Approach Considerations
Aortic dissection is usually diagnosed by using imaging techniques before the result of blood work is interpreted. The choice of imaging techniques depends in part on whether or not the patient is hemodynamically stable.
Chest radiography is the initial imaging technique and may or may not reveal any abnormality. Computed tomography (CT) is useful in hemodynamically stable patients; emergency CT angiography (CTA) with three-dimensional (3D) reconstruction is rapidly becoming the diagnostic test of choice. Magnetic resonance imaging (MRI) is as accurate as CT and may benefit patients who have adverse reactions to the use of intravenous (IV) contrast agents. For hemodynamically unstable patients, echocardiography is ideal.
Aortography is still considered by some as the diagnostic criterion standard test for aortic dissection. However, it is being replaced by newer imaging modalities. For more information on imaging in this disorder, see Aortic Dissection Imaging.
All patients with suspected thoracic aortic dissection should undergo 12-lead electrocardiography (ECG). However, ECG often demonstrates a nonspecific abnormality or normal results.
Blood Studies
A complete blood count (CBC), serum chemistry studies, and cardiac marker assays should be performed. On the CBC, leukocytosis may be present, which usually represents a stress state. Decreases in hemoglobin and hematocrit values are ominous findings suggesting that the dissection is leaking or has ruptured.
Elevation of the blood urea nitrogen (BUN) and creatinine levels may indicate involvement of the renal arteries or prerenal azotemia resulting from blood loss or associated dehydration (mainly when the BUN-to-creatinine ratio exceeds 20). Patients with dissection involving the renal arteries may also exhibit hematuria, oliguria, or even anuria (< 50 mL/day).
Myocardial muscle creatine kinase isoenzyme, myoglobin, and troponin I and T levels are elevated if the dissection has involved the coronary arteries and caused myocardial ischemia. The lactate dehydrogenase level may be elevated because of hemolysis in the false lumen.
Measurement of the degradation products of plasma fibrin and fibrinogen can facilitate the diagnosis of acute aortic dissection. In symptomatic patients, aortic dissection with a patent false lumen should be considered if the plasma fibrin degradation product (FDP) level is 12.6 μg/mL or higher; the possibility of dissection with complete thrombosis of the false lumen should be considered if the FDP level is 5.6 μg/mL or higher. [2]
Some authors suggest that a D-dimer assay should be a part of the initial workup if aortic dissection is suspected. [19] A negative result makes the presence of the disease less likely.
Smooth-Muscle Myosin Heavy-Chain Assay
A smooth-muscle myosin heavy-chain assay is performed in the first 24 hours. Increased levels in the first 24 hours are 90% sensitive and 97% specific for aortic dissection. levels are highest in the first 3 hours. A cutoff of 2.5 has a sensitivity of 91%, a specificity of 98%, and an accuracy rate of 96%, respectively.
The smooth muscle myosin heavy-chain assay has greater sensitivity and specificity than transthoracic echocardiography (TTE), CT, and aortography, but it has less sensitivity and specificity than transesophageal echocardiography (TEE), MRI, and helical CT.
Chest Radiography
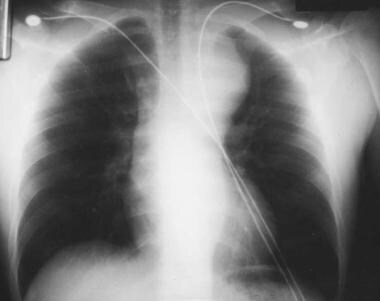
Although chest radiography is not the definitive imaging study for aortic dissection, it should be performed as the initial imaging technique if it is readily available at the bedside and does not cause delay in obtaining CT or MRI. [3] Chest radiography (see the images below) may or may not reveal any abnormality. Widening of the mediastinum is the classic finding.
In 2000, the International Registry of Acute Aortic Dissection published data on 464 patients. Chest radiography showed a widened mediastinum in approximately 62% of patients. [5]
If the patient is hemodynamically stable and cooperative, an anteroposterior (AP) radiograph can be obtained at bedside. A widened mediastinum is sometimes difficult to identify on a portable AP radiograph. Look for a mediastinal width greater than 8 cm on the AP view.
A tortuous aorta, common in hypertensive patients, may be hard to distinguish from a widened mediastinum. If in doubt, a good posterior-anterior radiograph is recommended. The differential diagnosis of a widened mediastinum includes tumor, adenopathy, lymphoma, and enlarged thyroid.
