Medical Care
In patients with pulmonary atresia with ventricular septal defect (PA-VSD) a ductal-dependent circulation, prostaglandin E2 is often required to keep the ductus arteriosus open in the early neonatal period until surgery can be performed. A neonate who is ill may require fluid and acidosis management, but mechanical ventilation is rarely needed.
Medical treatment with digitalis, diuretics, and other agents may be indicated in patients with congestive heart failure (CHF) resulting from increased pulmonary blood flow. Phlebotomy to relieve the adverse effects of extreme polycythemia in very hypoxic patients is rarely performed. In patients with CHF and increased work of breathing, a high-energy diet is required. Rarely, a patient may require placement of a nasogastric tube to achieve the goals of energy intake.
Consultations
The following specialists can help guide appropriate evaluation and treatment of young patients with PA-VSD [6] :
-
Pediatric cardiologists
-
Pediatric cardiac anesthesiologists
-
Pediatric surgeons
-
Geneticists
Also see guidelines from the European Society of Cardiology (ESC) (2020) for management of adults with PA-VSD.
Surgical Care
There is extreme variation of the anatomy, which will require individualized approach for each patient who has pulmonary atresia with ventricular septal defect (PA-VSD). A lack of consensus exists regarding surgical management of adult patients owing to debate over optimal treatment. [2]
Indications
Criteria for complete surgical repair of PA-VSD are as follows:
-
The native pulmonary arteries provide all or most pulmonary blood flow with oxygen saturations more than 75%.
-
The native pulmonary arteries must supply at least 10 segments, the equivalent of one lung.
-
If additional major collaterals are identified, test the level of arterial oxygen saturation after occlusion of the collaterals in the catheterization laboratory. If the oxygen saturation remains greater than 75%, then coil occlusion of the collaterals is carried out.
-
The Nakata index is used to guide decision making for surgical repair. it is defined as the cross-sectional area (in mm2) of the left and right pulmonary arteries just before the lobar branches, divided by the total body surface area (BSA). Good candidates for complete repair are those with a Nakata index above 200 mm2/BSA.
Contraindications
Contraindications for complete surgical repair of pulmonary atresia with ventricular septal defect include (1) hypoplastic or absent central pulmonary arteries and (2) inadequate peripheral arborization of pulmonary arteries.
Preoperative details
The use of blood is a consideration in any patient who is to undergo a major surgical procedure. Thus, evaluation for the concomitant presence of other medical problems such as DiGeorge syndrome must be performed. [10] DiGeorge syndrome may be present in patients with conotruncal abnormalities such as pulmonary atresia with ventricular septal defect (PA-VSD). When blood is used in these patients, it should be previously irradiated to avoid problems during transfusion.
In a retrospective study, Jia et al assessed the predictive value of preoperative cardiac computed tomography angiography (CTA) for survival in patients with PA-VSD and major aortopulmonary collateral arteries (PA-VSD-MAPCAs). [11] They analyzed PA-VSD-MAPCA patients with preoperative CTA who underwent both right ventricular outflow tract reconstruction and MAPCA unifocalization (n = 24) or pulmonary artery rehabilitation (n = 28). They found that in PA-VSD-MAPCA patients, preoperational high pulmonary vein index (PVI) and native pulmonary artery presence were morphologic predictors of a significant survival advantage. [11]
In a different retrospective review (2004-2017) of data from 65 neonates and young infants, Lee et al found comparable overall survival between staged repair and primary repair, as well as less frequent postrepair reinterventions. [12]
