Asam gelugor Leaves
Garcinia atroviridis Griff. ex. T. Anderson
Clusiaceae
DEFINITION
Asam gelugor leaves consist of the powder of dried leaves of Garcinia atroviridis (Clusiaceae).
SYNONYM
Information and data have not been established.
VERNACULAR NAMES
Asam gelugor (English); asam gelugur, asay gelugor, gelugor (Malay); suan zhu (Chinese) [1, 2, 3, 4]
CHARACTER
| Colour | Dark green |
| Odour | Odourless |
| Taste | Bitter |
IDENTIFICATION
Plant morphology
G. atroviridis is a perennial tree growing to a height of more than 10-25 m. Trunk is long hardwood, fluted at the base with dull grey, carcked and fissured or smooth grey bark and drooping branches; inner bark produces a little transparent or yellow sap. Leaves coriaceous, pointed at the tip and abruptly tapered at the base, 15 x 4cm – 25 x 7 cm, elongated oval, reddish young shoots when young and glossy dark green when mature. Inflorescence pedicellate, borne on the terminal of the twigs; calyx 4, yellow in colour, concave in shape and spreading orbicular; corolla 4 crimson, obovate and fleshy. Male flowers in short few-flowered racemes with numerous stamens in whorls round the pistillode on a fleshy receptacle. Female flowers solitary, large with an ovoid ribbed, 8-16 celled ovary; stigma, pileate, subtetragonal, convex in shape with deep red in colour; staminodes attached to an annulus. Fruits depressed globose, 6-10cm diameter with broadly sunken concave apex, with 12-16 ribs and shallow grooves, green when young and turn to bright yellow when ripe. Seeds 1.5cm long, surrounded by acidic, bright orange pulp [2, 5, 6].
Microscopy
Powdered material consists of adaxial epidermis cells with straight to curve anticlinal wall and abaxial epidermis cells with straight to wavy anticlinal wall, paracytic stomata cells on the abaxial surface; fragment of parenchyma cells and abundant of calcium oxalate crystals (solitary and druse forms). Other features observed include the presence of fiber; annular and reticulate vessels.
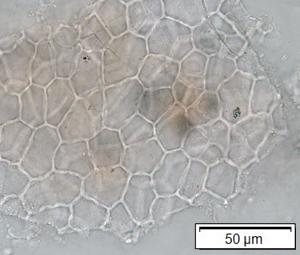
