Approach Considerations
The first step in evaluating the child with edema is to establish whether nephrotic syndrome is present, because hypoalbuminemia can occur in the absence of proteinuria (such as protein-losing enteropathy), and edema can occur in the absence of hypoalbuminemia (for example, in angioedema, capillary leak, venous insufficiency, or congestive heart failure).
In order to establish the presence of nephrotic syndrome, laboratory tests should confirm (1) nephrotic-range proteinuria, (2) hypoalbuminemia, and (3) hyperlipidemia. Therefore, initial laboratory testing should include the following [66] :
-
Urinalysis
-
Urine protein quantification (by first-morning urine protein/creatinine ratio or 24-hour urine protein measurement)
-
Serum albumin
-
Lipid panel
Once the diagnosis of nephrotic syndrome has been established, the next step is to determine whether the nephrotic syndrome is primary (idiopathic) or secondary to a systemic disorder and, if idiopathic nephrotic syndrome (INS) has been confirmed, whether signs of chronic kidney disease, kidney insufficiency, or other renal disorders exclude the possibility of minimal change nephrotic syndrome (MCNS). Therefore, in addition to the above tests, the following should be selectively included in the workup [66] :
-
Complete blood cell (CBC) count
-
Metabolic panel (serum electrolytes, blood urea nitrogen [BUN] and creatinine, calcium, phosphorus, and ionized calcium levels)
-
Testing for human immunodeficiency virus (HIV), hepatitis B and C viruses
-
Complement studies (C3, C4)
-
Antinuclear antibody (ANA), anti–double-stranded DNA antibody, anti-neutrophil cytoplasmic antibodies (in selected patients)
Patients with INS lose vitamin D–binding protein, which can result in low vitamin D levels, and thyroid-binding globulin, which can result in low thyroid hormone levels. Consideration should be given, especially in the child with frequently relapsing or steroid-resistant nephrotic syndrome, to testing for 25-OH-vitamin D; free T4; and thyroid-stimulating hormone (TSH).
Other tests and procedures in selected patients may include the following:
-
Genetic studies
-
Kidney ultrasonography
-
Chest radiography
-
Mantoux test or Quantiferon gold test to exclude tuberculosis prior to initiation of corticosteroid
-
Kidney biopsy (age < 1 year or >12 years, or other selected circumstances)
Age is an important factor in the diagnostic evaluation of nephrotic syndrome. Children younger than 1 year who present with nephrotic syndrome should be evaluated for congenital/infantile nephrotic syndrome. In addition to the tests listed above, infants should have the following tests:
-
Congenital infection (syphilis, rubella, toxoplasmosis, cytomegalovirus infection, HIV infection)
-
Kidney biopsy
-
Genetic tests for NPHS1, NPHS2, WT1, and PLCE1 as guided by biopsy findings and clinical presentation; presence of extrarenal syndromic findings might indicate other genetic testing, such as LAMB2 (Pierson syndrome), LMX1B (nail-patella syndrome), and SMARCAL1 ( Schimke immuno-osseous dysplasia)
Occasionally, a patient with nephrotic syndrome either presents with or develops clinical signs of an acute abdomen, which is frequently due to peritonitis. The diagnosis can usually be made clinically and confirmed by bacteriologic examination of the peritoneal fluid aspirate. The organism most often responsible for the peritonitis is Streptococcus pneumoniae; however, enteric bacteria may also cause peritonitis. Treatment is medical rather than surgical. The clinical picture of an acute abdomen can also occur in extreme hypovolemia.
Urine Studies
Microscopic hematuria is present in 20% of cases of INS and cannot be used to distinguish between MCNS and other forms of glomerular disease.
Red blood cell casts, if present, are suggestive of acute glomerulonephritis, such as postinfectious nephritis, or a nephritic presentation of chronic glomerulonephritis, such as membranoproliferative glomerulonephritis (MPGN). Granular casts may be present and are non-specific as to etiology.
The presence of macroscopic (gross) hematuria is unusual in MCNS and suggests another cause, such as MPGN, or a complication of INS, such as renal vein thrombosis.
Lipiduria may be seen in the form of detached lipid laden renal tubular epithelial cells (oval fat bodies).
Urine protein quantification
A first-morning urine protein/creatinine ratio is more easily obtained in young children than a 24-hour urine study, is possibly more reliable, and excludes orthostatic proteinuria. A urine protein/creatinine ratio of more than 2-3 mg/mg is consistent with nephrotic-range proteinuria.
A 24-hour urine protein level of more than 40 mg/m2/hr also defines nephrotic-range proteinuria.
Blood Studies
Serum albumin levels in nephrotic syndrome are generally less than 2.5 g/dL. Values as low as 0.5 g/dL may occur.