Elhedai et al performed a systematic review and meta-analysis comparison of staged repair (n = 167) versus single-stage complete repair (n = 97) for PA-VSD comprising data from 264 patients. [13] They found higher total mortality and a higher rate of freedom from right ventricular outflow tract reintervention in the group who underwent staged repair. The staged repair and complete repair groups had comparable operative and early postoperative mortality, postoperative ventilation duration, need for postoperative extracorporeal membrane oxygenation (ECMO) support, transcatheter reintervention, unplanned reoperation, and length of hospital stay. [13]
Staged repair
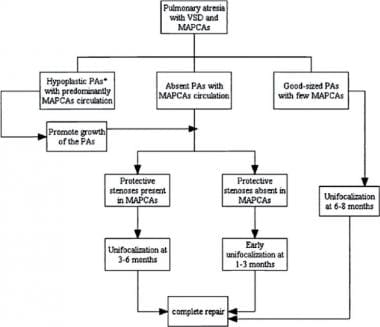 Pulmonary Atresia With Ventricular Septal Defect. Management algorithm for patients with pulmonary atresia with ventricular septal defect (PA-VSD) and major aortopulmonary collateral arteries (MAPCAs), based on the nature of pulmonary vascular supply. PAs = pulmomary arteries. Courtesy of Elsevier (Gupta A, Odim J, Levi D, Chang RK, Laks H. Staged repair of pulmonary atresia with ventricular septal defect and major aortopulmonary collateral arteries: Experience with 104 patients. J Thorac Cardiovasc Surg. 2003;126(6):1746–52).
Pulmonary Atresia With Ventricular Septal Defect. Management algorithm for patients with pulmonary atresia with ventricular septal defect (PA-VSD) and major aortopulmonary collateral arteries (MAPCAs), based on the nature of pulmonary vascular supply. PAs = pulmomary arteries. Courtesy of Elsevier (Gupta A, Odim J, Levi D, Chang RK, Laks H. Staged repair of pulmonary atresia with ventricular septal defect and major aortopulmonary collateral arteries: Experience with 104 patients. J Thorac Cardiovasc Surg. 2003;126(6):1746–52).
The aims of the staged approach (see the image above) in a patient with PA-VSD are to increase the flow of the native pulmonary arteries by establishing a direct continuity between the aorta or the right ventricle (RV) and the small PA, thereby stimulating its growth. This is followed by unifocalization of the collaterals in both lungs and, finally, closing the VSD and establishing RV-PA continuity.
The main advantage of staged palliation is that it breaks the entire repair into smaller and better tolerated procedures.
Increasing pulmonary blood flow can be accomplished either by performing a shunt between the aorta or one of its branches and one of the PAs, or by using a nonvalved conduit between the RV and main PA if it was of adequate size.
Direct aortopulmonary shunts (eg, Waterston shunt, Pott shunt) were used in the past, but these were subsequently found to create severe distortion, scarring, interruption of the PAs, and, on occasion, pulmonary hypertension. Thus, the use of these shunts has fallen into disfavor.
The modified Blalock-Taussig shunt is most commonly used, and it is connected from the subclavian or innominate artery to the PA (when anatomy permits). A central ascending aorta to the main PA shunt (Melbourne shunt) can also be considered in the presence of confluent hypoplastic main PAs.
The second stage involves ligation and transplantation of MAPCAs. Ligation is performed when there is a dual blood supply to the same segment of the lung from a native pulmonary artery as well as from the major aortopulmonary collateral arteries (MAPCAs). MAPCAs need to be transplanted when they are the only source of blood supply to a bronchopulmonary segment, there is no stenosis, and they are not hypertensive. [14]
Diagnostic angiography must be performed to establish the size and distribution of the MAPCAs. Using this road map, the surgeon will then establish anastomotic communications between these vessels and, often, the native PAs.
Initial unifocalization attempts are usually deferred until the patient is age 3-6 months. Early unifocalization around age 1-3 months can be performed when protective stenosis in the MAPCAs is absent and congestive heart failure symptoms continue to worsen despite maximal medical therapy. Flow can also be reduced by percutaneous coil occlusion in the cardiac catheterization laboratory.