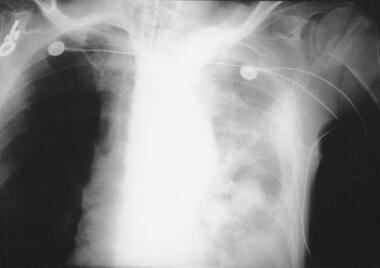
If an aortic dissection ruptures, blood can extravasate into the ipsilateral pleural space, causing a hemothorax (see the image below).
With type A dissection, an abnormal aortic contour is observed in a minority of patients. An abnormal (ie, blunted) aortic knob was observed in 66% of patients in one study. Ring sign (displacement of the aorta >5 mm past the calcified aortic intima) is considered a specific radiographic sign.
Other radiologic abnormalities seen on chest radiography include the following:
-
Pleural effusion
-
Left apical cap
-
Tracheal deviation to the right
-
Depression of left mainstem bronchus
-
Esophageal deviation
-
Loss of the paratracheal stripe
The International Registry for Aortic Dissection revealed that more than 12% of the chest radiographs of patients with aortic dissection were read as normal. [5] Several studies concluded that the overall diagnosis of aortic dissection is not determined by any one sign; rather, a combination of all findings leads to suspicion of dissection.
Computed Tomography
CT with contrast is used more frequently in emergency department (ED) settings. CT is useful only in hemodynamically stable patients, because of its lack of portability and its potential limitations in patients with contraindications to intravenous contrast agents.
A 2014 guideline from the American College of Radiology recommended CTA as the definitive test in most patients with suspicion of aortic dissection. [3] CTA provides detailed anatomic definition of the dissection as well as information on plaque formation. Prospective studies have shown a sensitivity of 83-94% and a specificity of 87-100%. However, 3D reconstruction may not be available in smaller centers. [20]
Spiral (helical) CT is associated with a higher rate of detection and better resolution than incremental CT scanning. Faster scanners have decreased the acquisition time to the range of a breath hold, resulting in less motion artifact from breathing. High-quality two-dimensional (2D) and 3D reconstructions are possible with spiral CT, greatly adding to the usefulness of this imaging modality.
More important, imaging information, including the type of lesion, location of the pathologic lesion, extent of the disease, and evaluation of the true and false lumen can be assessed quickly and help the surgeon plan the operation. This information helps determine if hypothermic circulatory arrest is necessary for surgery; this procedure increases the complexity, length, morbidity, and mortality associated with surgery.
One study reported that extended hypothermic circulatory arrest times, as a consequence of disease severity, contribute to a higher mortality rate, as do redo surgery and the overall extent of disease. Emergency surgery and extracardiac arteriopathy are associated with increased risk of neurological injury. Age, however, was not associated with increased risk for neurologic injury or mortality. [21]
Drawbacks include the following:
-
Transportation of a patient in potentially unstable condition from the ED, even for the relatively short time needed for this procedure, places the patient at risk
-
CTA requires the injection of iodinated contrast, which may harm a patient who has impaired renal function or an allergy to contrast media
-
CT provides no information on aortic regurgitation
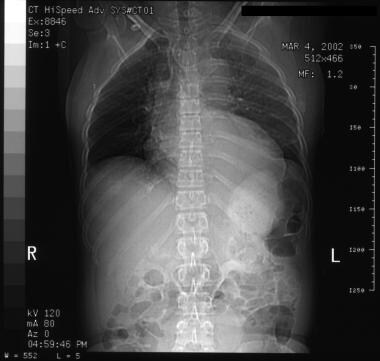
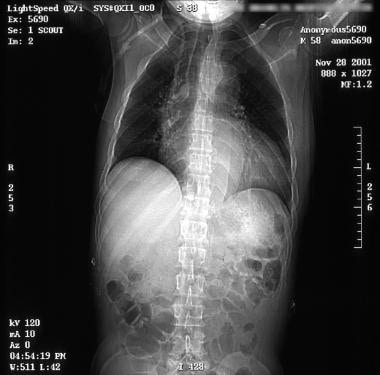
See the images below for CT scans showing aortic dissection.
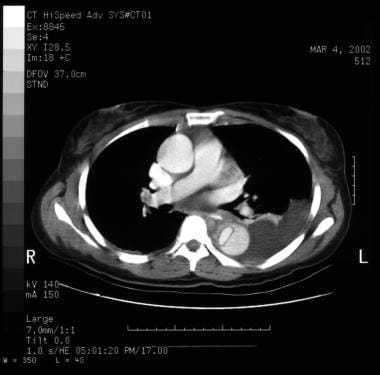 Aortic dissection. Left subsegmental atelectasis and left pleural effusion. Flap at lower right of image.
