Advertisement
Advertisement
August 2021
Interventional Approach to Renal Mass Incidentally Found on Imaging
Moderator: Gloria Salazar, MD;
Panelists: Kirema I. Garcia-Reyes, MD; and Vincent Vidal, MD, PhD
CASE PRESENTATION
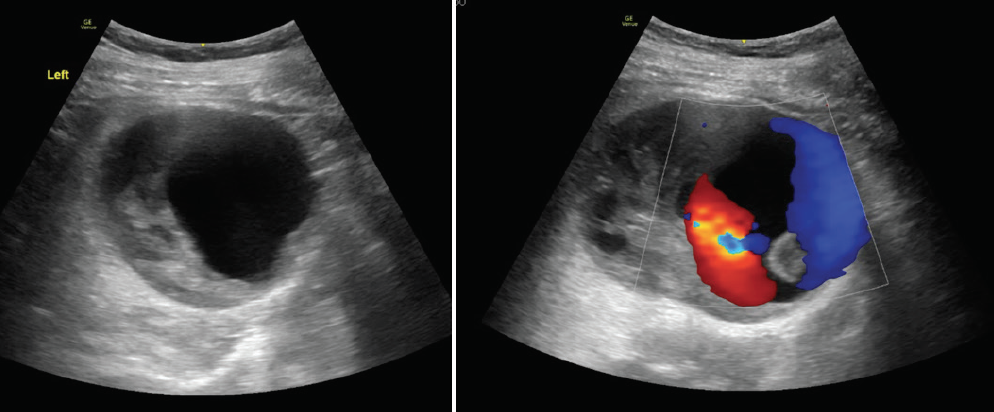
A woman in her late 60s with a history of hyperlipidemia and hypertension presented with left leg pain for the past 4 months. A mass was detected incidentally on an ultrasound performed for recurrent urinary tract infections (UTIs) (Figure 1). She had been experiencing recurrent UTIs for the past 5 to 6 years. She reported no abdominal or flank pain.
How would you approach this case? Would you require any additional consults? Is there any role for surgical resection?
Dr. Vidal: In this case, I suspect a diagnosis of renal sporadic angiomyolipoma (AML) because they are unilateral, typically identified in adults with a mean age of 45 years, and have a strong female predilection (female/male ratio of 4:1). I would admit the patient to the hospital, meet with her to discuss the options, and confirm the diagnosis with additional imaging, excluding other diagnoses such as malignant tumors.
Based on the images provided, it appears that there is a direct link with the vein, but it is not a typical arteriovenous fistula (AVF) because there is no large dilated drainage vein. My hypothesis is that a small aneurysm bled and created a “false communicating aneurysm” in the hematoma. I wonder why the patient did not report any flank pain. Pain in the leg is not a classic symptom, even with large AML.
For now, I think that there is no role for surgical resection, but I would share this case with urologist colleagues to get a multidisciplinary perspective.
Dr. Garcia-Reyes: I would urgently obtain a CTA of the abdomen and pelvis to further characterize the ultrasound findings of a mass with a pseudoaneurysm. Without further information, it is too early to request any additional consults or plan for surgical excision before pursuing additional imaging. Due to the risk of pseudoaneurysm rupture, I would consider admission to expedite the workup and treatment.
CASE CONTINUED
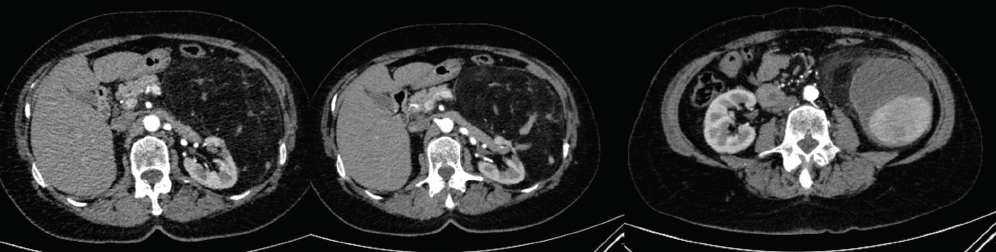
CTA and digital subtraction angiography were performed (Figure 2 and Figure 3), demonstrating enlarged arterially enhancing mass, consistent with a large aneurysm, and with areas of thrombosis within the sac. In addition, there were fat-containing lesions consistent with AML.
What would you do next? Would you attempt percutaneous direct puncture or endovascular puncture?
Dr. Garcia-Reyes: CTA revealed a large, fat-containing mass arising from the left kidney, which is most compatible with a giant renal AML, and a large, partially thrombosed pseudoaneurysm along its inferior margin. Renal AMLs are at risk of spontaneous hemorrhage, especially if > 4 cm or if they have internal pseudoaneurysms, which puts this patient at a high risk for bleeding. She needs intervention as soon as possible, and I would favor an endovascular approach to concomitantly treat both.
