Practice Essentials
Laparoscopic pelvic lymph node dissection (LPLND) is used in the diagnosis and staging of prostate cancer as well as bladder, penile, and urethral malignancies. The goal of LPLND in prostate cancer is to exclude high-risk patients from noncurative therapy and to stage high-risk patients at the time of prostatectomy. [1, 2] Pelvic lymphadenectomy remains the criterion standard for detecting metastatic spread to the pelvic lymph nodes. [3]
Overview of prostate cancer pelvic lymphadenectomy
The introduction of prostate-specific antigen (PSA) measurement as a screening test for prostate cancer created a dramatic shift or stage migration, which led to the diagnosis of prostate cancer in earlier stages of the disease. As a result, more patients are undergoing a variety of treatments for prostate cancer, including radical prostatectomy (open, laparoscopic, robotic), brachytherapy, external beam radiotherapy, intensity-modulated radiation therapy (IMRT), cryosurgery, microwave thermotherapy, and high-frequency ultrasound therapy.
Because the stage at the time of diagnosis is typically lower, the need for pelvic lymphadenectomy in staging patients with prostate cancer has decreased. Nevertheless, pelvic lymphadenectomy remains an important piece of the cancer armamentarium for urologic surgeons. The principles outlined in this article are also pertinent to robotic laparoscopic lymphadenectomy, which is commonly performed at the time of laparoscopic or robotic prostatectomy.
Despite refinements in surgical technique, radical prostatectomy remains an operative procedure that is associated with significant morbidity. Complications, including urinary incontinence, impotence, and fecal incontinence, [4] dictate a conservative approach in patients shown to have metastatic disease. In addition, decisions concerning nonsurgical treatment of prostate cancer using external beam radiation, brachytherapy, or adjuvant hormonal therapy may be altered in patients with microscopic metastatic disease. Thus, the goal of LPLND is to exclude high-risk patients from noncurative therapy and to stage high-risk patients at the time of prostatectomy performed robotically or laparoscopically.
Since the establishment of LPLND as a staging procedure in patients with prostate cancer, indications have been expanded to include the staging of bladder, penile and urethral malignancies. [5, 6] In these other types of genitourinary cancers, the presence of metastatic disease also has a tremendous impact on therapeutic choices.
The following image illustrates 2 examples of port placement in LPLND.
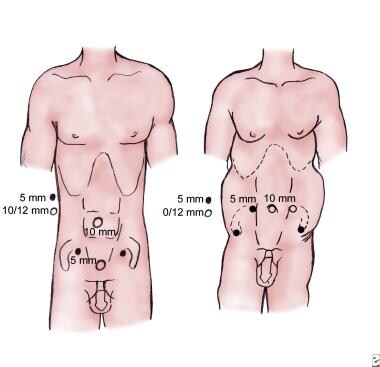 Two variations of port placement are used commonly, the diamond and the fan configurations. In the diamond configuration (left), two 10-mm trocars are used. One is placed at the umbilicus, and the second is placed approximately 4-6 cm above the symphysis pubis in the midline. Two additional 5-mm trocars are used, one near the McBurney point in the midclavicular line on both sides. In the fan position (right), 5 trocars are used. A 10-mm trocar is placed at the umbilicus for the laparoscope. A second 10-mm trocar is placed on the left side, and a 5-mm trocar is placed on the right side at the level of the umbilicus, lateral to the inferior epigastric vessels, in line with the anterior superior iliac crest. Two additional 5-mm trocars are placed laterally, midway between the umbilicus and the symphysis pubis.
Two variations of port placement are used commonly, the diamond and the fan configurations. In the diamond configuration (left), two 10-mm trocars are used. One is placed at the umbilicus, and the second is placed approximately 4-6 cm above the symphysis pubis in the midline. Two additional 5-mm trocars are used, one near the McBurney point in the midclavicular line on both sides. In the fan position (right), 5 trocars are used. A 10-mm trocar is placed at the umbilicus for the laparoscope. A second 10-mm trocar is placed on the left side, and a 5-mm trocar is placed on the right side at the level of the umbilicus, lateral to the inferior epigastric vessels, in line with the anterior superior iliac crest. Two additional 5-mm trocars are placed laterally, midway between the umbilicus and the symphysis pubis.
See Prostate Cancer, Prostate-Specific Antigen, and Laparoscopic and Robotic Radical Prostatectomy for more information on these topics.