The last stage is usually performed between ages 1 to 3 years and involves closure of the VSD with or without fenestration, and establishment of continuity between the RV outflow tract and the PAs. The RV systolic pressure (RVSP) after correction should be less than 75% of that in the left ventricle. [15]
Complete repair
The objective of complete repair is to create an unrestricted continuity between the RV outflow tract and the PA tree using nonvalved or valved conduits. Subsequently, all extracardiac sources of pulmonary blood flow need to be ligated. The atrial septal defects (ASDs) and ventricular septal defects need to be closed. After correction, it is desirable to have a right ventricular pressure that is low, as close to normal as possible.
Various approaches have been devised to achieve a complete surgical repair, including the following:
-
If a patient meets all the criteria for complete repair, single-stage unifocalization of pulmonary blood supply and complete intracardiac repair is the procedure of choice.
-
Single-stage unifocalization and postponement of VSD closure to a second operation is an option.
-
Sequential unilateral unifocalization followed by intracardiac repair is preferred in some patients.
Heart catheterization
More recently, new techniques and devices are being used to treat stenoses and insufficiency of the RV-PA) conduits and/or homografts in these patients. After complete surgical repair of the condition, patients require close follow-up and, on occasion, angioplasties and stent placement to overcome stenotic segments. In current practice, valved stents can be inserted during a heart catheterization, without the need to open the chest, in the presence of severe pulmonary valve insufficiency or stenosis. These percutaneous valves can be implanted using fluoroscopic guidance.
Heart-lung transplantation
In patients with completely atretic main, left, and right PAs, heart-lung transplantation is a viable option.
Complications of surgery include the following:
-
Bronchospasm occurs after the unifocalization surgery because of tracheobronchial epithelial ischemia. This complication significantly contributes to early postoperative morbidity and mortality rates.
-
Pulmonary parenchymal reperfusion injury, pulmonary hemorrhage, and phrenic nerve injury.
-
Aortic regurgitation or aortic root dilation may occur.
-
Restenoses of the shunts and neopulmonary arteries may occur, and subsequent interventions may be required.
Follow-up
Monitor patients for adequacy of repair and postoperative complications. Perform echocardiography on a regular basis, paying special attention to surgically created shunts, residual shunts, and the flow through right ventricular outflow tract conduits. Patients will require antibiotic prophylaxis for subacute bacterial endocarditis (SBE) for an indefinite period.
-
Pulmonary Atresia With Ventricular Septal Defect. Management algorithm for patients with pulmonary atresia with ventricular septal defect (PA-VSD) and major aortopulmonary collateral arteries (MAPCAs), based on the nature of pulmonary vascular supply. PAs = pulmomary arteries. Courtesy of Elsevier (Gupta A, Odim J, Levi D, Chang RK, Laks H. Staged repair of pulmonary atresia with ventricular septal defect and major aortopulmonary collateral arteries: Experience with 104 patients. J Thorac Cardiovasc Surg. 2003;126(6):1746–52).
-
Pulmonary Atresia With Ventricular Septal Defect. Anteroposterior still image obtained from angiography in the aortic arch of a 3-week-old infant born with pulmonary atresia with ventricular septal defect (PA-VSD) who is receiving a prostaglandin E infusion. A patent ductus arteriosus (PDA) is seen supplying confluent branch pulmonary arteries. Courtesy of Dr Thomas Forbes.
-
Pulmonary Atresia With Ventricular Septal Defect. Anteroposterior angiographic view in the aortic arch of a 3-week-old infant born with pulmonary atresia with ventricular septal defect (PA-VSD) who is receiving a prostaglandin E infusion. A patent ductus arteriosus (PDA) is seen supplying confluent branch pulmonary arteries. Courtesy of Dr Thomas Forbes.
-
Pulmonary Atresia With Ventricular Septal Defect. Anteroposterior still image obtained from angiography in a 3.5-mm right modified Blalock–Taussig (BT) shunt in the previous patient at age 4 months. There is a patent BT shunt with mild proximal right upper lobe and right lower lobe branch stenoses. Courtesy of Dr Thomas Forbes.