Dr. Vidal: In this approach, I prefer endovascular versus direct puncture because I am interested to see the global angio architecture of the entire kidney. I would like to first verify that there is no large drainage vein. Second, I would verify if the site of the fistula is dependent or not on the vascularization of the AML. The remaining question is whether both the fistula and the AML be treated at the same time. I would favor addressing both directly.
Given the angiographic findings with venous fistula, what embolic agents would you favor?
Dr. Vidal: I would choose an embolic agent with a strong anchoring, such as a short plug or detachable coils that are well oversized (25%). The implantation will be as close as possible to the link site. If the flow continues after 10 minutes, I would probably finish occlusion by gently anchoring a small amount of Onyx 34 (Medtronic) in the packing of the coils. The goal is to have the smallest length of coiling in the artery to avoid renal infarction.
Dr. Garcia-Reyes: I routinely treat renal AMLs with either an ethanol–ethiodized oil mixture or small particles. However, in the setting of an AVF or significant venous shunting, I would steer away from those embolics. I would treat the large pseudoaneurysm first, likely with coils, and reassess flow with a repeat angiogram postembolization. If significant shunting is still present, I would attempt to catheterize the shunt and shut it down, controlling the outflow with balloon occlusion. If there is persistent flow to the AML once the fistula is closed, I would consider using N-butyl cyanoacrylate–ethiodized oil or ethylene vinyl alcohol copolymer to treat the mass.
APPROACH OF THE MODERATOR
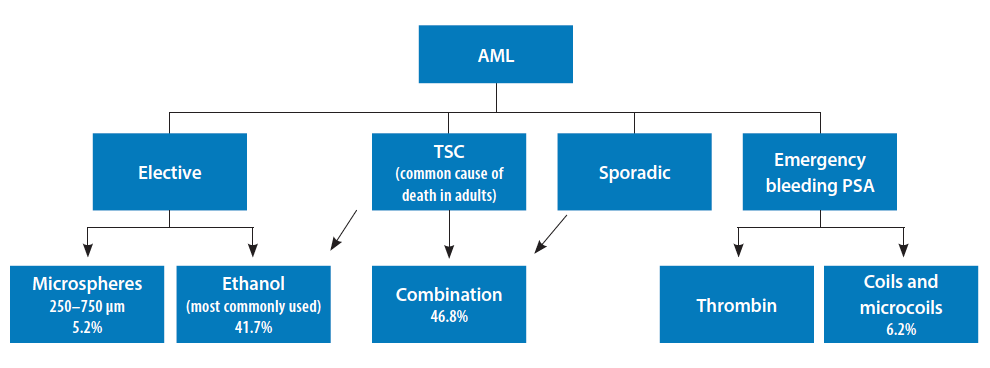
Given the imaging findings, the differential diagnosis in this patient included sporadic AML or liposarcoma associated with renal pseudoaneurysm. Drs. Garcia-Reyes and Vidal provided pertinent discussion about the workup and management of this patient with a complex AML, highlighting the trade-off in choosing embolic agents that provide thrombogenicity without the risk of nontargeted embolization. Therefore, given that AML is the most common benign renal tumor, and it has a size-related propensity to hemorrhage,1 we elected to perform an endovascular approach to further evaluate the mass and determine the extent of possible venous drainage pattern, as already mentioned by this panel. Transcatheter arterial embolization of AMLs is a minimally invasive treatment for both emergent and prophylactic indications.2 In patients with AML, the risk of hemorrhage is most strongly related to aneurysm formation and extent of internal vascularity rather than size. Superselective embolization avoids injury to the normal renal parenchyma while devascularizing the AML.3 An algorithm for the choice of embolic materials for treatment of AMLs is shown in Figure 4.4-7
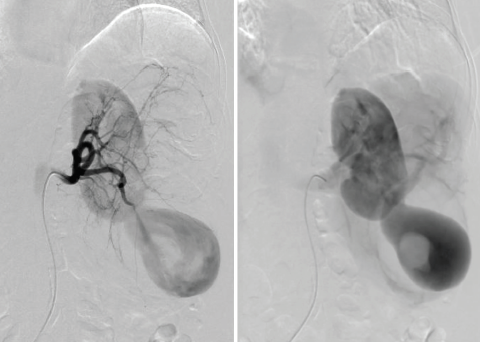
In this patient, selective left renal arteriography demonstrated a large pseudoaneurysm with shunting to the renal vein and inferior vena cava (Figure 3).
