Reference Range
The major circulating form of vitamin D is 25-hydroxyvitamin D (25(OH)D); thus, the total serum 25(OH)D level is currently considered the best indicator of vitamin D supply to the body from cutaneous synthesis and nutritional intake.
The reference range of the total 25(OH)D level is 25-80 ng/mL. [1]
Interpretation
There are two principal forms of vitamin D: D2 and D3. Many of the currently available assays measure and report on both vitamin D2 and D3 metabolites. This can be useful in studies evaluating the contribution of vitamin D2 and D3 to overall vitamin D status. 25-hydroxyvitamin D (25(OH)D) is the major circulating form of vitamin D; thus, the total serum 25(OH)D level is currently considered the best indicator of vitamin D supply to the body from cutaneous synthesis and nutritional intake.
One exception is that 25(OH)D levels do not indicate clinical vitamin D status in patients with chronic renal failure or type 1 vitamin D-dependent rickets or when calcitriol (1,25-dihydroxyvitamin D) is used as a supplement.
Interpretation of 25(OH)D can be challenging owing to wide variability in patient’s weight, ethnicity, assays, laboratory procedures and validation of reference ranges. [2, 3, 4]
Vitamin D deficiency
Vitamin D deficiency is defined by most experts as a serum 25(OH)D level of less than 20 ng/mL (50 nmol/L).
Vitamin D insufficiency
Vitamin D insufficiency has been defined as a serum 25(OH)D level of 21-29 ng/mL (52-72 nmol/L). This is based on the observed physiological changes in calcium absorption and parathyroid hormone levels that occur with changes in vitamin D levels.
In healthy postmenopausal women with serum 25(OH)D levels averaging 34.4 ng/mL (86.5 nmol/L), intestinal calcium absorption increased from 45% to 65% compared to those with mean 25(OH)D levels of 20 ng/mL (50.1 nmol/L). [5]
Chapuy et al reported an inverse correlation between serum intact parathyroid hormone (iPTH) and serum 25(OH)D. Serum iPTH held a stable plateau level when serum 25(OH)D values exceeded 31 ng/mL (78 nmol/L) but started to rise when the serum 25(OH)D values fell below this level. [6]
Vitamin D sufficiency
Vitamin D sufficiency has been defined as serum 25(OH)D levels of 30 ng/mL (75 nmol/L) and above based on analysis of observational studies of vitamin D and various health outcomes.
Bischoff-Ferrari et al reviewed evidence from studies that evaluated the thresholds of serum 25(OH)D levels and their relation to bone mineral density (BMD), lower-extremity function, dental health, and the risk of falls, fractures, and colorectal cancer. Their conclusion based on all endpoints was that 25(OH)D levels of 30 ng/mL (75 nmol/L) or greater are more effective in achieving positive outcomes than lower levels. The best outcomes were seen with 25(OH)D levels from 36-40 ng/mL (90-100 nmol/L). [7]
Vitamin D toxicity
Vitamin D toxicity is observed when serum 25(OH)D levels are greater than 150 ng/mL (374 nmol/L).
Assays for serum 25-hydroxyvitamin D
Currently, there are several assays for measuring serum 25(OH)D. Each of them has its strengths and weaknesses.
In the early 1970s, Belsey et al and Haddad et al developed a competitive binding assay to measure serum vitamin D levels. This assay used vitamin D–binding protein (DBP) obtained from rat serum as the binding agent and exploited the capacity of vitamin D in test samples to displace bound [3 H]25(OH)D3 from DBP. [8, 9] This assay was limited by issues related to the required extraction and purification processes; as a result, this method has been replaced by others as indicated below.
Antibody-based assays have been available since the early 1980s. [10] Since then, various antibody-based methods have been in use, including radioimmunoassay (RIA), enzyme-linked immunosorbent assay (ELISA), and chemiluminescent assay. [11] In general, these assays depend on an antibody that recognizes 25(OH)D2 and 25(OH)D3 in equimolar proportions and the competitive displacement of tracer 25(OH)D (25(OH)D that has been tagged with a radioactive isotope such as 125-I or a chemiluminescent ligand) by acetonitrile extracted samples. The acetonitrile extraction eliminates the need for chromatographic purification of vitamin D prior to its actual assay. [12, 13] The advantages include good precision and ease of use.