Aortic dissection. Left subsegmental atelectasis and left pleural effusion. Flap at lower right of image.
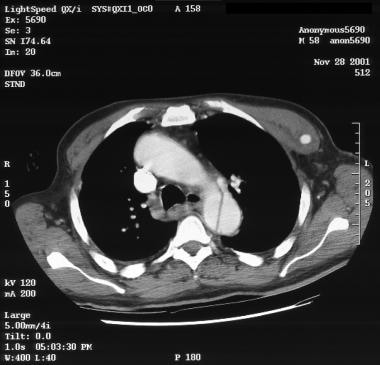
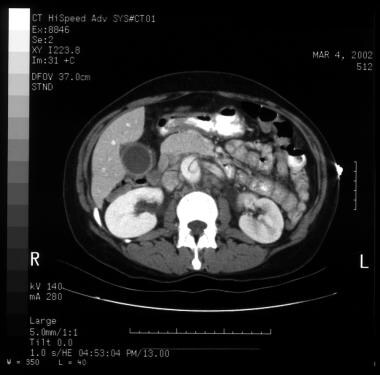
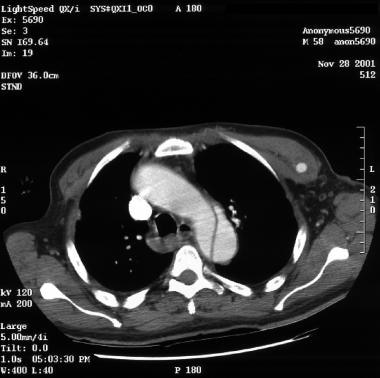
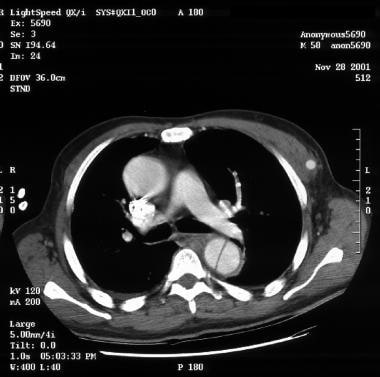
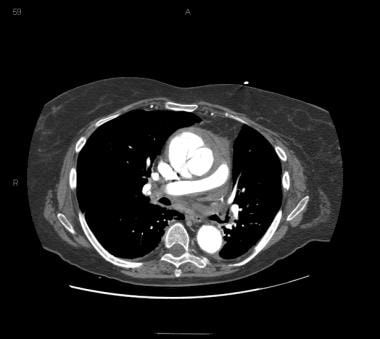 Patient with an ascending type A aortic dissection showing the intimal flap. Image courtesy of Kaiser-Permanente.
Patient with an ascending type A aortic dissection showing the intimal flap. Image courtesy of Kaiser-Permanente.
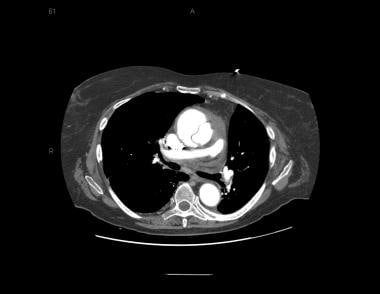 Patient with an ascending type A aortic dissection showing the intimal flap. Image courtesy of Kaiser-Permanente.
Patient with an ascending type A aortic dissection showing the intimal flap. Image courtesy of Kaiser-Permanente.
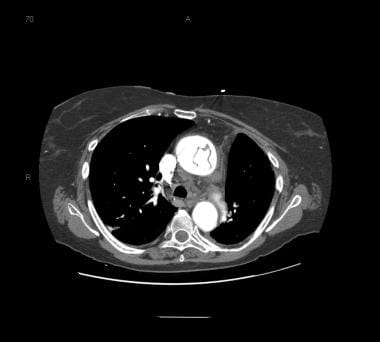 Patient with an ascending type A aortic dissection showing the intimal flap. Image courtesy of Kaiser-Permanente.
Patient with an ascending type A aortic dissection showing the intimal flap. Image courtesy of Kaiser-Permanente.