-
Pulmonary Atresia With Ventricular Septal Defect. Anteroposterior angiographic view in a 3.5-mm right modified Blalock–Taussig (BT) shunt in the previous patient at age 4 months. There is a patent BT shunt with mild proximal right upper lobe and right lower lobe branch stenoses. Courtesy of Dr Thomas Forbes.
-
Pulmonary Atresia With Ventricular Septal Defect. Lateral still image obtained from angiography in the previous patient at age 21 months. The infant underwent Rastelli operation with placement of a 15-mm pulmonary homograft. In this image, there is free homograft insufficiency without stenosis. Courtesy of Dr Thomas Forbes.
-
Pulmonary Atresia With Ventricular Septal Defect. Lateral angiographic view in the previous patient at age 21 months. The infant underwent Rastelli operation with placement of a 15-mm pulmonary homograft. The presence of free homograft insufficiency with no stenosis is observed. Courtesy of Dr Thomas Forbes.
-
Pulmonary Atresia With Ventricular Septal Defect. Lateral still image obtained from angiography in a 7-year-old boy born with pulmonary atresia with ventricular septal defect (PA-VSD) who underwent Rastelli operation with a 17-mm right ventricle to pulmonary artery (RV-PA) homograft. There is mild proximal conduit stenosis and free conduit insufficiency. The right ventricle appears moderately dilated. Courtesy of Dr Thomas Forbes.
-
Pulmonary Atresia With Ventricular Septal Defect. Lateral angiographic view in a 7-year-old boy born with pulmonary atresia with ventricular septal defect (PA-VSD) who underwent Rastelli operation with a 17-mm right ventricle to pulmonary artery (RV-PA) homograft. There is mild proximal conduit stenosis and free conduit insufficiency. The right ventricle appears moderately dilated. Courtesy of Dr Thomas Forbes.
-
Pulmonary Atresia With Ventricular Septal Defect. Lateral still image from angiography in the previous patient at age 8 years following Melody valve placement in the prestented 17-mm right ventricle to pulmonary artery (RV-PA) homograft. The Melody valve appears in good position. There is no Melody valve insufficiency. Courtesy of Dr Thomas Forbes.
-
Pulmonary Atresia With Ventricular Septal Defect. Lateral angiographic view in the previous patient at age 8 years following Melody valve placement in the prestented 17-mm right ventricle to pulmonary artery (RV-PA) homograft. The Melody valve appears in good position. There is no Melody valve insufficiency. Courtesy of Dr Thomas Forbes.
-
Pulmonary Atresia With Ventricular Septal Defect. Left anterior oblique ventriculogram in a patient (same patient as in the next image) with pulmonary atresia with ventricular septal defect (PA-VSD). The angiogram shows the left and right ventricles with a large malalignment VSD between them. The only outflow from the heart is the aorta. No evidence of pulmonary blood flow is observed arising from the ventricles directly to the lungs. Asc Ao = ascending aorta; Desc Ao = descending aorta; LV = left ventricle; and RV = right ventricle.
-
Pulmonary Atresia With Ventricular Septal Defect. Anteroposterior view of an aortogram in a patient (same patient as in the previous image) with pulmonary atresia with ventricular septal defect (PA-VSD). The pulmonary circulation is supplied by collateral vessels (Collaterals) that arise from the descending aorta (Desc Ao).
-
Pulmonary Atresia With Ventricular Septal Defect. Short-axis parasternal view (1) and diagram (3) in a patient with pulmonary atresia and ventricular septal defect (PA-VSD). Short-axis parasternal view (2) and diagram (4) in a patient with normal anatomy. LA = left atrium; PA = pulmonary artery; PV = pulmonary valve; RA = right atrium; RV = right ventricle; and TR = tricuspid valve.