Indications for pelvic imaging studies
Noninvasive imaging technologies such as computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET) can be useful in the diagnosis of metastatic disease in numerous malignancies. However, these imaging modalities are inadequate to reliably diagnose pelvic lymph node involvement in most patients with prostate cancer and can yield false-positive findings in the setting of infection or inflammation of the prostate after biopsy. [7, 8]
The utility of capromab pendetide imaging in the management and staging of prostate cancer continues to be explored. [9] The appropriate techniques for obtaining images, the clinical indications, and its use in clinical staging of the pelvic spread of prostate cancer are still being optimized. Once additional information is gathered through careful clinical trials, capromab pendetide scans may be a complementary diagnostic tool to PSA levels, Gleason score determined by prostate biopsy findings, and digital rectal examination.
Hacker et al evaluated the use of fluorocholine combined in-line PET-CT scanning in patients with a PSA level of more than 10 ng/mL, Gleason sum greater than 7, and negative bone scan findings and found that PET scanning was not useful in detecting occult lymph node metastasis in clinically localized prostate cancer. [10]
Hruby et al investigated whether visualization of the prostate drainage system with free indocyanine green would identify more lymph node metastases than super-extended pelvic lymph node dissection in intermediate- and high-risk patients with prostate cancer. The authors reported that fluorescence-targeted pelvic lymph node dissection allows for the lymphatic drainage of the prostate to be identified with great reliability. They concluded that since only the nodes draining the prostate are removed, the absolute number of removed nodes is decreased while diagnostic accuracy is increased. [11]
Indications for pelvic lymphadenectomy
Pelvic lymphadenectomy remains the criterion standard for detecting metastatic spread to the pelvic lymph nodes. [12] The indications for staging pelvic lymphadenectomy before prostatectomy include the following:
-
Prebiopsy serum prostate-specific antigen (PSA) level greater than 20 ng/mL
-
Gleason sum equal to 8 or greater
-
Five or more positive systematic sextant biopsies or total linear involvement of 28% or more
-
Palpably advanced local disease, clinical stages T3 and T4
-
Positive seminal vesicle biopsy
-
Enlargement of pelvic lymph nodes as evident on pelvic imaging
Partin tables
Using standard nomograms, such as the Partin tables below, we can predict that 24-51% of patients with a PSA level greater than 20 ng/mL and a Gleason sum of 8-10 will have nodal disease and would not be candidates for curative resection.
Table 1. Multivariate Logistic Regression Analysis for Prediction of Pathologic Stage Using Prostate-Specific Antigen, Gleason Score, and Clinical Stage (TNM): Prediction of Organ-Confined Disease (Percentage) (Open Table in a new window)
Prostate-Specific Antigen Level (ng/mL) |
||||||||||||||
Gleason Score |
0 – 4 Clinical Stage |
4.1 – 10 Clinical Stage |
||||||||||||
T1a |
T1b |
T1c |
T2a |
T2b |
T2c |
T3a |
T1a |
T1b |
T1c |
T2a |
T2b |
T2c |
T3a |
|
2-4 |
100 |
85 |
92 |
88 |
76 |
82 |
— |
100 |
78 |
82 |
83 |
67 |
71 |
— |
5 |
100 |
78 |
81 |
81 |
67 |
73 |
— |
100 |
70 |
71 |
73 |
56 |
64 |
43 |
6 |
100 |
68 |
69 |
72 |
54 |
60 |
42 |
100 |
53 |
59 |
62 |
44 |
48 |
33 |
7 |
— |
54 |
55 |
61 |
41 |
46 |
— |
100 |
39 |
43 |
51 |
32 |
37 |
26 |
8-10 |
— |
— |
— |
48 |
31 |
— |
— |
— |
32 |
31 |
39 |
22 |
25 |
12 |
Prostate-Specific Antigen Level (ng/mL) |
||||||||||||||
Gleason Score |
10.1 – 20 Clinical Stage |
>20 Clinical Stage |
||||||||||||
T1a |
T1b |
T1c |
T2a |
T2b |
T2c |
T3a |
T1a |
T1b |
T1c |
T2a |
T2b |
T2c |
T3a |
|
2-4 |
100 |
— |
— |
61 |
52 |