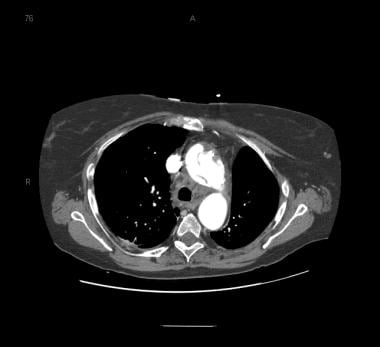 Patient with an ascending type A aortic dissection showing the intimal flap. Image courtesy of Kaiser-Permanente.
Patient with an ascending type A aortic dissection showing the intimal flap. Image courtesy of Kaiser-Permanente.
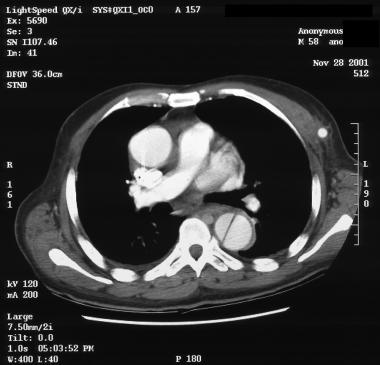
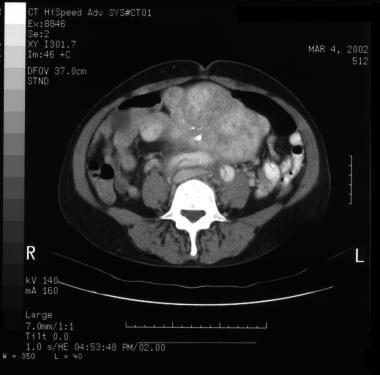
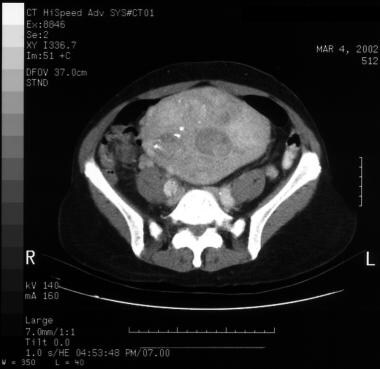
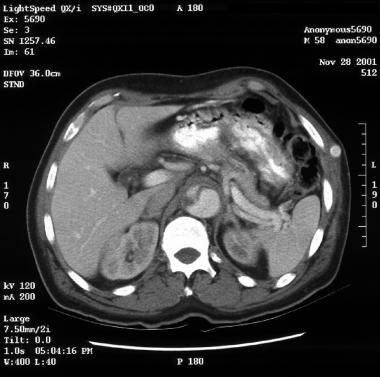
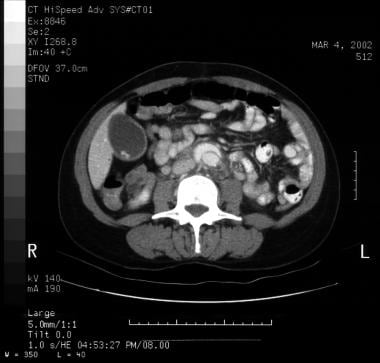
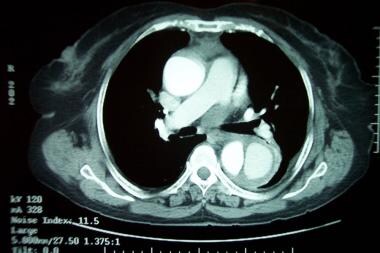
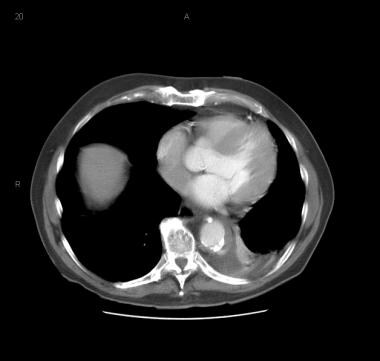
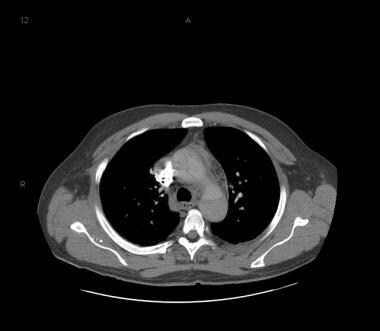 Patient with a type A aortic dissection involving the ascending and descending aorta. Image courtesy of Kaiser-Permanente.
Patient with a type A aortic dissection involving the ascending and descending aorta. Image courtesy of Kaiser-Permanente.