The first liquid-chromatography (LC)–based method was presented in 1977. [14] It was based on the distinctive ultraviolet (UV) spectral absorption peak of vitamin D metabolites at 265 nm. Via UV detection, this assay allowed direct quantization of not only total 25(OH)D but also 25(OH)D2 and 25(OH)D3 separately. Over the years, this LC-based method has developed into the latest LC-chromatography–tandem mass spectrometry (LC-MS/MS), which is currently considered the criterion standard of vitamin D assays. In this method, a mass spectrometer serves as the metabolite detector, instead of UV light. It measures 25(OH)D2 and 25(OH)D3 with much higher sensitivity and specificity than the traditional LC methods. [15]
Despite these advances in methodologies over the last 40 years, there is still wide and unacceptable interassay and interlaboratory variability that has the potential to affect physicians’ clinical decisions. This has raised a call for assay standardization. [3, 16] To address this issue, the Vitamin D External Quality Assurance Scheme (DEQAS; Charing Cross Hospital, London, UK) has been created to monitor and ensure the reliability of vitamin D assays.
However, until a unified method has been established, physicians need to be cautious in interpreting results of vitamin D levels.
Collection and Panels
Specimen: Blood (0.25 mL room-temperature serum)
Container: Red-top tube, serum separator tube (if a serum separator tube is used, allow to clot at room temperature; centrifuge and remove from the gel within 48 hours)
Collection method: Routine venipuncture
Other instructions: Collect blood into a standard red-top tube; allow blood to clot at room temperature; centrifuge and separate the serum from the cells immediately
Panels: 25-hydroxyvitamin D (25(OH)D) is not included in routine comprehensive metabolic panels; it should be ordered as a separate blood test
Background
Description
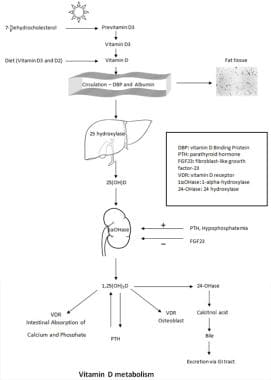
Vitamin D is a group of fat-soluble compounds with a four-ringed cholesterol backbone; it is now recognized as a prohormone.
Vitamin D exists in two major forms: vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol). The precursor for vitamin D2 is a plant sterol ergosterol. D2 can be synthesized by ultraviolet irradiation of ergosterol from yeast. Similarly, vitamin D3 is synthesized in the body when sunlight (ultraviolet B, wavelength 280-315 nm) photoisomerizes 7-dehydroxycholesterol found in the skin. D3 is also found in animal-based foods (eg, fatty fish, liver, milk, eggs).
Vitamin D2 and D3, regardless of the source, are biologically inactive. They are transformed into the biologically active molecule 1,25 dihydroxyvitamin D. After being synthesized in the skin or absorbed (in chylomicrons) from the gastrointestinal (GI) tract, most vitamin D is bound to specific carrier proteins in the blood (vitamin D–binding protein [DBP] and albumin) and transported to the liver. In the liver, vitamin D is hydroxylated by the enzyme 25-hydroxylase (CYP2R1) to become 25(OH)D. 25(OH)D is the major circulating form of vitamin D. From the liver, 25(OH)D is transported to the kidneys via the same carrier proteins as above.
1,25 dihydroxyvitamin D (1,25(OH)2 D) is formed when 25(OH)D is hydroxylated by the enzyme 1α-hydroxylase (CYP27B1), which is located in the mitochondria of proximal tubules of the kidney. 1,25(OH)2 D is the biologically active form of vitamin D. As a result of 1 and 25 hydroxylation, the prohormone vitamin D has been transformed into an active hormone.
