Approach Considerations
Many laboratories do not routinely test for Cryptosporidium, and, in many instances, the tests used to evaluate for this organism are insensitive. [1] Studies in the United States have documented cryptosporidiosis in about 4% of stools sent for parasitologic examination, while, overall, about 13% of stool studies submitted for parasitologic studies in developing countries reveal Cryptosporidium oocysts.
Cryptosporidium infection can be difficult to diagnose via standard methods and is often missed unless specific tests are performed. Traditionally, it was diagnosed via microscopic examination with special staining techniques (eg, acid-fast staining, direct fluorescent antibody [DFA], enzyme immunoassays, or immunochromatographic tests for detection of Cryptosporidium antigens). [49] Currently, PCR multiplex molecular tests have increased sensitivity and specificity.
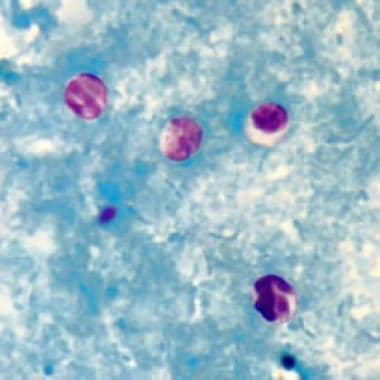
 Modified acid-fast stain of stool shows red oocysts of Cryptosporidium parvum against the blue background of coliforms and debris.
Modified acid-fast stain of stool shows red oocysts of Cryptosporidium parvum against the blue background of coliforms and debris.
Urea, electrolytes, and liver function tests
Diarrhea causes volume depletion, so urea and electrolyte tests are used to assess electrolyte and volume replacement requirements. Elevated alkaline phosphatase and glutamyl transpeptidase without hyperbilirubinemia are typical signs of biliary involvement.
Imaging studies
Imaging studies are not indicated as a first-line diagnostic approach in cryptosporidiosis but can aid in the diagnosis. Abdominal radiography and computed tomography (CT) scanning are nonspecific, but may reveal distended loops of bowel, air-fluid levels, and disrupted bowel motility.
When indicated, as guided by symptoms, ultrasonography or CT scanning may reveal an enlarged gallbladder with a thickened wall, dilated or irregular intrahepatic and extrahepatic biliary ducts, and a normal or stenotic distal common bile duct. Cholangiography may reveal beading of the common bile duct or papillary stenosis.
In cases of respiratory involvement, chest radiography is unremarkable, with modest infiltrates or increased bronchial markings.
Stool Tests
Processing
Unconcentrated fresh specimens can be examined with wet mount preparations, but they are not recommended owing to high infectivity and unreliability. Despite different methods available, the formalin ethyl acetate concentration method is the most sensitive and widely used. [50] Optimal centrifugation time and speed are critical for concentrating Cryptosporidium oocysts. Commercial fecal concentration tubes are available that decrease processing time and supplies needed for concentrating specimens (eg, Fecal Parasite Concentrator, Evergreen Scientific). Immunomagnetic separation commercial kits are also available. Polyvinyl alcohol (PVA)–preserved specimens are not acceptable for modified acid-fast staining or antigen-detection assays for detection of Cryptosporidium.
Types of tests
Since the oocyst is small (4-6 µm), identification requires staining prior to light microscopy. [49] Modified acid-fast staining procedure is useful for the identification of oocysts (which may be difficult to detect with routine stains, such as trichrome). Cryptosporidium species stain a pinkish-red color on a uniformly green background. Unlike the modified Ziehl-Neelsen acid-fast stain, this stain does not require the heating of reagents for staining. Other studies have found the fluorogenic stain auramine-phenol to be a more sensitive and faster option, which has been adopted by many laboratories as the standard staining method. [49, 51]
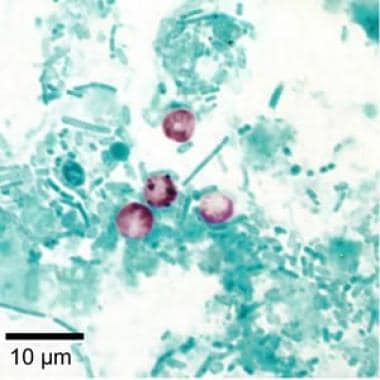 Cryptosporidium parvum oocysts revealed with modified acid-fast stain. Against a blue-green background, the oocysts stand out with a bright red stain. Image courtesy of CDC DPDx parasite image library.
Cryptosporidium parvum oocysts revealed with modified acid-fast stain. Against a blue-green background, the oocysts stand out with a bright red stain. Image courtesy of CDC DPDx parasite image library.
The gold standard for stool examination is the immunofluorescence assay, which is based on oocyst cell wall antigens targeted by specific fluorescent monoclonal antibodies. The main disadvantages for this method are the inability to process large amounts of samples and the need for a specialized microscope and technician. Antigen detection via enzyme-linked immunosorbent assays (ELISA) or enzyme immunoassay (EIA) has also been developed with variable sensitivities and specificities depending on the commercial kit used, limiting its use in epidemiological studies. Chalmers et al demonstrated that enzyme-linked immunosorbent assays (ELISA) and immunofluorescent tests (IFA) have sensitivities above 90%, significantly higher than that of modified Ziehl-Neelsen stains (75%). [51, 52]
In the past few years, various PCR-based commercial multiplex molecular assays to detect Cryptosporidium species have been approved by the FDA. These tests can detect various pathogens (eg, parasites, bacteria, viruses) that cause diarrhea. They are more expensive, but are highly sensitive and are not readily available in all laboratories. [49, 50, 53]
Specimen examination
Concentrated sediment of fresh (within 30 min after passage of stools) or formalin-preserved stool may be used. Other types of clinical specimens, such as duodenal fluid, bile, and pulmonary samples (induced sputum, bronchial wash, biopsies) may also be stained.