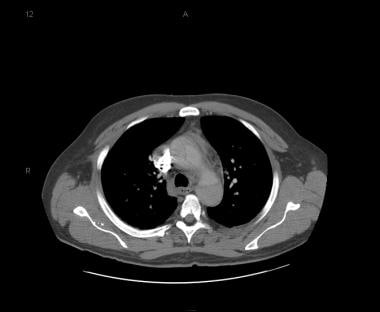 Patient with a type A aortic dissection involving the ascending and descending aorta. Image courtesy of Kaiser-Permanente.
Patient with a type A aortic dissection involving the ascending and descending aorta. Image courtesy of Kaiser-Permanente.
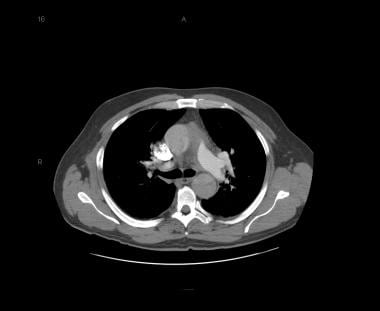 Patient with a type A aortic dissection involving the ascending and descending aorta. Image courtesy of Kaiser-Permanente.
Patient with a type A aortic dissection involving the ascending and descending aorta. Image courtesy of Kaiser-Permanente.
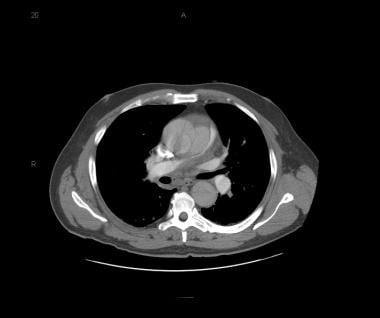 Patient with a type A aortic dissection involving the ascending and descending aorta. Image courtesy of Kaiser-Permanente.
Patient with a type A aortic dissection involving the ascending and descending aorta. Image courtesy of Kaiser-Permanente.
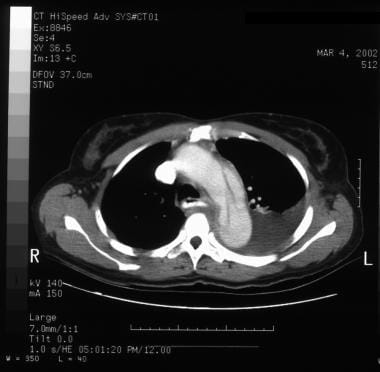
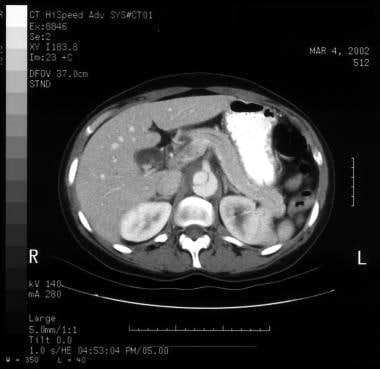
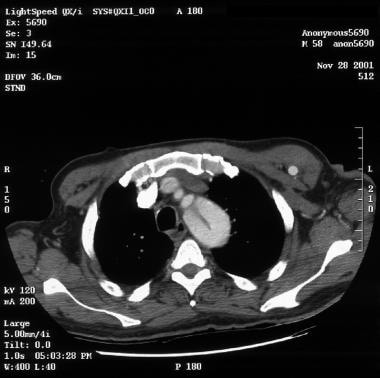
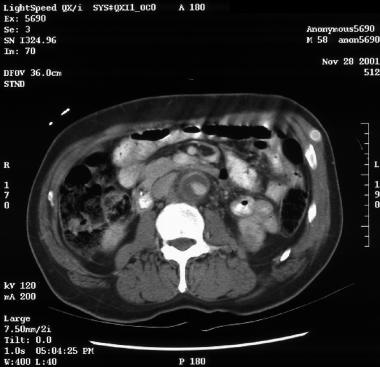
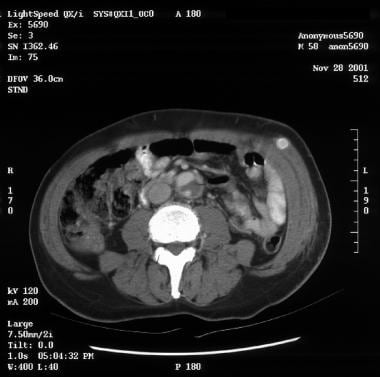
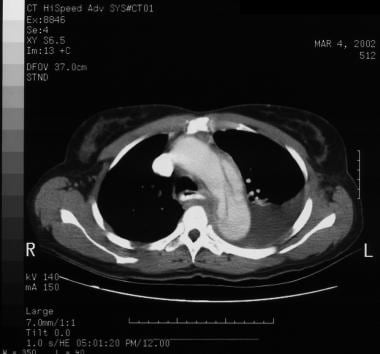
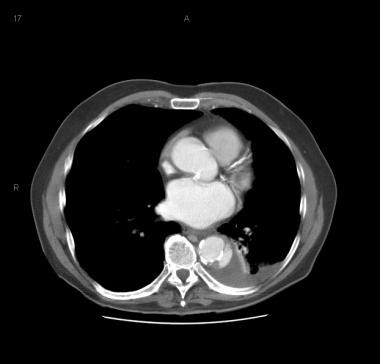 Patient showing a type B aortic dissection with extravasation of blood into the pleural cavity. Image courtesy of Kaiser-Permanente.