1,25(OH)2 D is a steroidlike hormone. In target cells, such as classic steroid hormones, it binds to a specific cytoplasmic VDR; the vitamin D bound to VDR then translocates to the nucleus, where its effects are initiated at a transcriptional level. [17] The main established actions of 1,25(OH)2 D collectively increase calcium in the body and modulate the skeleton. It increases the intestinal absorption of calcium and phosphate, decreases renal excretion of calcium and phosphate, suppresses PTH production, and regulates osteoblast function and bone resorption. It has been suggested that 1,25(OH)2 D has roles beyond the calcium-skeletal axis. [17]
The identification of VDRs in various cells has prompted the investigation of vitamin D in immunomodulation, cancer prevention and therapy, autoimmune disease, cardiovascular disease, and other nonskeletal organs.
The synthesis of 1,25(OH)2 D is tightly regulated by PTH, serum calcium, serum phosphate, and fibroblast-like growth factor 23 (FGF-23). Increased levels of PTH and hypophosphatemia stimulate the enzyme 1α-hydroxylase and, subsequently, the synthesis of 1,25(OH)2 D. FGF-23 is a circulating hormone synthesized by osteocytes and osteoblasts. 1,25(OH)2 D and phosphate intake stimulates the synthesis of FGF-23, which, in turn, inhibits 1,25(OH)2 D production, reduces the expression of renal sodium–phosphate transporters, and activates the metabolizing of active 1,25(OH)2 D to the inactive metabolite 24,25(OH)2 D. [18]
As a fat-soluble molecule, vitamin D is stored in adipose tissue; however, the exact mechanism by which vitamin D is regulated and mobilized from adipose tissue has not been elucidated at this time. Most vitamin D products are excreted through bile into the gut. Very little is eliminated via kidneys (see graph below).
Indications/Applications
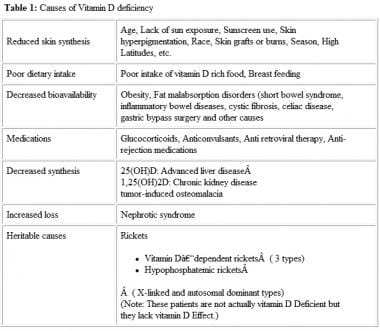
Measurement of 25(OH)D levels can be considered to evaluate suspected vitamin D toxicity or deficiency. Deficiency can result from several factors and affect people of all ages and health (see image below). [19] Serial monitoring is also recommended during treatment with vitamin D to prevent hypercalcemia. [20]
Current guidelines do not recommend screening the general population for deficiency. The Endocrine Society recommends a measurement of 25(OH)D for those at risk, which include pregnant patients. [20] On the other hand, the American College of Obstetricians and Gynecologists advocates testing only for pregnant patients at high-risk for deficiency, such as vegetarians, those with limited sun exposure, and members of ethnic minorities. [21]
Considerations
The serum level of 25(OH)D is currently considered the best indicator of total body vitamin D stores. However, it is unclear if this reflects the actual physiological state of vitamin D activity in the body. It is possible that there are effects of vitamin D that occur intracellularly independent of the measured serum level of vitamin D. Thus, there is a need for future research that more specifically relates the measured levels of D in blood with the vitamin D activities that are mediated through the VDR effects on transcription.
25(OH)D and 1,25(OH)2 D, when transported in the circulation, are bound primarily to DBP and albumin. A very small fraction (0.02%–0.05% of 25(OH)D and 0.2%-0.6% of total 1,25(OH)2 D) remains free or unbound. Physiological and pathological changes in the concentrations of DBP and albumin, such as in pregnancy, in malnutrition, in liver disease, in nephrotic syndrome, and in association with medications, among others, can affect the total and free fractions of 25(OH)D.
It is controversial whether determination of free vitamin D metabolites would be important. In a recent population-based cohort study with 2085 participants (blacks and whites), black Americans had lower levels of total 25(OH)D and vitamin D–binding protein compared with whites. [22] However, the 2 groups had similar concentrations of estimated bioavailable 25(OH)D. How the lower vitamin D–binding protein levels affect the risk of fracture is unknown.
Until now, owing to the lack of data on the physiological and clinical impact of free vitamin D, total 25(OH)D remains the best indicator of total body vitamin D stores and its availability for biologic functions.
-
Causes of vitamin D deficiency.
-
Vitamin D metabolism.