— |
— |
— |
— |
33 |
20 |
7 |
— |
— |
5 |
100 |
49 |
55 |
58 |
43 |
37 |
26 |
— |
— |
24 |
32 |
— |
3 |
— |
6 |
— |
36 |
41 |
44 |
28 |
37 |
19 |
— |
— |
22 |
14 |
1 |
4 |
5 |
7 |
— |
24 |
24 |
36 |
19 |
24 |
14 |
— |
— |
7 |
18 |
4 |
5 |
3 |
8-10 |
— |
11 |
— |
29 |
14 |
15 |
9 |
— |
— |
3 |
3 |
— |
2 |
2 |
TNM = tumor, nodes, and metastases (cancer staging). |
||||||||||||||
Numbers represent the percentage predictive probability (95% confidence interval) of the patient having a given final pathologic stage based on a multinomial log-liner regression of all 3 variables. [13] |
||||||||||||||
Table 2. Multivariate Logistic Regression Analysis for Prediction of Pathologic Stage Using Prostate-Specific Antigen, Gleason Score, and Clinical Stage (TNM): Prediction of Lymph Nodal Status (Percentage) (Open Table in a new window)
Prostate-Specific Antigen Level (ng/mL) |
||||||||||||||
Gleason Score |
0 – 4 Clinical Stage |
4.1 – 10 Clinical Stage |
||||||||||||
T1a |
T1b |
T1c |
T2a |
T2b |
T2c |
T3a |
T1a |
T1b |
T1c |
T2a |
T2b |
T2c |
T3a |
|
2-4 |
0 |
2 |
< 1 |
1 |
2 |
4 |
— |
0 |
2 |
1 |
1 |
2 |
5 |
— |
5 |
0 |
4 |
1 |
2 |
4 |
8 |
— |
0 |
4 |
1 |
2 |
5 |
10 |
8 |
6 |
0 |
8 |
2 |
3 |
9 |
17 |
15 |
0 |
9 |
2 |
4 |
11 |
19 |
16 |
7 |
— |
15 |
2 |
7 |
18 |
31 |
— |
0 |
18 |
3 |
8 |
20 |
34 |
28 |
8-10 |
— |
— |
— |
13 |
32 |
— |
— |
— |
30 |
5 |
15 |
35 |
53 |
50 |
Prostate-Specific Antigen Level (ng/mL) |
||||||||||||||
Gleason Score |
10.1 – 20 Clinical Stage |
>20 Clinical Stage |
||||||||||||
T1a |
T1b |
T1c |
T2a |
T2b |
T2c |
T3a |
T1a |
T1b |
T1c |
T2a |
T2b |
T2c |
T3a |
|
2-4 |
0 |
— |
— |
1 |
3 |
— |
— |
— |
— |
6 |
2 |
7 |
— |
— |
5 |
0 |
5 |
3 |
2 |
6 |
13 |
11 |
— |
— |
9 |
3 |
— |
29 |
— |
6 |
— |
11 |
4 |
5 |
13 |
22 |
20 |
— |
— |
8 |
9 |
18 |
53 |
31 |
7 |
— |
21 |
7 |
9 |
24 |
39 |
35 |
— |
— |
24 |
11 |
44 |
62 |
55 |
8-10 |
— |
41 |
— |
17 |
40 |
59 |
54 |
— |
— |
41 |
35 |
76 |
73 |
65 |
TNM = tumor, nodes, and metastases (cancer staging). |
||||||||||||||
Numbers represent the percentage predictive probability (95% confidence interval) of the patient having a given final pathologic stage based on a multinomial log-liner regression of all 3 variables. [13] |
||||||||||||||
Because of the lower average stage at prostate cancer diagnosis, some have argued that pelvic lymphadenectomy may no longer be necessary in patients undergoing radical prostatectomy. Wyler et al conducted a retrospective review of 123 patients with organ-confined high-risk prostate cancer who underwent LPLND at the time of laparoscopic radical prostatectomy and concluded that LPLND should still be used in patients at high risk for metastasis. [14] In this series, the mean PSA level was 15 ng/mL and the mean Gleason score was 4+3. The investigators found that 17% of patients had lymph node metastases, and the overall complication rate was 4%.
Genitourinary malignancies including bladder, penile, and urethral cancer
Other genitourinary malignancies that require pelvic lymphadenectomy often need a more extensive nodal dissection that includes removing the common iliac, external iliac, and obturator lymph nodes. [15] Transitional cell carcinoma of the bladder with lymphatic spread often has an aggressive clinical course. As with prostate cancer, noninvasive techniques to determine nodal status are inadequate, and pelvic lymphadenectomy is the most accurate way to definitively establish the presence of metastatic disease in the lymph nodes.
Given the poor survival rate in patients with aggressive bladder cancer, those with enlarged lymph nodes on pelvic imaging studies may be candidates for laparoscopic staging before radical cystoprostatectomy. Patients who choose bladder-sparing treatment with chemotherapy, radiation therapy, or partial cystectomy and those who desire orthotopic neobladder construction may also benefit from staging LPLND.