Lipid panel findings are typically as follows:
-
Elevated total cholesterol, low-density lipoprotein (LDL)-cholesterol
-
Elevated triglycerides with severe hypoalbuminemia
-
High-density lipoprotein (HDL)-cholesterol (normal or low)
The patient with INS, even MCNS, can present with acute kidney injury as a result of intravascular volume depletion or bilateral renal vein thrombosis. Elevated BUN and creatinine levels and signs of chronic kidney disease (such as poor growth, anemia, acidosis, hyperkalemia, hyperphosphatemia, and elevated parathyroid hormone) suggest a chronic glomerular disease other than MCNS, such as one of the following:
-
Membranous nephropathy (MN)
-
MPGN
-
Immunoglobulin (Ig)A nephropathy
Serum sodium levels may be low in patients with INS because of hyperlipidemia (pseudohyponatremia), as well as dilution due to water retention. Total calcium levels are low because of hypoalbuminemia, but ionized calcium levels are normal.
An increased hemoglobin level and hematocrit indicate hemoconcentration and intravascular volume depletion. The platelet count is often increased.
HIV, hepatitis B virus, and hepatitis C virus are important secondary causes of nephrotic syndrome. Consequently, screening for these viruses should be performed in selected patients. Consider checking liver enzymes, such as alanine aminotransferase (ALT) and aspartate aminotransferase (AST), when screening for liver disease.
Low complement levels (C3, C4) are found in postinfectious glomerulonephritis, C3 glomerulonephritis/MPGN, and lupus nephritis.
ANA and anti–double-stranded DNA antibody assays are used to screen for SLE in patients with systemic symptoms (fever, rash, weight loss, joint pain) or in any patient with nephrotic syndrome who presents in later school-age or adolescent years when the incidence of lupus is higher.
Genetic Testing
Genetic testing is especially useful in syndromic nephrotic syndrome (NS), congenital and infantile NS, and steroid-resistant nephrotic syndrome (SRNS). The detection of a monogenic cause of NS can prevent the unnecessary use of immunosuppressive medications since genetic NS is generally unresponsive to such treatment. Conversely, the absence of a known genetic cause might justify further treatment with non-steroidal immunosuppressive agents.
Additionally, knowledge of the presence or absence of a genetic cause can allow the practitioner to offer guidance to famillies regarding clinical course and prognosis, as well as the risk of recurrence after transplantation. Finally, identification of a known genetic cause for NS may allow further genetic counseling regarding family planning and antenatal screening. [67] Because approximately 30% of children with SRNS may have a single-gene cause of their disease, it is recommended that all children with SRNS (as well as all patients with congenital, infantile, and syndromic NS) undergo genetic testing when possible. [6]
High-throughput, next-generation sequencing (NGS) has made genetic testing more accurate, cost-efficient, timely, and practical. NGS allows screening of large gene panels for monogenic causes of NS. [68] The figure below outlines a practical approach to genetic tesing in childhood NS.
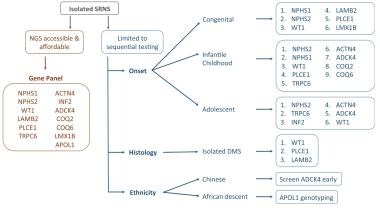 Mutational screening in children with isolated steroid-resistant nephrotic syndrome (SRNS). If next-generation sequencing (NGS) technology is accessible, screening should utilize a gene panel including, but not limited to, the most common monogenic causes of SRNS. If NGS technology is not available, genes should be screened in numerical order of frequency per age group. Ethnicity and histologic findings should trigger preferential screening of certain genes. DMS = diffuse mesangial sclerosis. Courtesy of Pediatric Nephrology (Open Access journal) [Preston R, et al. Genetic testing in steroid-resistant nephrotic syndrome: why, who, when and how? Pediatr Nephrol. 2019 Feb;34(2):195-210.]
Mutational screening in children with isolated steroid-resistant nephrotic syndrome (SRNS). If next-generation sequencing (NGS) technology is accessible, screening should utilize a gene panel including, but not limited to, the most common monogenic causes of SRNS. If NGS technology is not available, genes should be screened in numerical order of frequency per age group. Ethnicity and histologic findings should trigger preferential screening of certain genes. DMS = diffuse mesangial sclerosis. Courtesy of Pediatric Nephrology (Open Access journal) [Preston R, et al. Genetic testing in steroid-resistant nephrotic syndrome: why, who, when and how? Pediatr Nephrol. 2019 Feb;34(2):195-210.]