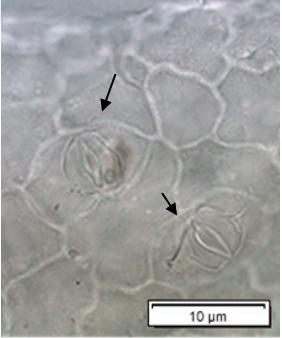
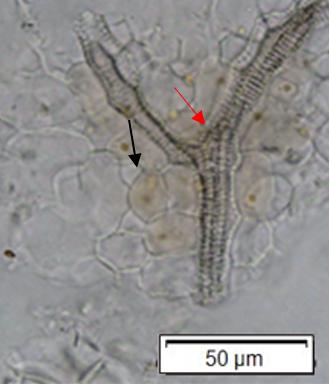
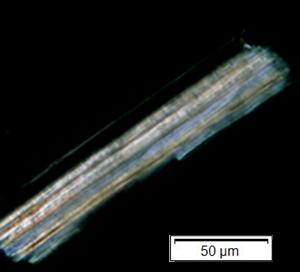
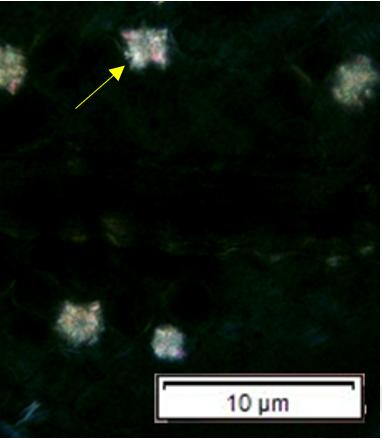
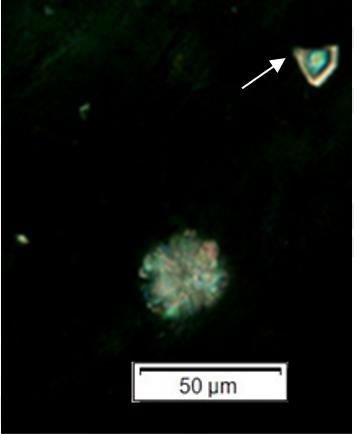
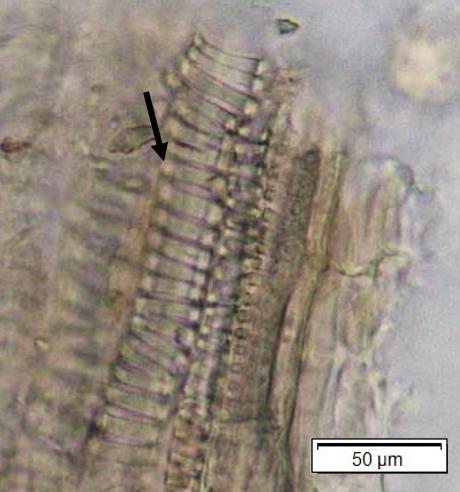

Figure 2 : Microscopic characters of G. atroviridis leaves powder of 0.355 mm size. (a) adaxial epidermis cells with straight to curve anticlinal wall (magnification 20×); (b) abaxial epidermis cells with straight to wavy anticlinal wall and the presence of paracytic stomata (black arrow) (magnification 40×); (c) parenchyma cells (black arrow) and parenchyma bundle sheath (red arrow) (magnification 20×); (d) fiber fragments (magnification 20×); (e) druse calcium oxalate crystals under polarized filter (yellow arrow) (magnification 40×); (f) solitary calcium oxalate crystals under polarized filter (white arrow) (magnification 20×); (g) annular vessel (black arrow) (magnification 20×); (h) reticulate vessel (magnification 40×). [Scale bars: b, e, h = 10 μm; a, c, d, f, g = 50 μm]
Thin Layer Chromatography (TLC)
| Test solution | Weigh about 1.0 g of G. atroviridis dried leaves powder of 0.355 mm size in 100 mL conical flask. Add 10 mL of water : ethanol (1 : 1) and sonicate the mixture for 15 mins. Centrifuge the mixture and collect the supernatant. |
| Standard solution | Dissolve rhoifolin standard (CAS no.: 17306-46-6) in methanol to produce a standard concentration of 1.0 mg/mL. |
| Stationary phase |
HPTLC Silica gel 60 F254, 10 x 10 cm |
| Mobile phase | Ethyl acetate : water : formic acid : acetic acid; (40 : 8 : 3.5 : 5.5) (v/v/v/v) |
| Application |
|
| Development distance | 8 cm |
| Drying | Air drying |
| Detection | UV at 366 nm after derivatization with natural product-polyethyleneglycol (NP-PEG) reagent by dipping method |
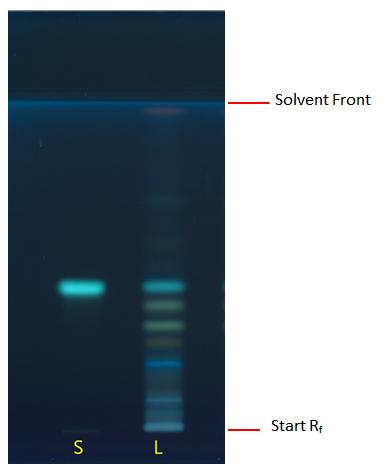
Figure 3 : TLC chromatogram of rhoifolin (S), ethanol (50%) extract of G. atroviridis dried leaves powder (L) observed under UV at 366 nm after derivatisation.