Carcinoma of the penis has a characteristic pattern of nodal spread depending on the location and depth of the primary lesion. [16] Laparoscopic extended pelvic lymphadenectomy may be used in the initial staging of penile cancer. [17]
LPLND in the staging of urethral cancer has been recommended for patients who meet the following criteria:
-
Radiologic evidence of pelvic lymphadenopathy not accessible to fine-needle aspiration biopsy
-
Preceding exenterative surgery in tumors of the proximal urethra
-
Locally invasive distal urethral lesions
The use of laparoscopic pelvic node dissection for bladder cancer, urethral cancer, and penile cancer continues to develop, and further study is necessary to establish definitive indications.
Contraindications to LPLND
Contraindications to LPLND include severe cardiopulmonary disease, bowel obstruction, active infection, and uncorrected coagulopathy.
Conditions that may be considered relative contraindications include morbid obesity, previous abdominal surgery, hiatal hernia, history of pelvic fractures, hip replacement, and large pelvic or intra-abdominal masses. Relative contraindications are related to the surgeon's experience with laparoscopic surgery and LPLND.
Preparation
Preoperative laboratory testing
A preoperative metastatic evaluation is performed in all patients before laparoscopic pelvic lymph node dissection (LPLND). This should include a prostate-specific antigen (PSA) level.
Studies have also investigated the use of several other laboratory markers (ie, serum tartrate-resistant acid phosphatase [TRACP], alkaline phosphatase [ALP], prostatic acid phosphatase [PACP]) that may indicate metastatic disease, including bone metastasis. [18] In these studies, mean serum TRACP, PACP, ALP, and PSA levels were significantly higher in all patients with bone metastases than in those without. PSA and PACP levels increased significantly with the clinical stage of the disease, whereas TRACP and ALP levels increased significantly only in stage D2. Serum TRACP levels correlated significantly with the extent of disease on bone scans.
Of the markers evaluated, PSA, ALP, and TRACP were significant predictors of bone metastasis; using these 3 markers could eliminate the need for almost 70% of bone scans. Consequently, elevations of PSA, ALP, and TRACP levels indicate metastatic disease and may eliminate the need for pelvic node dissection in the staging of patients with prostate cancer. [18]
Preoperative imaging studies
Preoperative metastatic imaging evaluation before laparoscopic pelvic lymphadenectomy includes the following studies:
-
Chest radiography
-
Bone scanning
-
Pelvic imaging with computed tomography (CT) or magnetic resonance imaging (MRI) in some patients. (If the preoperative CT scan reveals lymphadenopathy, fine-needle aspiration can be considered; positive findings may eliminate the need for pelvic node dissection.)
Preparation in the surgical suite
After the induction of general anesthesia, an orogastric tube is placed and pneumatic compression stockings applied to the lower extremities. All pressure points are padded, including the patient's heels and arms. The arms are tucked at the patient's side and secured. The chest and pelvis are secured with strips of 3-inch tape, and a mid-thigh safety strap is used.
A sterile scrub is performed from the xiphoid process to the pubis and from the right to the left anterior axillary lines. The penis and scrotum are prepared and draped into the sterile field. A bladder catheter is placed to decompress the bladder, reducing the risk of injury during trocar placement. If pneumoscrotum is a concern, a sterile gauze roller bandage is wrapped around the penis, scrotum, and catheter to prevent carbon dioxide accumulation in the penis and scrotum, although this is an uncommon complication.
The patient is placed in the supine position, and the operating room table is moved to approximately 10° in the Trendelenburg position for initial Veress needle placement and insufflation. After pneumoperitoneum is established, the patient can be placed in the extreme Trendelenburg position, allowing the intestines to vacate the pelvis.
The operating surgeon stands on the contralateral side of the node dissection, with the first assistant positioned on the ipsilateral side. A video screen placed at the foot of the table allows visualization of both sides of the procedure by all members of the surgical team.
Complication prevention
Some surgeons do not use bowel preparation for laparoscopic surgery. Other surgeons favor bowel preparations before surgery. For those who favor bowel preparation, one regimen is explained here.
Patients are started on clear liquids the afternoon before the procedure, and 2 enemas are administered the evening before surgery. Enemas decompress the colon, facilitating the lymph node dissection. Should a difficult dissection be anticipated based on the patient history, full mechanical and antibiotic bowel preparation may be performed the day before surgery. Broad-spectrum antibiotics are administered parenterally 1 hour before surgery.