Patient showing a type B aortic dissection with extravasation of blood into the pleural cavity. Image courtesy of Kaiser-Permanente.
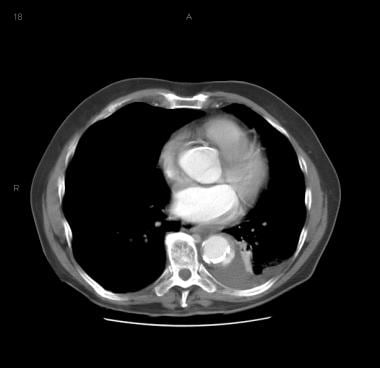 Patient showing a type B aortic dissection with extravasation of blood into the pleural cavity. Image courtesy of Kaiser-Permanente.
Patient showing a type B aortic dissection with extravasation of blood into the pleural cavity. Image courtesy of Kaiser-Permanente.
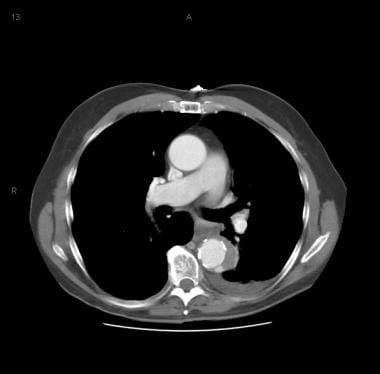 Patient showing a type B aortic dissection with extravasation of blood into the pleural cavity. Image courtesy of Kaiser-Permanente.
Patient showing a type B aortic dissection with extravasation of blood into the pleural cavity. Image courtesy of Kaiser-Permanente.
Echocardiography
Echocardiography is an important imaging modality for detecting aortic dissection. [22, 4] It also is useful in diagnosing cardiac tamponade and aortic regurgitation.
TEE has greater sensitivity (97-99% vs 80%) and specificity (97-100% vs 90%) than TTE. [4] TTE is most useful in ascending aortic dissections, especially those closest to the aortic root and within a few centimeters of the aortic valve. Sensitivity is highest in this location. TTE is much less accurate for arch and descending aortic dissections.
TEE is as accurate as CT and MRI in terms of sensitivity and specificity, and it can be used at the bedside, which makes it ideal for hemodynamically unstable patients. It is a relatively quick study to perform and relatively noninvasive. However, obesity, a narrow intercostal space, pulmonary emphysema, and mechanical ventilation decrease the accuracy of TEE. False-positive results with TEE are about 10%. [22, 4]
In one study, color Doppler TEE using biplanar views detected intimal flaps in 18 of 18 patients with type A dissections and in 10 of 39 with type B dissections. [23] Intimal tears were detected in 15 (83%) patients with type A and in 35 (90%) patients with type B (both confirmed by surgery or angiography). Intimal entry was detected in 16 of 18 patients using biplanar views and in 34 of 39 patients using single planar views.
Using longitudinal views, only two intimal entries were detected in 18 patients. Using biplanar views, the intimal entries in two patients with type B entries were not visualized by TEE because the dissection was in the aortic arch and was obscured by the trachea and the left mainstem bronchus. The intimal entries in two patients with type B entries were in the abdominal aorta. [23]
Color Doppler TEE using biplanar views has the advantages of additional acoustic windows, ease of spatial orientation, more accurate visualization of the entry, and ease of application.
