz nevus.
Pigmented spindle cell nevus (Reed nevus)
Pigmented spindle cell nevus (Reed nevus) is either a junctional or a superficial compound proliferation. By definition, the melanocytes are predominantly or exclusively spindled and pigmented (see the image below). The lymphohistiocytic host response contains numerous heavily pigmented melanophages.
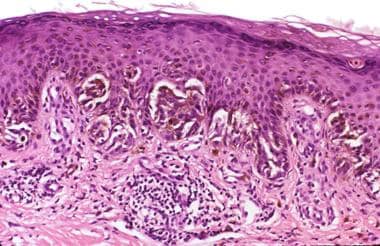 Pathology of Spitz nevi. Pigmented spindle cell nevus (Reed nevus). Vertically oriented pigmented spindled melanocytes along the junction. Melanophages in the papillary dermis.
Pathology of Spitz nevi. Pigmented spindle cell nevus (Reed nevus). Vertically oriented pigmented spindled melanocytes along the junction. Melanophages in the papillary dermis.
Notoriously, in all forms of Spitz nevi, features that overlap with those of melanoma may be present, as follows. [33, 34]
Small numbers of large marked, severe, or strikingly atypical epithelioid melanocytes may be seen. The nuclei of these cells are more often vesicular than hyperchromatic (see the image below).
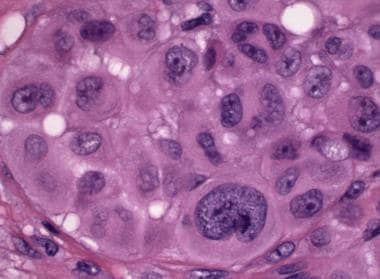 Pathology of Spitz nevi. Spitz nevus, compound type. This tumor from the arm of a 4-year-old contains a dense epithelioid melanocytic proliferation with features that overlap with those of melanoma: scattered markedly atypical epithelioid melanocytes.
Pathology of Spitz nevi. Spitz nevus, compound type. This tumor from the arm of a 4-year-old contains a dense epithelioid melanocytic proliferation with features that overlap with those of melanoma: scattered markedly atypical epithelioid melanocytes.
Pagetoid intraepithelial growth may occur, but if such growth is present, it is typically most prominent in the central portion of the tumor and often manifests as transepidermal elimination or expulsion of nests of melanocytes rather than single cells; this manifestation must be distinguished from "consumption of the epidermis," a phrase used to describe the thinned epithelium overlying junctional nests and associated epidermal atrophy that favors melanoma over Spitz nevus. [35] Consumption of the epidermis has also been reported in ALK-fused atypical Spitz tumors. [19]
The degree of pagetoid growth of single melanocytes in Reed nevus, particularly in children, may be greater than that seen in some examples of melanoma in situ (see the image below).
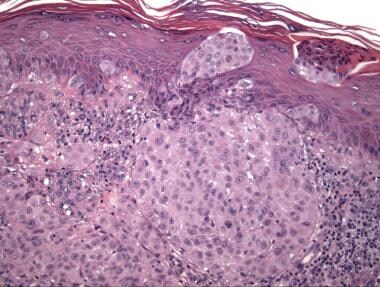 Pathology of Spitz nevi. Spitz nevus, compound type. This tumor from the arm of a 4-year-old contains a dense epithelioid melanocytic proliferation with features that overlap with those of melanoma: pagetoid growth and transepidermal elimination of melanocytic nests resembling so-called consumption of the epidermis.
Pathology of Spitz nevi. Spitz nevus, compound type. This tumor from the arm of a 4-year-old contains a dense epithelioid melanocytic proliferation with features that overlap with those of melanoma: pagetoid growth and transepidermal elimination of melanocytic nests resembling so-called consumption of the epidermis.
Mitotic figures within junctional or dermal melanocytes are often present (see the image below). The rules for assessing mitoses are the same as for all melanocytic tumors. That is, mitotic figures in nevi are generally rare in number, not clustered, not atypical in appearance, and confined to the dermal epidermal junction and superficial dermal component. Conversely, high numbers or "hot spots" of mitoses, atypical forms, deep mitoses, or suprabasilar mitoses suggest melanoma.