High Performance liquid Chromatography (HPLC)
| Test solution | Weigh about 1.0 g of G. atroviridis dried leaves powder of 0.355 mm size in 100 mL conical flask. Add 10 mL of water : ethanol (1 : 1) and sonicate the mixture for 15 mins. Centrifuge the mixture and collect the supernatant. Take 50 μL supernatant and dilute with 150 μL water : ethanol (1 : 1) | ||||||||||||||||||
| Standard solution | Dissolve rhoifolin standard [CAS no.: 17306-46-6] in methanol to produce a standard concentration of 1.0 mg/mL. | ||||||||||||||||||
| Chromatographic system |
Detector: 335 nm |
||||||||||||||||||
| Mobile phase (gradient mode) |
|
||||||||||||||||||
| System suitability requirement |
Perform at least five replicate injections of the standard solutions (1 mg/mL). The requirements of the system suitability parameters are as follow:
|
||||||||||||||||||
| Acceptance criteria |
|
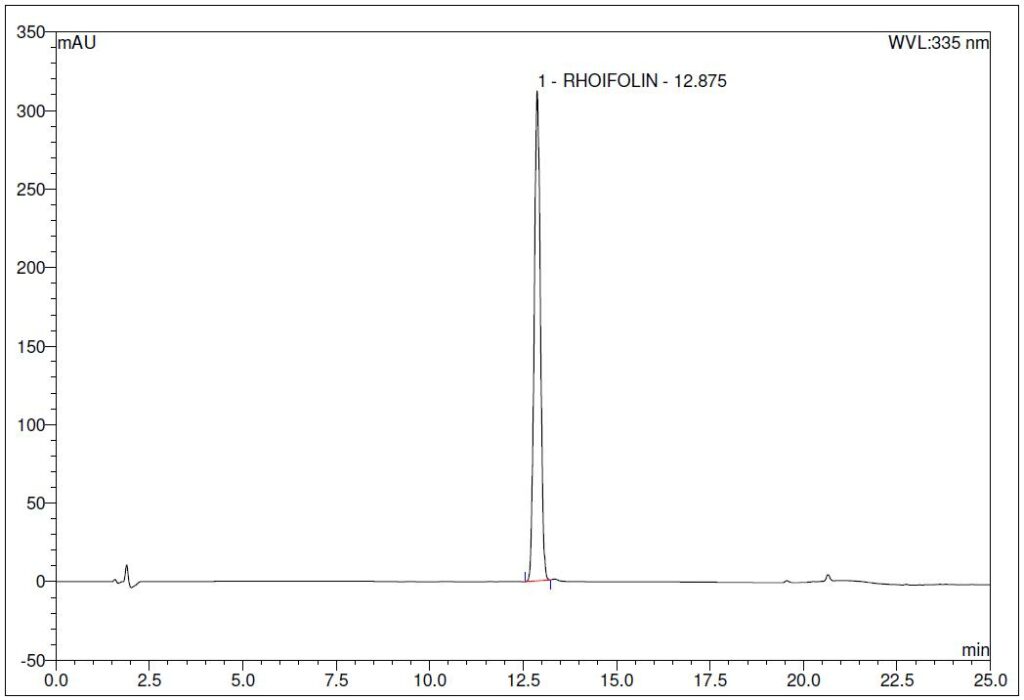
(a)
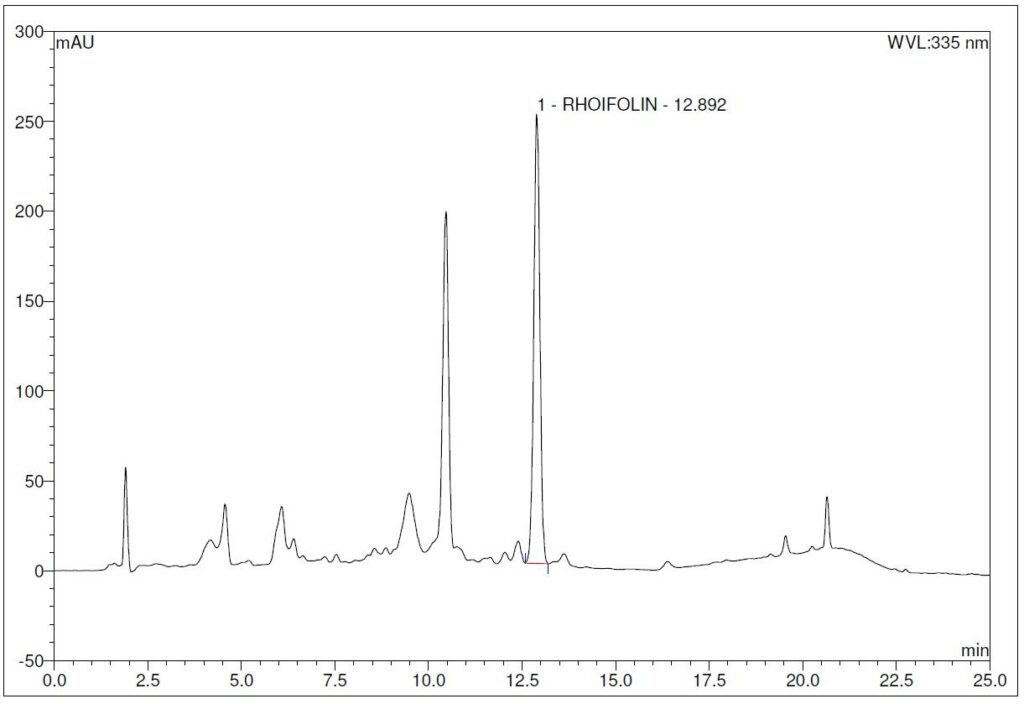
(b)
Figure 4 : Whole HPLC chromatogram of (a) rhoifolin standard solution (1.0 mg/mL) at tr = 12.88 min and (b) ethanol (50%) extract of G. atroviridis dried leaves powder showing a peak corresponding to rhoifolin standard solution at tr = 12.89 min.