When gene panel testing is not available, patients with infantile or congenital NS should be tested for mutations in NPHS1 and WT1. If the results are normal, testing for mutations in NPHS2 and PLCE1 should be considered. Although LAMB2 and LMX1B are generally associated with syndromic NS, isolated cases of congenital NS have been reported. Genetic testing for WT1 mutations also should be considered in patients who present with NS and extrarenal features of Denys-Drash syndrome (NS, pseudohermaphroditism or genitourinary tract anomalies, and Wilms tumor) or Frasier syndrome (NS, pseudohermaphroditism or genitourinary tract anomalies, and gonadoblastoma).
Infants with NS and neurologic or visual disturbances should be considered candidates for LAMB2 testing (Pierson syndrome). [30] The presence of other extrarenal findings might indicate further genetic testing, such as LMX1B (nail-patella syndrome) and SMARCAL1 (Schimke immuno-osseous dysplasia).
In patients who are initially or subsequently unresponsive to steroid treatment, in addition to kidney biopsy, consideration should be given to testing for mutations in podocin (NPHS2), ACTN4, and TRPC6.
Imaging Studies
Kidney ultrasonography
Kidney ultrasonography findings are usually nonspecific. The kidneys are typically enlarged due to interstitial edema. Increased echogenicity is usually indicative of medical renal disease other than MCNS, in which echogenicity is usually normal. A finding of small kidneys suggests chronic kidney disease other than MCNS and is often accompanied by elevated serum creatinine levels.
Chest radiography
Chest radiography is indicated in the child with respiratory symptoms. Pleural effusions are common although pulmonary edema is rare.
Chest radiography also should be considered before steroid therapy to rule out tuberculosis (TB) infection, especially in the child with a history of exposure to an affected close relative, a positive or previously positive Mantoux test, or prior treatment for TB.
Mantoux Test
A Mantoux test (purified protein derivative [PPD] test) should be performed before steroid treatment to rule out TB infection.
Mantoux testing can be performed concurrent to starting steroids, as treatment with steroids for 48 hours prior to reading the PPD test does not mask a positive result and the risk associated with 2 days of steroid therapy is minimal. If test results are positive, steroids should be stopped immediately.
In children with a positive PPD test, previously positive PPD test, or prior treatment for TB, chest radiography should be performed.
Kidney Biopsy
A kidney biopsy is not indicated for the first presentation of INS in children aged 1-12 years unless the history, physical findings, or laboratory results indicate the possibility of secondary nephrotic syndrome or primary nephrotic syndrome other than MCNS. Kidney biopsy is indicated in patients younger than 1 year, when genetic forms of congenital nephrotic syndrome are more common, and in patients older than 12 years, when chronic glomerular diseases such as FSGS have a higher incidence. [69]
A kidney biopsy is also indicated if the patient has any of the following:
-
Symptoms of systemic disease (eg, fever, rash, joint pain)
-
Laboratory findings indicative of secondary nephrotic syndrome (eg, positive ANA result, positive anti–double-stranded DNA antibody findings, low complement levels)
-
Elevated creatinine levels unresponsive to correction of intravascular volume depletion
-
A relevant family history of kidney disease
Finally, in patients who are initially or subsequently unresponsive to steroid treatment, kidney biopsy should be performed, because steroid unresponsiveness has a high correlation with prognostically unfavorable histopathological findings, such as those associated with FSGS or membranous glomerulonephritis (MGN).
Histologic Findings
If a kidney biopsy is performed, various histopathological findings can be present, depending on the etiology of the nephrotic syndrome. A detailed discussion of the various types of INS and histologic findings is beyond the scope of this article. Briefly, the most common histologic types of INS are as follows.
Minimal change nephrotic syndrome
MCNS indicates glomerular morphology that on light microscopic examination is little different from normal. Minimal mesangial hypercellularity may be present. Immunofluorescent microscopy (IF) is usually negative for Ig deposits.
Occasionally, mesangial IgM deposition may be seen on IF. Some consider the presence of IgM to indicate a separate entity (IgM nephropathy), whereas others consider this to be a variant of MCNS. The presence of IgM may indicate a more difficult course of nephrotic syndrome, with frequent relapses, steroid dependence, or steroid resistance, although the overall prognosis is still usually favorable. The only significant change seen on electron microscopy (EM) is effacement of discrete podocyte foot processes and the appearance of a continuous sheet of cytoplasm. [70]
Diffuse mesangial proliferation
Diffuse mesangial proliferation (DMP) refers to increased mesangial matrix and increased mesangial hypercellularity. IF findings are negative and EM reveals the typical foot process effacement of MCNS. Patients with DMP have an increased incidence of steroid resistance, although whether these patients are at increased risk for progression to kidney failure is unclear. [70]
Focal glomerulosclerosis
FSGS describes a lesion in which, as seen on light microscopy (LM), discrete segments of the glomerular tuft reveal sclerosis (segmental); some glomeruli are involved, and others are spared (focal).