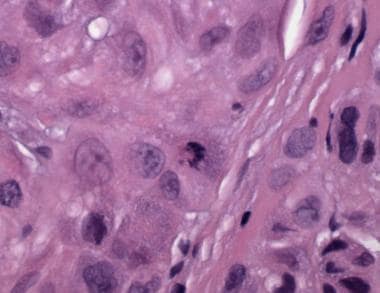 Pathology of Spitz nevi. Spitz nevus, compound type. This tumor from the arm of a 4-year-old contains a dense epithelioid melanocytic proliferation with features that overlap with those of melanoma: many dermal mitoses.
Pathology of Spitz nevi. Spitz nevus, compound type. This tumor from the arm of a 4-year-old contains a dense epithelioid melanocytic proliferation with features that overlap with those of melanoma: many dermal mitoses.
However, because Spitz nevi often exhibit a rapid growth phase, the presence of numerous typical mitoses within the superficial dermal component of an otherwise classic Spitz nevus does not specifically suggest melanoma.
Because Spitz nevi usually arise in children and young adults, solar elastosis is usually absent. Thus, spitzoid proliferations, especially pure spindle cell or pure epithelioid cell melanocytic proliferations on skin with severe solar elastosis usually represent melanoma.
Immunohistochemistry
In cases in which the tumor cell lineage is uncertain on hematoxylin-eosin (H&E) staining, melanocytic markers may be used to stain Spitz nevi. The most sensitive marker is polyclonal S100. Other melanocytic markers include SOX-10, Melan-A (MART-1), HMB-45, MiTF, tyrosinase, and NKI/C3.
Immunohistochemical (IHC) studies have traditionally provided limited utility in the diagnosis of Spitz nevi. Practices and institutions vary regarding the extent to which IHC studies are used in selected cases when the differential diagnosis includes atypical Spitz tumor or spitzoid melanoma. [20] In such cases, IHC studies may include the following:
-
Assessment of the staining pattern of melanocytic markers, such as HMB-45 (staining of the deep dermal component in melanoma vs absent or prompt loss of staining with descent in the dermal component of nevi) [36] or S100A6 (strong, diffuse immunopositivity in Spitz nevi; often absent or weak staining in melanoma and Reed nevus) [37]
-
Assessment of cell proliferation, as measured by cell proliferation markers such as Ki-67; a proliferative index greater than 5% supports melanoma; however, a lower proliferative index does not exclude melanoma, and Ki-67 labeling greater than 10% has been documented in Spitz nevus [38]
-
Immunohistochemical staining for mitoses using PHH3 labels fewer cells than by Ki-67 but labels approximately twice the number of cells compared to manual counting of mitoses
-
Immunohistochemical staining for loss of p16 expression (see below)
Ancillary molecular diagnostic studies to assist in the distinction between Spitz nevus and spitzoid melanoma include fluorescence in situ hybridization (FISH) and array comparative genomic hybridization (aCGH). [39] FISH testing was initially offered as 4-probe panel targeting chromosomes 6 and 11. However, a fifth probe for 9p21 (corresponding to p16NInk4/CDKN2a) confers greater diagnostic sensitivity for spitzoid neoplasms. [40] Homozygous (rather than heterozygous) deletion of 9p21 by FISH represents an adverse prognostic factor and correlates with complete (rather than partial) loss of immunohistochemical expression of p16. [41, 42]
aCGH addresses the inherent limitations of FISH but, to date, has only been validated and offered on a limited basis. [43]
Loss of BAP-1 and kinase fusions can be identified using immunohistochemistry (BRAF, ALK); however, these alone are not diagnostically specific.