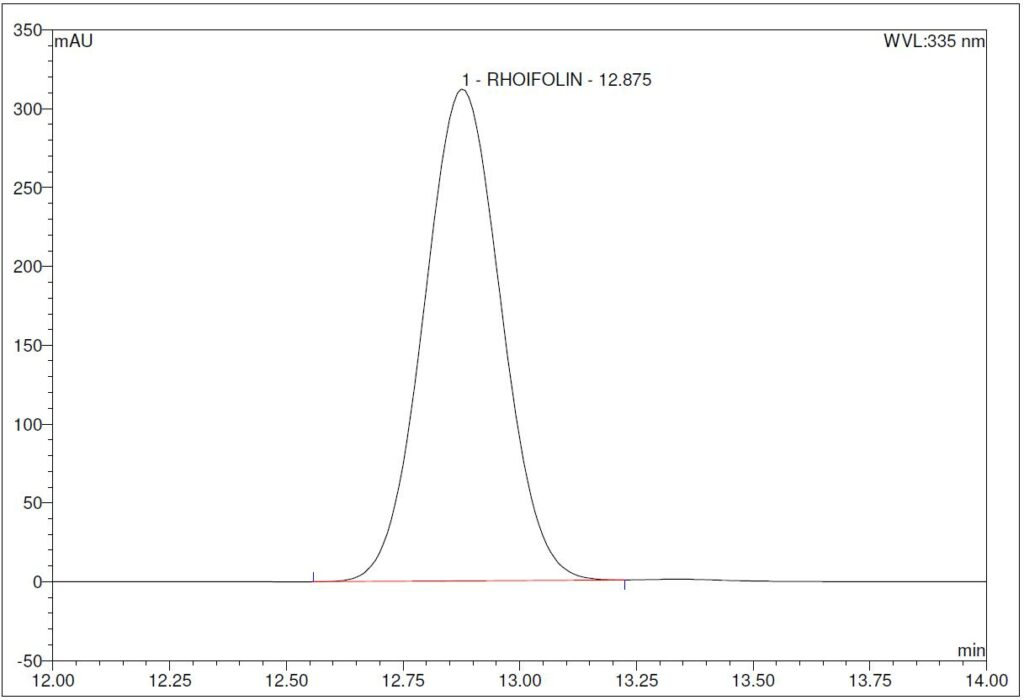
(a)
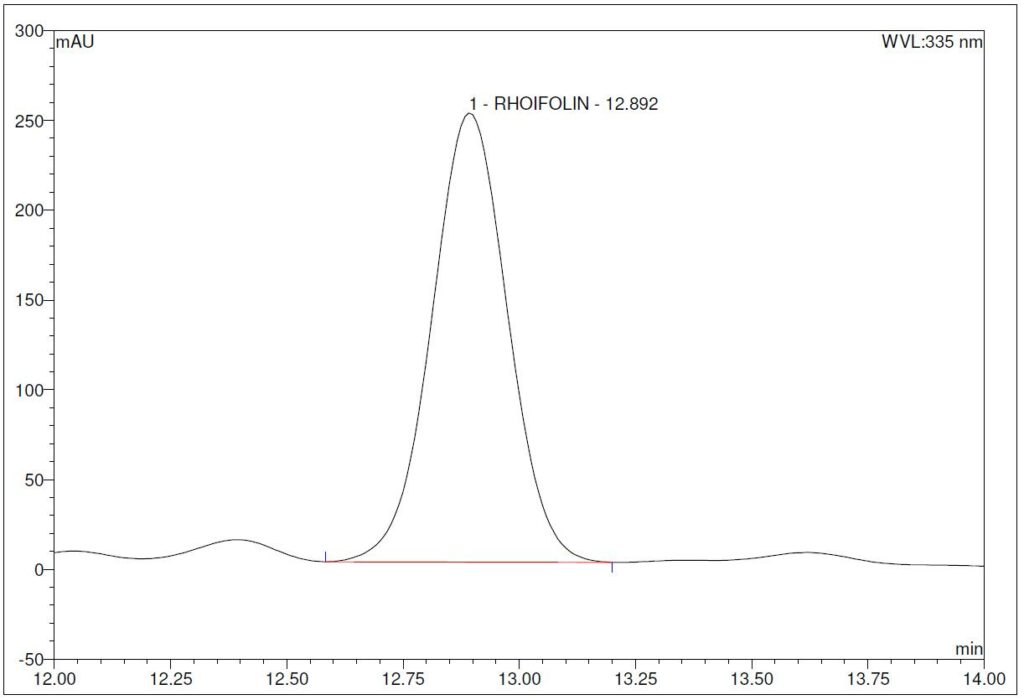
(b)
Figure 5 : HPLC chromatogram highlighting the elution region of (a) rhoifolin standard solution (1.0 mg/mL) at tr = 12.88 min and (b) ethanol (50%) extract of G. atroviridis dried leaves powder showing a peak corresponding to rhoifolin standard solution at tr = 12.89 min.
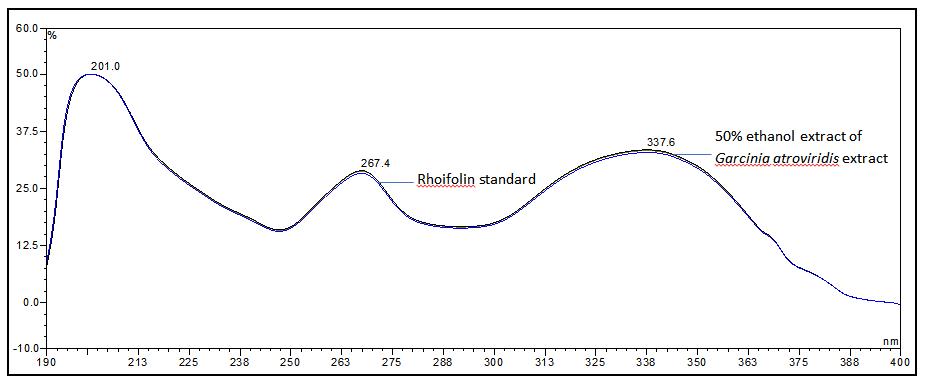
Figure 6 : UV spectra of rhoifolin standard solution (1.0 mg/mL) and ethanol (50%) extract of G. atroviridis dried leaves powder.