Technique
Insufflation of abdominal wall
A Veress needle is introduced into the abdominal cavity through the base of the umbilicus. This site is chosen for initial insufflation, because all fascial layers fuse into one single layer. A relatively low-flow, low-pressure carbon dioxide state (< 8 mm Hg, < 1 L/min) is used until the entire abdominal wall is elevated, indicating proper placement of the Veress needle. Additional ports are placed at a pressure of 20 mm Hg, and pressure is then decreased to 15 mm Hg during the node dissection.
Many different approaches are used to gain access to the abdomen. Veress needle access, the open technique, the Hasson technique, or modified open techniques are all viable options. Once the initial trocar is placed and the underlying tissue is inspected for injury, the secondary ports are placed under direct vision.
The Hasson technique may be useful for initial port placement in patients with a history of extensive or multiple abdominal surgical procedures or a history of peritonitis. The Hasson system consists of a trumpet valve with tying struts, a cone-shaped sleeve, and a blunt-tipped obturator. A 2- to 3-cm incision is made in the skin at the insertion site, the preperitoneal fat is swept off the fascia, and the fascia is incised. The peritoneum is elevated and opened with a pair of forceps and opened sharply. The peritoneum is entered, and the trocar is inserted and secured. The pneumoperitoneum is established through the Hasson trocar, and the abdomen is inspected with the laparoscope.
Trocar placement
Two variations of trocar placement are commonly used in laparoscopic pelvic lymph node dissection (LPLND) (see the image below). The diamond configuration uses two 10-mm ports, one at the umbilicus and one approximately 4-6 cm above the symphysis pubis in the midline. Two 5-mm ports are placed near the McBurney point in the midclavicular line on both sides.
 Two variations of port placement are used commonly, the diamond and the fan configurations. In the diamond configuration (left), two 10-mm trocars are used. One is placed at the umbilicus, and the second is placed approximately 4-6 cm above the symphysis pubis in the midline. Two additional 5-mm trocars are used, one near the McBurney point in the midclavicular line on both sides. In the fan position (right), 5 trocars are used. A 10-mm trocar is placed at the umbilicus for the laparoscope. A second 10-mm trocar is placed on the left side, and a 5-mm trocar is placed on the right side at the level of the umbilicus, lateral to the inferior epigastric vessels, in line with the anterior superior iliac crest. Two additional 5-mm trocars are placed laterally, midway between the umbilicus and the symphysis pubis.
Two variations of port placement are used commonly, the diamond and the fan configurations. In the diamond configuration (left), two 10-mm trocars are used. One is placed at the umbilicus, and the second is placed approximately 4-6 cm above the symphysis pubis in the midline. Two additional 5-mm trocars are used, one near the McBurney point in the midclavicular line on both sides. In the fan position (right), 5 trocars are used. A 10-mm trocar is placed at the umbilicus for the laparoscope. A second 10-mm trocar is placed on the left side, and a 5-mm trocar is placed on the right side at the level of the umbilicus, lateral to the inferior epigastric vessels, in line with the anterior superior iliac crest. Two additional 5-mm trocars are placed laterally, midway between the umbilicus and the symphysis pubis.
If the patient is obese, the fan configuration may be more appropriate. In the fan position, 5 trocars are used. A 10-mm trocar is placed at the umbilicus for the laparoscope. A second 10-mm trocar is placed on the left side, and a 5-mm trocar is placed on the right side at the level of the umbilicus, lateral to the inferior epigastric vessels, in line with the anterior superior iliac crest. Two additional 5-mm trocars are placed laterally, midway between the umbilicus and the symphysis pubis (see the image above).
Pelvic anatomy inspection
A 0° laparoscope is used for trocar placement and inspection of the abdomen. The node dissection is performed with a 30° laparoscope. Start the dissection on the side with the highest likelihood of malignant lymph node involvement. Adhesions are divided sharply to expose the pelvic structures.
The patient is rotated approximately 30° toward the surgeon, elevating the side of initial dissection, and the operating room table is placed in a 25-30° Trendelenburg position, allowing the bowel to fall away from the side of the lymphadenectomy. The medial umbilical ligament, iliac vessels, internal inguinal ring, vas deferens, and cord structures are identified. The line of peritoneal incision is then determined. With the scrotum prepared into the surgical field, the surgeon can apply traction to the testicle on the side of dissection to identify the spermatic cord structures as they enter the internal inguinal ring.