Prognosis and Predictive Factors
As a variant of melanocytic nevus, Spitz nevus is a benign neoplasm. For classic cases, complete excision is not necessarily mandated, especially considering their frequent location on the face and/or requirement for general anesthesia in young children. Recent survey data indicate that reexcision is performed approximately half the time. [44]
A minority of Spitz nevi persist or recur at the biopsy site, sometimes manifesting clinically 1 or more years after the original biopsy. In the evaluation of a recurrent tumor with spitzoid features, it is imperative to compare the findings with those from the original biopsy, because recurrent nevi (pseudomelanoma), particularly Spitz nevi, often exhibit a microscopic appearance that is similar to, if not indistinguishable from, melanoma. [45]
Missed melanoma represents a leading cause of medical malpractice cases; many of these are initially misdiagnosed as Spitz nevi. [20] (See Microscopic Findings and Immmunohistochemistry above). Melanoma is curable if caught early, but it is consistently fatal if left untreated. Moreover, melanoma disproportionately affects younger individuals compared to the more common non-melanoma skin cancers.
Regional lymph node involvement by atypical spitzoid tumors has been increasingly documented, many in children. [46, 25] Despite the presence of metastatic disease, in many of these cases, the follow-up to date has been more favorable than would be expected in comparison with conventional melanomas of similar tumor burden.
Thus, although each case must be evaluated individually and comprehensively, clinical experience suggests that there is a differential diagnosis for regional lymphatic involvement by melanocytes that includes spitzoid melanoma (possibly divisible into prognostically distinct subsets—for example, spitzoid melanoma of childhood), [47] other forms of melanoma, AST, [3, 24] and various forms of nodal nevi.
Whenever feasible, obtaining one or more additional opinions on the pathologic diagnosis and following an interdisciplinary management approach are prudent in these problematic cases.
-
Pathology of Spitz nevi. Spitz nevus, compound type. Classic location on the cheek of a child.
-
Pathology of Spitz nevi. Spitz nevus.
-
Pathology of Spitz nevi. Halo Spitz nevus (biopsy confirmed).
-
Pathology of Spitz nevi. Spitz nevus, compound type (hematoxylin-eosin stain). Symmetrical, circumscribed, dome-shaped, flat-bottomed profile at scanning magnification.
-
Pathology of Spitz nevi. Spitz nevus, compound type (hematoxylin-eosin stain). Epidermal hyperplasia with vertically oriented spindle cells within vertically oriented junctional nests ("banana clusters"). Clefting/retraction around junctional nests.
-
Pathology of Spitz nevi. Spitz nevus (hematoxylin-eosin stain). Vertically oriented spindled junctional melanocytes and Kamino body.
-
Pathology of Spitz nevi. Spitz nevus (hematoxylin-eosin stain). Spindled melanocytes and coalescent Kamino bodies.
-
Pathology of Spitz nevi. Spitz nevus, intradermal/desmoplastic type (hematoxylin-eosin stain). High-domed, symmetrical, flat-bottomed profile at scanning magnification. This nevus may be further classified as polypoid.
-
Pathology of Spitz nevi. Spitz nevus, intradermal/desmoplastic type (hematoxylin-eosin stain). Spindled and epithelioid melanocytes with prominent eosinophilic nucleoli, associated with fibrotic/desmoplastic stroma.
-
Pathology of Spitz nevi. Pigmented spindle cell nevus (Reed nevus) on the thigh of a woman.
-
Pathology of Spitz nevi. Pigmented spindle cell nevus (Reed nevus). Vertically oriented pigmented spindled melanocytes along the junction. Melanophages in the papillary dermis.
-
Pathology of Spitz nevi. Spitz nevus, compound type. This tumor from the arm of a 4-year-old contains a dense epithelioid melanocytic proliferation with features that overlap with those of melanoma: pagetoid growth and transepidermal elimination of melanocytic nests resembling so-called consumption of the epidermis.
-
Pathology of Spitz nevi. Spitz nevus, compound type. This tumor from the arm of a 4-year-old contains a dense epithelioid melanocytic proliferation with features that overlap with those of melanoma: many dermal mitoses.
-
Pathology of Spitz nevi. Spitz nevus, compound type. This tumor from the arm of a 4-year-old contains a dense epithelioid melanocytic proliferation with features that overlap with those of melanoma: scattered markedly atypical epithelioid melanocytes.