PURITY TESTS
The purity tests, except for foreign matter tests, are based on G. atroviridis dried leaves powder of 0.355 mm particle size.
| Foreign Matter |
| Not more than 2% |
| Ash Contents | |
| Total ash | Not more than 6% |
| Acid-insoluble ash | Not more than 1% |
| Water soluble ash | Not less than 0.5% |
| Loss on Drying |
| Not more than 10% |
| Extractive Values | |
| Water-soluble extracts | |
| Hot method | Not less than 39% |
| Cold method | Not less than 32% |
| Ethanol-soluble extracts | |
| Hot method | Not less than 28% |
| Cold method | Not less than 14% |
SAFETY TEST
The safety tests are based on G. atroviridis dried leaves powder of 0.355 mm particle size.
| Heavy Metals | |
| Arsenic | Not more than 5.0 mg/kg |
| Mercury | Not more than 0.5 mg/kg |
| Lead | Not more than 10.0 mg/kg |
| Cadmium | Not more than 0.3 mg/kg |
| Microbial Limits | |
| Total bacterial count | Not more than 105 cfu/g |
| Total yeast and mould count | Not more than 104 cfu/g |
| Bile-tolerant gram negative | Not more than 104 cfu/g |
| Specific Pathogens | |
| Salmonella spp. | Absent in 25 g |
| Escherichia coli | Absent in 1 g |
| Staphylococcus aureus | Absent in 1 g |
| Pseudomonas aeruginosa | Absent in 1 g |
CHEMICAL CONSTITUENTS
Ethyl acetate fraction from methanol extract of G. atroviridis leaves contains flavonone (e.g., naringenin) and carbonyl compounds (e.g., garcinia acid) [7].
Essential oil of G. atroviridis leaves contains sesquiterpenes (e.g α-selinene, β-selinene, α-himachalene, α-humulene, β-caryophyllene, (E)-β-farnesene, α-guaiene), and phenolics (e.g α-linolenic acid, pentadecanoic acid, selin-6-en-4α-ol, epiglobulol and phytol) [8].
MEDICINAL USES
Uses described in folk medicine, not supported by experimental or clinical data
Traditionally, juice from the leaves of G. atroviridis is given to woman after childbirth. A decoction prepared from the leaves and roots is applied to the ear for earache [9].
Biological and pharmacological activities supported by experimental data
Antioxidant activity
Water extract of G. atroviridis leaves showed antioxidant activity with EC50 value of 29.22 ± 0.77 μg/mL compared to ascorbic acid (EC50 = 3.22 ± 0.37 μg/mL) using 2,2-azinobis 3-ethylbenzothiazoline 6-sulfonate (ABTS) assay [10].
Methanol extract of G. atroviridis leaves showed antioxidant activity with EC50 value of 22.95 ± 0.22 μg/mL compared to ascorbic acid (EC50 = 3.22 ± 0.37 μg/mL) using ABTS assay [10].
Ethyl acetate fraction from methanol extract of G. atroviridis leaves (100 ppm) showed 59% antioxidant activity compared to butylated hydroxyl anisole (BHA) (100ppm) with 61% antioxidant activity using DPPH assay [11].
Antihyperlipidimic activity
Aqueous extract of G. atroviridis leaves (1000 mg/kg) administered orally in poloxamer 407-induced hyperlipidemic activity male Sprague Dawley rats (180 – 250 g) for 3 days. After 58 hrs of treatment, the extract lowered the total glyceride (TG) level to 5.77 ± 1.58 mmol/L compared to atorvastatin (3.71 ± 0.86 mmol/L) and hyperlipidemic control (10.90 ± 2.59 mmol/L); total cholesterol level to 6.87 ± 1.01 mmol/L compared to atorvastatin (6.34 ± 0.69 mmol/L) and hyperlipidemic control (11.61 ± 1.25 mmol/L); and low-density lipoproteins (LDL) level to 4.01 ± 0.26 mmol/L compared to atorvastatin (3.44 ± 0.17 mmol/L) and hyperlipidemic control (4.24 ± 0.17 mmol/L). The extract increased the high-density lipoproteins (HDL) level to 0.68 ± 0.06 mmol/L compared to atorvastatin (0.62 ± 0.04 mmol/L) and hyperlipidemic control (0.55 ± 0.05 mmol/L) [10].
Antiproliferative activity
Essential oil of G. atroviridis leaves inhibited the growth of human breast cancer cell line (MCF-7) with half maximal inhibitory concentration (IC50) value of 71 μg/mL using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The combination treatment of essential oil of G. atroviridis leaves (concentrations 10 and 25 μg/mL) and tamoxifen (10 and 25 μM) induced more cancer cell death (30–46% (p < 0.05) of growth inhibition within 72 hour post-treatment) compared to using tamoxifen alone [8].