Given the size and venous drainage pattern, we had the following concerns: (1) Which embolic agent is most appropriate and safe to use in this patient, and (2) should we do additional evaluation of the venous drainage? Subsequently, using a microcatheter, superselective catheterization of the lower pole branches was performed with a 0.021-inch microcatheter, and access was gained into the pseudoaneurysm sac with demonstration of a large communication with venous drainage into the renal vein (Figure 5A) postcontrast injection. At that point, we elected to use Concerto 3D and Helix detachable large-diameter coils with long lengths (Medtronic), oversizing so that coils would not extend into the draining veins. In addition, we placed a balloon occlusion catheter from a transfemoral venous approach to avoid nontargeted embolization of coils. We then proceeded to use a combination of Concerto 3D and Helix coils (Figure 5B) ranging from 8 to 20 mm in diameter in long lengths (up to 50 cm) until occlusion of pseudoaneurysm sac was achieved (Figure 5C).
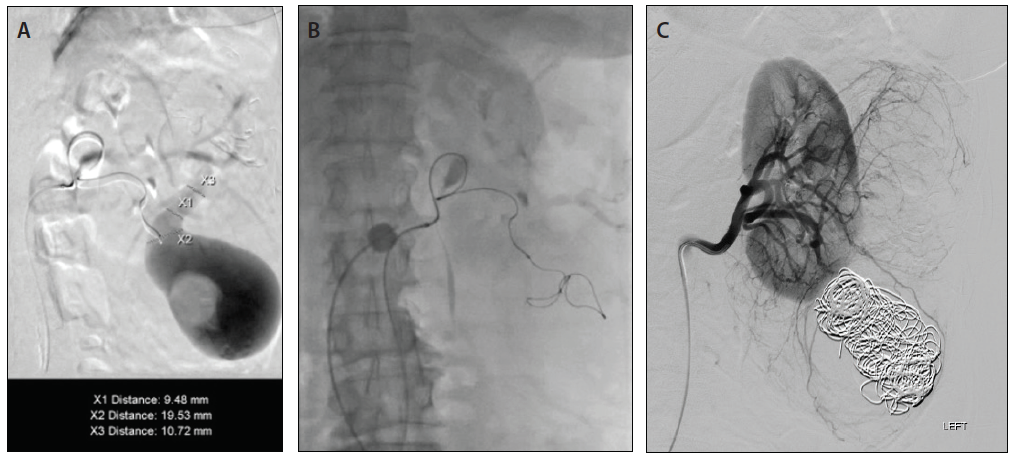
Figure 5. Superselective catheterization of aneurysmal sac demonstrating large filling defect consistent with thrombosis and a large communicating draining vein, diameters ranging from 9.4 to 19.5 mm (A). Deployment of a Concerto 3D (20-mm diameter) coil into the aneurysmal sac (B). Final left renal arteriography after coiling of aneurysmal sac demonstrated occlusion of pseudoaneurysm without venous drainage and mid pole AML, treated at a later intervention (C).
In summary, we presented a complex renal pseudoaneurysm with venous shunting in which we evaluated the trade-off between volume filling and thrombosis of pseudoaneurysm with stable delivery against nontargeted embolization. Therefore, we elected to use large-diameter coils and lengths with precise positioning, framing, and packing of pseudoaneurysm since we were unable to use liquid embolics.
1. Rimon U, Duvdevani M, Garniek A, et al. Large renal angiomyolipomas: digital subtraction angiographic grading and presentation with bleeding. Clin Radiol. 2006;61:520-526. doi: 10.1016/j.crad.2006.02.003
2. Chatziioannou A, Gargas D, Malagari K, et al. Transcatheter arterial embolization as therapy of renal angiomyolipomas: the evolution in 15 years of experience. Eur J Radiol. 2011;81:2308-2312. doi: 10.1016/j.ejrad.2011.06.003
3. Chan CK, Yu S, Yip S, et al. The efficacy, safety and durability of selective renal arterial embolization in treating symptomatic and asymptomatic renal angiomyolipoma. Urology. 2011;77:642-648. doi: 10.1016/j.urology.2010.08.040
4. Shepherd CW, Gomez MR, Lie JT, et al. Causes of death in patients with tuberous sclerosis. Mayo Clin Proc. 1991;66:792-796. doi: 10.1016/s0025-6196(12)61196-3
5. Murray TE, Doyle F, Lee M. Transarterial embolization of angiomyolipoma: a systematic review. J Urol. 2015;194:635-639. doi: 10.1016/j.juro.2015.04.081
6. Takebayashi S, Horikawa A, Arai M, et al. Transarterial ethanol ablation for sporadic and non-hemorrhaging angiomyolipoma in the kidney. Eur J Radiol. 2009;72:139-145. doi: 10.1016/j.ejrad.2008.06.017
7. Corso R, Carrafiello G, Rampoldi A, et al. Pseudoaneurysm after spontaneous rupture of renal angiomyolipoma in tuberous sclerosis: successful treatment with percutaneous thrombin injection. Cardiovasc Intervent Radiol. 2005;28:262-264. doi: 10.1007/s00270-004-0154-x
Advertisement
Advertisement