The main drawback of TEE is its strong dependence on operator experience. Other drawbacks are that false-positive results can occur from reverberations in the ascending aorta and that the upper ascending aorta and arch may not be visualized well, leading to false-negative results. TEE cannot be performed in patients with esophageal varicosities or stenosis. If the findings are negative and clinical suspicion remains high, a second diagnostic test is recommended.
Magnetic Resonance Imaging
MRI has approximately 98% sensitivity and specificity for detecting thoracic aortic dissection. It is the most sensitive method for diagnosing aortic dissection and has similar specificity to CT.
MRI shows the site of intimal tear, type and extent of dissection, and presence of aortic insufficiency, as well as the surrounding mediastinal structures. Other benefits are that MRI requires no contrast medium and no ionizing radiation. It is the preferred modality for patients with renal failure and those with an allergy to iodine, as well as for imaging chronic dissections and postsurgical follow-up. Its use in acute dissection is limited because it is not portable. [24]
A study of 53 consecutive patients with clinically suspected aortic dissection found that for type A dissection, MRI was 100% sensitive and specific, while TEE was 100% sensitive and 78% specific. In patients with type B dissection, MRI was 100% sensitive and specific, whereas TEE was 90% sensitive and 97% specific. [25]
With regard to epiphenomena, visualization of the site and spatial extent of the intimal flap with TEE was 78% specific in the ascending aorta, 94% in the aortic arch, and 92% in the descending aorta. MRI and TEE were equal in the detection of the site of entry of the aortic dissection. Sensitivity in the detection and localization of intraluminal thrombi was 75% for TEE and 100% for MRI. [25]
Contrast-enhanced magnetic resonance angiography (CE-MRA) is an accurate noninvasive imaging modality. It has the advantage of being able to evaluate the aortic valve more effectively than CTA can.
Drawbacks include the following:
-
MRI is not readily available at most institutions, requiring transportation of patients in unstable condition away from the ED
-
MRI requires much more time to acquire images than CT does
-
Patients with permanent pacemakers cannot undergo MRI (however, most patients with prosthetic heart valves or coronary stents can safely undergo MRI)
Aortography
Aortography has been the diagnostic criterion standard study for aortic dissection, but it is difficult to perform in patients with hemorrhage, shock, and/or cardiac tamponade. Aortography is being replaced by newer imaging modalities because of risks associated with invasiveness and adverse reactions to intravenous contrast agents.
Aortography (see the image below) leads to accurate diagnosis of aortic dissection in over 95% of patients and aids the surgeon in planning the repair operation because blood vessels of the arch can be assessed easily.
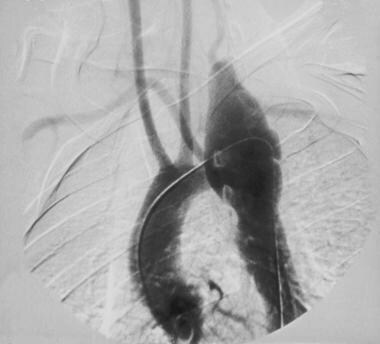 Angiogram demonstrating dissection of the aorta in a patient with aortic dissection presenting with hemothorax.
Angiogram demonstrating dissection of the aorta in a patient with aortic dissection presenting with hemothorax.
Benefits include visualization of the true and false lumens, intimal flap, aortic regurgitation, and coronary arteries.
Drawbacks include the following:
-
The procedure is invasive
-
The patient must be transported to the radiology department, leaving the ED
-
The use of contrast media may be harmful to patients who have renal insufficiency or an allergy to iodine
-
The false lumen and intimal flap may not be visualized if the false channel is thrombosed, which can lead to misdiagnosis
-
Simultaneous opacification of the true and false lumens may make discerning the presence of a dissection difficult
Electrocardiography
All patients with suspected thoracic aortic dissection should have a 12-lead ECG. However, the ECG often demonstrates a nonspecific abnormality or normal results (approximately 31% of patients). One study reported normal findings in 63 (90%) of 70 patients.
In acute thoracic aortic dissection, the ECG changes can mimic those seen in acute cardiac ischemia. In the presence of chest pain, these signs can make distinguishing dissection from acute myocardial infarction very difficult (see the image below). Keep this in mind when administering thrombolytics to patients with chest pain.