Clinical studies
Information and data have not been established.
SAFETY INFORMATION
Preclinical studies (Toxicology studies)
Acute oral toxicity.
Oral single dose acute toxicity study on female Sprague Dawley rats (aged between 8 and 12 weeks old) using aqueous extract of G. atroviridis leaves showed no toxic effect on the parameters observed, including behaviours, body weight, food and water intake. All rats were observed for 14 days prior to necropsy. No death was found throughout the study period. Necropsy revealed no significant abnormality but several histological lesions were found. No-observed-adverse-effect level (NOAEL) is less than 2,000 mg/kg body weight. [12]
Others (Adverse reactions, contraindications, side effects, warning, precautions)
Information and data have not been established.
DOSAGE
Information and data have not been established.
STORAGE
Store below 30°C. Protect from light and moisture.
REFERENCES
- Umberto Q. CRC world dictionary of medicinal and poisonous plants: Common names, scientific names, eponyms, synonyms, and etymology. Vol. 1 A-B. Boca Raton, Florida: CRC Press. 2012; p. 294.
- Lim TK. Edible medicinal and non-medicinal plants volume 2: Fruits. Dordrecht Heidelberg New York London: Springer, 2012; p. 21-28.
- Mustapha NMA, Mahmood NZN, Ali NAM, Haron N. Khazanah Perubatan Melayu: Tumbuhan Ubatan. Jilid 2: Institut Penyelidikan Perhutanan Malaysia 2017; p.20-25. ISBN 978-967-0622-96-5.
- Ling GK. Malaysian Herbs Chinese Edition. Volume 1. Selangor: Goh Kong Ling. 2004; p. 210.
- Pangsuban S, Bamroongrugsa N, Kanchanapoom K and Nualsri C. An evaluation of the sexual system of Garcinia atroviridis (Clusiaceae), based on reproductive features. Songklanakarin Journal of Science and Technology 2007;29(6):1457–1468.
- Ngernsaengsaruay C. Lectotypifications of Three Names in Garcinia, Syninymy of Garcinia pedunculata and Detailed Descriptions of Three Species in Garcinia Section Brindonia (Clusiaceae). Diversity. 2022;14(7):556.
- Muchtaridi M, Nuwarda RF, Ikram EHK, Abdul Rahim AS, Gazzali AM, Wahab HA. Neuraminidase Inhibitor of Garcinia atroviridis L. Fruits and Leaves Using Partial Purification and Molecular Characterization. Molecules. 2022;27(3):949.
- Tan W-N, Lim J-Q, Afiqah F, Nik Mohamed Kamal NNS, Abdul Aziz FA, Tong W-Y, et al. Chemical composition, and cytotoxic activity of Garcinia atroviridis Griff. ex T. Anders. essential oils in combination with tamoxifen. Natural Product Research. 2018;32(7):854-8.
- Burkill IH. A dictionary of the economic product of the Malay Peninsula. Vol. 1. London: Published on behalf of the Governments of the Straits Settlements and Federated Malay States by the Crown Agents for the Colonies. 1935; p. 1047.
- Al-Mansoub MA, Asmawi MZ, Murugaiyah V. Effect of extraction solvents and plant parts used on the antihyperlipidemic and antioxidant effects of Garcinia atroviridis: a comparative study. Journal of the Science of Food and Agriculture. 2014;94(8):1552-8.
- Abdullah A, Bakhari N, Osman H. Study on the Relationship of the Phenolic, Flavonoid and Tannin Content to the Antioxidant Activity of Garcinia Atroviridis. Universal Journal of Applied Science. 2013; 1:95-100.
- Nor Azlina Z, Terence Tan YC, Siau Thien C, Nur Salsabeela R, Pusphawathy K, Raja Nazatul Izni RSH, Wan Nurul Nadia WS, Zubaira K, Lalitha Suganthi S. Acute oral toxicity study of selected Malaysian medicinal herbs on Sprague Dawley rats. Institute for Medical Research, Ministry of Health; 2021. Report no.: NON-GLP/2021/03/01.