Exposure of external iliac artery and vein
Using electrocautery through the laparoscopic scissors and a curved grasper for counter traction, an incision is made just lateral to the medial umbilical ligament, from the pubic bone to the common iliac artery (see the following image). Dissection of the peritoneum off underlying structures is then accomplished to expose the vas deferens, which is isolated, clipped, and divided between surgical clips. All lymphatic tissue is divided with either electrocautery or ultrasonic energy to prevent lymphocele formation. Placing the point of both laparoscopic instruments in the loose areolar tissue just beneath the incised vas and moving the instruments in opposite directions (one cranially and the other caudally) exposes the underlying external iliac artery and vein. The above operative maneuver not only exposes the external iliac vessels but also moves the ureter cranially, away from the dissection, thus decreasing the likelihood of injury.
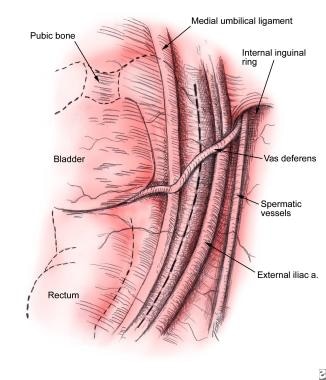 The medial umbilical ligament, iliac vessels, internal inguinal ring, and testicular cord structures are identified. The line of peritoneal incision is just lateral to the medial umbilical ligament. A curved grasper and laparoscopic scissors are used to open the peritoneum along a line from the pubic bone to the common iliac artery.
The medial umbilical ligament, iliac vessels, internal inguinal ring, and testicular cord structures are identified. The line of peritoneal incision is just lateral to the medial umbilical ligament. A curved grasper and laparoscopic scissors are used to open the peritoneum along a line from the pubic bone to the common iliac artery.
The boundaries of the dissection are identical to the open procedure—the circumflex iliac artery inferiorly, the internal iliac artery superiorly, the external iliac vein laterally, and the obturator nerve medially. The lateral dissection starts with identification of the external iliac vein, which is identified by looking for pulsation from the external iliac artery (see the image below). The fatty tissue inferior to the arterial pulsation is elevated, and, using gentle blunt dissection in a cephalocaudal direction, with the tip of the irrigator aspirator, the vein is exposed.
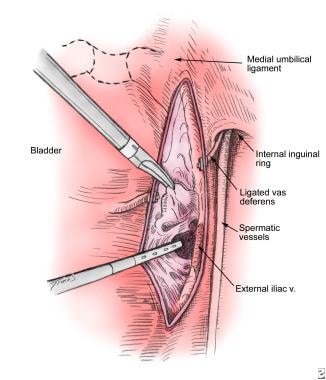 Pulsation from the external iliac artery helps to identify the position of the external iliac vein. The fatty tissue inferior to the arterial pulsation is elevated and bluntly dissected off the anterior surface of the vein in a cephalocaudal direction with the tip of the irrigator aspirator. The vein can then be retracted laterally to expose the underlying lymphatic tissue. The dissection is carried laterally to the pelvic sidewall under the vein.
Pulsation from the external iliac artery helps to identify the position of the external iliac vein. The fatty tissue inferior to the arterial pulsation is elevated and bluntly dissected off the anterior surface of the vein in a cephalocaudal direction with the tip of the irrigator aspirator. The vein can then be retracted laterally to expose the underlying lymphatic tissue. The dissection is carried laterally to the pelvic sidewall under the vein.
The loose connective and lymphatic tissue is elevated off the vein and dissected free from the level of the bifurcation of the common iliac vein to the pubic bone and medially until the obturator internus muscle is observed. At the pubic bone, an accessory obturator vein is often encountered. The dissection should terminate proximal to the branch of the obturator vein to minimize the risk of troublesome bleeding.
The lymphatic tissue can usually be dissected off the pelvic sidewall with a combination of blunt and sharp dissection. The surgical assistant can medially elevate the nodal packet to facilitate dissection from the pelvic wall. Accessory blood vessels and lymphatic channels should be clipped and divided.
Once the lateral portion of the dissection is complete, attention is directed to the portion of the lymph node packet near the medial umbilical ligament and decompressed bladder wall. The assistant retracts the nodal packet laterally to allow development of the plane between the medial umbilical ligament and nodal tissue using blunt dissection. After defining and isolating the medial and lateral borders, the apex of the nodal packet near the pubic bone is clipped and divided. Cephalad retraction of the distal portion of the nodal package then provides a clear view of the obturator nerve (see the following image).
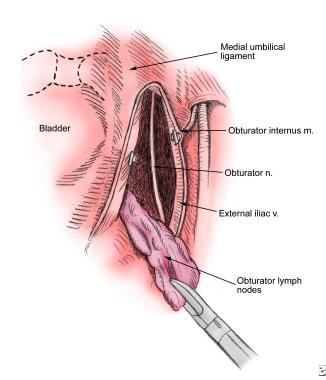 The medial and lateral borders have been developed. The apex of the nodal packet is clipped near the pubic bone. Cephalad retraction of the packet while sweeping bluntly with the irrigator-aspirator tip will tease the packet from the obturator nerve and pelvic sidewall. Large lymphatic channels and small blood vessels can be clipped. The remaining pedicle is clipped, and the packet is freed.