Adhesion of the glomerular tuft to the Bowman capsule (synechiae) is observed. Glomerular hypertrophy is common. Interstitial fibrosis and tubular atrophy are often present and correlate with the severity of disease.
IF reveals IgM and C3 trapped in the sclerotic areas. As in MCNS, EM reveals effacement of the podocyte foot processes. Additionally, EM reveals obliteration of capillary lumens by fine granular and lipid deposits.
A subtype of FSGS, in which the glomerular tufts demonstrate collapse of capillaries (collapsing glomerulopathy) on LM, has a poorer prognosis and high rate of progression to end-stage kidney disease (ESKD).
FSGS is not a specific disease entity but a pattern of injury that can be associated with INS but can also be found in a wide variety of other conditions, including HIV nephropathy, heroin nephropathy, reflux nephropathy, obstructive uropathy, renal hypoplasia, hypertension, obesity, and Alport syndrome.
As always, clinical and histopathologic correlations must be made when considering the findings evident on kidney biopsy. [70]
Membranoproliferative glomerulonephritis
MPGN is also known as mesangiocapillary glomerulonephritis. Glomeruli are typically lobulated in appearance on LM and demonstrate mesangial proliferation. Silver staining may reveal characteristic duplication of the glomerular basement membrane ("tram-track" appearance). IF findings reveal characteristic capillary deposition of C3.
Three types of MPGN are recognized and can be distinguished by EM findings according to the location of immune deposits. Type 1 is subendothelial; type 2 has ribbon-like, dense intramembranous deposits; and type 3 is subendothelial and subepithelial. Some controversy surrounds the existence of type 3 MPGN as a distinct entity or a variant of type 1. [71]
Membranous nephropathy
MN is a rare finding in INS of childhood, comprising only approximately 1% of biopsies, whereas in adult INS, MN can be found in 25-40% of cases. LM typically reveals thickening of the glomerular basement membrane. Silver staining may demonstrate characteristic "spikes," resulting from protrusion of basement membrane around immune deposits. IF shows fine granular IgG and complement staining along the periphery of the glomerular capillary wall. EM reveals subepithelial electron-dense deposits. [72]
Histopathologic staging
Various staging schemes are recognized for the different histologic lesions of INS. In general, when referring to kidney biopsy, the severity and chronicity of the disease is determined by the extent of tubulointerstitial fibrosis. The greater the extent of fibrosis, the greater the irreversibility of the disease and the poorer the prognosis, regardless of histolopathological subtype.
-
Schematic drawing of the glomerular barrier. Podo = podocytes; GBM = glomerular basement membrane; Endo = fenestrated endothelial cells; ESL = endothelial cell surface layer (often referred to as the glycocalyx). Primary urine is formed through the filtration of plasma fluid across the glomerular barrier (arrows); in humans, the glomerular filtration rate (GFR) is 125 mL/min. The plasma flow rate (Qp) is close to 700 mL/min, with the filtration fraction being 20%. The concentration of albumin in serum is 40 g/L, while the estimated concentration of albumin in primary urine is 4 mg/L, or 0.1% of its concentration in plasma. Courtesy of the American Physiological Society (www.the-aps.org) [Haraldsson B, Nystrom J, Deen WM. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev. 2008 Apr;88(2):451-87.]
-
Mutational screening in children with isolated steroid-resistant nephrotic syndrome (SRNS). If next-generation sequencing (NGS) technology is accessible, screening should utilize a gene panel including, but not limited to, the most common monogenic causes of SRNS. If NGS technology is not available, genes should be screened in numerical order of frequency per age group. Ethnicity and histologic findings should trigger preferential screening of certain genes. DMS = diffuse mesangial sclerosis. Courtesy of Pediatric Nephrology (Open Access journal) [Preston R, et al. Genetic testing in steroid-resistant nephrotic syndrome: why, who, when and how? Pediatr Nephrol. 2019 Feb;34(2):195-210.]
Tables
What would you like to print?
- Overview
- Presentation
- DDx
- Workup
- Treatment
- Approach Considerations
- Corticosteroid Therapy
- Diuretic Therapy
- Antihypertensive Therapy
- Home Monitoring
- Frequently Relapsing and Steroid-Dependent Disease
- Steroid-Resistant Disease and Focal Segmental GS
- Investigational Treatments
- Side Effects of Drug Therapy
- Indications for Hospital Admission
- Diet and Activity
- Vaccination
- Consultations and Long-Term Monitoring
- Show All
- Guidelines
- Medication
- Questions & Answers
- Media Gallery
- References