The formalin ethyl acetate method is used to concentrate stool before staining with a modified acid-fast stain, because routine laboratory examination of stool for ova and parasites does not detect Cryptosporidium. [1] This technique allows for differentiation from fecal debris or yeast, which stains blue or green, versus oocysts, which counterstain pink or red. Careful examination of slides is imperative, as oocysts can easily be missed.
Because shedding may be intermittent, examine at least 3 stool specimens collected on separate days before considering the test results negative. Fecal leukocytes are not found in stool specimens, because invasion does not occur below the epithelial layer of the mucosa.
Other testing strategies include the following:
-
GI biopsy specimens can be used instead of stool specimens; a high concentration of oocysts is seen in the jejunum.
-
Electron microscopy of stool or biopsy specimens can also be performed for direct visualization of oocysts.
-
PCR and immunohistochemistry can be used on tissue samples, precluding the need for staining. [50]
-
Serologic detection of specific anti- Cryptosporidium antibodies is primarily used as a research or epidemiologic tool.
Evaluation of Immune Function
Lymphocyte subset analysis
CD4+ lymphocyte counts predict the duration of disease in patients infected with HIV. When the counts are greater than 150 cells/μL, the diarrhea is likely to resolve spontaneously. With lower counts, however, the diarrhea may be chronic. Counts are typically less than 50 cells/μL in patients with either biliary involvement or cholera-like syndromes.
HIV testing
Prolonged diarrhea caused by cryptosporidiosis may warrant HIV testing.
Primary immunodeficiencies
Children with chronic diarrhea from cryptosporidiosis should be screened for primary immunodeficiencies associated with depressed cellular immune function. The most commonly identified immunodeficiency is hyper-IgM syndrome, which can be identified by antibody screening. T-cell deficiencies can be identified by examining lymphocyte numbers and subsets. [54]
Secondary immunodeficiencies
In organ transplant recipients who are receiving immunosuppressive medications, immunosuppression should be minimized, and levels of tacrolimus and cyclosporine monitored to avoid toxicity. [43] Recently, malignancy, chemotherapy and CAR T cell therapy have also been associated with higher risk of cryptosporidium infection. [7, 8]
Abdominal Ultrasonography and ERCP
Dilated or irregular intrahepatic and extrahepatic bile ducts, along with a thickened gallbladder, as detected with abdominal ultrasonography, indicate biliary involvement.
Endoscopic retrograde cholangiopancreatography (ERCP) is often needed to diagnose sclerosing cholangitis or papillary stenosis.
ERCP identification of Cryptosporidium oocysts in bile or intracellular forms on biopsy confirms the diagnosis of biliary cryptosporidiosis. Papillary stenosis may be present and responds symptomatically to endoscopic sphincterotomy, often with stent placement.
Biopsy and Lavage
GI or liver biopsy
GI or liver biopsy may be indicated in cases of diagnostic uncertainty. Different parts of the intestinal tract may be affected. Liver biopsy findings may reveal the organism attached to bile duct epithelial cells. Concurrent infection with cytomegalovirus (CMV), Enterobacter cloacae, and microsporidia is common.
Bronchoalveolar lavage and lung biopsy
In patients with related symptoms, bronchoscopy may reveal the parasite in lavage fluid, in brushing specimens, and in biopsy specimens attached to the surface of bronchial mucosal cells or in macrophages. In most instances, another pulmonary pathogen, such as CMV or Pneumocystis jiroveci, is concurrently detected; however, in a series of four patients infected with HIV, Cryptosporidium was the only pathogen identified in the respiratory tract. Clear association with intestinal cryptosporidiosis or diarrhea has not been shown in these cases.
Histologic Findings
Histologic examination of the small intestine is not required to confirm the diagnosis of cryptosporidiosis, although the small intestine does show the parasite projecting from the brush border of the mucosal surface. Parasites may also be identified in bile or biliary tract biopsies.
Villous atrophy with blunting, epithelial flattening, and an increase in lamina propria lymphocytes are seen in patients with persistent cryptosporidiosis. In patients with heavier infection, crypt hyperplasia and marked infiltration with lymphocytes, plasma cells, and neutrophils are also noted.
-
Modified acid-fast stain of stool shows red oocysts of Cryptosporidium parvum against the blue background of coliforms and debris.
-
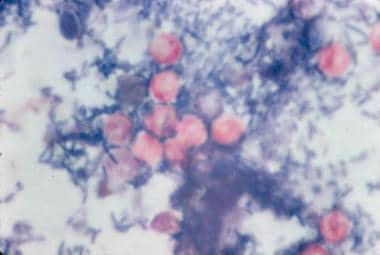
Hematoxylin and eosin stain of intestinal epithelium. The blue dots (arrows) represent intracellular Cryptosporidium organisms along the surface of the epithelial cells. Image courtesy of Carlos Abramowsky, MD, Professor of Pediatrics and Pathology, Emory University School of Medicine.
-
Cryptosporidium species oocysts are rounded and measure 4.2-5.4 µm in diameter. Sporozoites are sometimes visible inside the oocysts, indicating that sporulation has occurred on wet mount.
-
Cryptosporidium parvum oocysts revealed with modified acid-fast stain. Against a blue-green background, the oocysts stand out with a bright red stain. Image courtesy of CDC DPDx parasite image library.
-
Cryptosporidium oocysts revealed with modified acid-fast stain.