The medial and lateral borders have been developed. The apex of the nodal packet is clipped near the pubic bone. Cephalad retraction of the packet while sweeping bluntly with the irrigator-aspirator tip will tease the packet from the obturator nerve and pelvic sidewall. Large lymphatic channels and small blood vessels can be clipped. The remaining pedicle is clipped, and the packet is freed.
The remainder of the dissection involves cephalad retraction of the nodal package and blunt dissection of the remaining deep portion away from the pelvic sidewall. Keeping the obturator nerve in clear view is important to avoid injury. The superior aspect of the nodal packet is thinned, clipped, and divided.
Tissue extraction
The nodal tissue is removed through a 10/12-mm port, as shown in the image below. To prevent loss of the specimen, all specimens are removed in an impermeable sac.
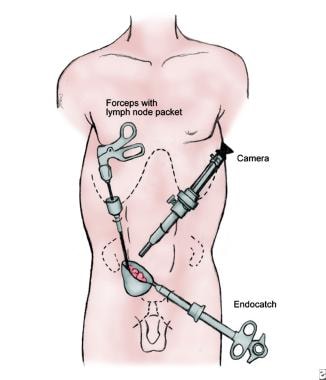 The nodal tissue is removed through one of the 10/12-mm trocar sites. To prevent loss of the specimen and to avoid potential seeding of the tract with tumor from positive lymph nodes, the specimen is retrieved in an impermeable sac.
The nodal tissue is removed through one of the 10/12-mm trocar sites. To prevent loss of the specimen and to avoid potential seeding of the tract with tumor from positive lymph nodes, the specimen is retrieved in an impermeable sac.
The specimen is passed off the field for immediate frozen section if prostatectomy or another treatment is planned under the same anesthetic. Although frozen section evaluation of lymph nodes yields acceptable sensitivity and specificity, the false-negative rate ranges from 15% to 19%. This is an important consideration for those undergoing additional surgery. After completion of the most suspicious side, the contralateral dissection is undertaken with the same technique.
Surgical closure
At the end of the procedure, the intra-abdominal pressure is decreased to 5 mm Hg and the obturator fossa on both sides is inspected carefully for adequate hemostasis. A complete evaluation of the pelvic structures is performed to determine the presence of injury to pelvic viscera or vessels. The fascia of the 10-mm trocar sites is closed under direct vision.
Extended laparoscopic dissection
Bladder, urethral, and penile cancers often require an extended nodal dissection. Unlike the standard or modified pelvic node dissection that removes the obturator and hypogastric nodes only, the extended dissection also incorporates the common and external iliac nodes. [14, 17, 19]
In the extended dissection, the peritoneal incision extends from the pubis (starting lateral to the medial umbilical ligament) and continues cranially along the line of Toldt. The vas deferens is observed over the medial umbilical ligament as it enters the internal inguinal ring. After the vas deferens has been divided, the medial umbilical ligament is traced back to the internal iliac artery and the ureter is identified as it crosses the common iliac vessels.
Lateral border
The lateral border of the dissection is developed from the pubis and the circumflex iliac vein to the level of the common iliac artery and medial to the genitofemoral nerve and external and internal iliac vessels. The dissection proceeds along the anterior border of the common iliac artery. The cephalad extent of the packet is secured with hemoclips and is rolled away from the iliac artery.
The genitofemoral nerve is identified as the lateral dissection continues. Any psoas branches from the common iliac artery are clipped and divided. The common iliac and external iliac arteries are displaced medially, exposing the bifurcation of the common iliac artery and nodal tissue. The obturator nerve is observed passing below this bifurcation. The external iliac vein is then identified, and lymphatic tissue is cleared from the pubis to the bifurcation of the common iliac vessels.
Medial border
The medial border of dissection starts with the ureter at the common iliac artery and continues along the lateral border of the medial umbilical ligament and bladder to the pubis. The lymphatic tissue is pulled from the sidewall using traction and suction from the irrigator aspirator. Large lymphatic channels are clipped and divided. The caudal limit of the extended dissection is the pubic bone. Care must be taken not to injure the superficial epigastric vein running medially and superiorly into the femoral vein.