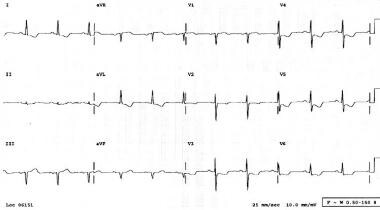 Electrocardiogram of a patient presenting to the ED with chest pain; this patient was diagnosed with aortic dissection.
Electrocardiogram of a patient presenting to the ED with chest pain; this patient was diagnosed with aortic dissection.
The incidence of abnormal ECG findings is greater in Stanford type A dissections than in other types of dissections. ST-segment elevation can be seen in Stanford type A dissections because the dissection interrupts blood flow to the coronary arteries. In one study, 8% of patients with type A dissections had ST-segment elevation, whereas no patients with type B dissections had ST-segment elevation. More commonly, the ECG abnormality is ST-segment depression.
If the dissection involves the coronary ostia, the right coronary artery is most commonly involved. This can result in ST-segment elevation in leads II, III, and aVF, a pattern similar to that seen in inferior wall infarctions.
-
Aortic dissection. CT scan showing a flap (right side of image).
-
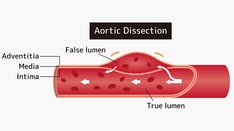
Aortic dissection. True lumen versus false lumen in an intimal flap.
-
Aortic dissection. Left subsegmental atelectasis and left pleural effusion. Flap at lower right of image.
-
Aortic dissection. Significant left pleural effusion.
-
Aortic dissection. CT scan showing a flap (center of image).
-
Aortic dissection. CT scan showing a flap (center of image).
-
Aortic dissection. CT scan showing a flap.
-
Aortic dissection. CT scan showing a flap.
-
Aortic dissection. Mediastinal widening.
-
Aortic dissection. CT scan showing a flap.
-
Aortic dissection. CT scan showing a flap.
-
Aortic dissection. CT scan showing a flap.
-
Aortic dissection. Thrombus and a patent lumen.
-
Aortic dissection. Thrombus.
-
Aortic dissection. True lumen and false lumen separated by an intimal flap.
-
Aortic dissection. Mediastinal widening.
-
Aortic dissection. CT scan showing a flap.
-
Aortic dissection. Intimal flap and left pleural effusion.
-
Image A represents a Stanford A or a DeBakey type 1 dissection. Image B represents a Stanford A or DeBakey type II dissection. Image C represents a Stanford type B or a DeBakey type III dissection. Image D is classified in a manner similar to A but contains an additional entry tear in the descending thoracic aorta. Note that a primary arch dissection does not fit neatly into either classification.
-
Aortic dissection.
-
Chest radiograph of a patient with aortic dissection. Image courtesy of Dr. K. London, University of California at Davis Medical Center.
-
Chest radiograph of a patient with aortic dissection presenting with hemothorax.
-
Chest radiograph demonstrating widened mediastinum in a patient with aortic dissection.
-
Angiogram demonstrating dissection of the aorta in a patient with aortic dissection presenting with hemothorax.
-
Electrocardiogram of a patient presenting to the ED with chest pain; this patient was diagnosed with aortic dissection.
-
Patient with an ascending type A aortic dissection showing the intimal flap. Image courtesy of Kaiser-Permanente.
-
Patient with an ascending type A aortic dissection showing the intimal flap. Image courtesy of Kaiser-Permanente.
-
Patient with an ascending type A aortic dissection showing the intimal flap. Image courtesy of Kaiser-Permanente.
-
Patient with an ascending type A aortic dissection showing the intimal flap. Image courtesy of Kaiser-Permanente.
-
Patient with a type A aortic dissection involving the ascending and descending aorta. Image courtesy of Kaiser-Permanente.
-
Patient with a type A aortic dissection involving the ascending and descending aorta. Image courtesy of Kaiser-Permanente.
-
Patient with a type A aortic dissection involving the ascending and descending aorta. Image courtesy of Kaiser-Permanente.
-
Patient with a type A aortic dissection involving the ascending and descending aorta. Image courtesy of Kaiser-Permanente.
-
Patient showing a type B aortic dissection with extravasation of blood into the pleural cavity. Image courtesy of Kaiser-Permanente.
-
Patient showing a type B aortic dissection with extravasation of blood into the pleural cavity. Image courtesy of Kaiser-Permanente.
-
Patient showing a type B aortic dissection with extravasation of blood into the pleural cavity. Image courtesy of Kaiser-Permanente.
-
Patient showing a type B aortic dissection with extravasation of blood into the pleural cavity. Image courtesy of Kaiser-Permanente.