Posterior border
Posteriorly, the border of dissection is the obturator nerve and internal iliac vessels. Using the irrigator-aspirator tip, the lymph node packet is dissected gently from the pubic ramus, and the obturator nerve is identified. Suction and dissection is applied parallel to the nerve to prevent avulsion injury. The obturator artery and vein are usually found medial to the nerve. Clipping and dividing the obturator vessels is usually unnecessary. The packet is dissected along the obturator nerve until the nerve passes posterior to the iliac vein, where the lateral dissection was performed. Multiple small vessels and lymphatic channels are in the packet under the obturator nerve, which can be clipped and divided. Application of electrocautery in this area results in powerful adduction of the thigh and may lead to inadvertent vascular injury.
Cephalad border
The cephalad margin is the common iliac artery. The branching of the common iliac artery and vein is observed in the lateral dissection. The node dissection continues along the medial surface of the internal iliac artery to the origin of the medial umbilical ligament, and the entire nodal packet is freed; clips are used to secure the pedicle.
Post-Procedure
Complications of laparoscopic pelvic lymph node dissection
Studies have compared postoperative complications of laparoscopic node dissection with those of the mini-laparotomy technique and the modified open technique. [20] Early reports indicated an approximate 15% overall complication rate for the transperitoneal laparoscopic approach. However, as laparoscopic training and experience have progressed, the overall complication rate has fallen and is similar to that associated with the mini-laparotomy approach.
Potential complications of laparoscopic pelvic lymph node dissection include hemorrhage, bladder or ureter injury, bowel perforation, deep venous thrombosis, pulmonary embolus, ileus, bowel obstruction, urinary retention, hypercarbia, obturator nerve injury, wound infection and dehiscence, lymphocele, and conversion to the open procedure. [21, 22, 23]
Most of these potential complications can be avoided with a careful technique, thorough inspection, and adequate repair of noted injuries during the procedure. Bowel perforation can be minimized by limiting the use of electrocautery in the area of the intestine that needs mobilization in order to expose the pelvic lymph nodes. A short burst of electrical energy is sufficient to create a full-thickness injury to the bowel. In addition, abrasions that occur during mobilization of the colon should be covered using horizontal mattress sutures placed laparoscopically. [24]
By carefully identifying the obturator nerve (located in the posterior aspect of the dissection) before placing clips or removing the lymph node packet, injury can usually be avoided. The nerve is injured most commonly during the division of the superior and inferior aspects of the node dissection.
Conversion to open surgery occurs most commonly after the iliac vessels have been injured. Small holes in the artery or vein can sometimes be closed laparoscopically. However, if any doubt exists about the quality of repair, the procedure should be converted to open surgery for closure of the vessel injury.
-
Two variations of port placement are used commonly, the diamond and the fan configurations. In the diamond configuration (left), two 10-mm trocars are used. One is placed at the umbilicus, and the second is placed approximately 4-6 cm above the symphysis pubis in the midline. Two additional 5-mm trocars are used, one near the McBurney point in the midclavicular line on both sides. In the fan position (right), 5 trocars are used. A 10-mm trocar is placed at the umbilicus for the laparoscope. A second 10-mm trocar is placed on the left side, and a 5-mm trocar is placed on the right side at the level of the umbilicus, lateral to the inferior epigastric vessels, in line with the anterior superior iliac crest. Two additional 5-mm trocars are placed laterally, midway between the umbilicus and the symphysis pubis.
-
The medial umbilical ligament, iliac vessels, internal inguinal ring, and testicular cord structures are identified. The line of peritoneal incision is just lateral to the medial umbilical ligament. A curved grasper and laparoscopic scissors are used to open the peritoneum along a line from the pubic bone to the common iliac artery.
-
Pulsation from the external iliac artery helps to identify the position of the external iliac vein. The fatty tissue inferior to the arterial pulsation is elevated and bluntly dissected off the anterior surface of the vein in a cephalocaudal direction with the tip of the irrigator aspirator. The vein can then be retracted laterally to expose the underlying lymphatic tissue. The dissection is carried laterally to the pelvic sidewall under the vein.
-
The medial and lateral borders have been developed. The apex of the nodal packet is clipped near the pubic bone. Cephalad retraction of the packet while sweeping bluntly with the irrigator-aspirator tip will tease the packet from the obturator nerve and pelvic sidewall. Large lymphatic channels and small blood vessels can be clipped. The remaining pedicle is clipped, and the packet is freed.
-
The nodal tissue is removed through one of the 10/12-mm trocar sites. To prevent loss of the specimen and to avoid potential seeding of the tract with tumor from positive lymph nodes, the specimen is retrieved in an impermeable sac.









