Hypoprothrombinemia
Hypoprothrombinemia also known as prothrombin deficiency, factor II deficiency or dysprothrombinemia, is a rare bleeding disorder that slows the blood clotting process 1. People with hypoprothrombinemia often experience prolonged bleeding following an injury, surgery, or having a tooth pulled. In severe cases of prothrombin deficiency, heavy bleeding occurs after minor trauma or even in the absence of injury (spontaneous bleeding). Women with prothrombin deficiency can have prolonged and sometimes abnormally heavy menstrual bleeding. Serious complications can result from bleeding into the joints, muscles, brain, or other internal organs. Milder forms of prothrombin deficiency do not involve spontaneous bleeding, and the condition may only become apparent following surgery or a serious injury.
Prothrombin (or factor II) deficiency is a blood disorder that affects the ability of the blood to clot properly. Symptoms of the deficiency include prolonged bleeding, especially after an injury or after surgery. Women with prothrombin deficiency may have heavy menstrual bleeding. The severity of the disease can vary, with some people experiencing severe bleeding without any known cause, and others only experiencing increased bleeding after a surgery or serious injury.
Inherited hypoprothrombinemia (prothrombin deficiency) is caused by changes (mutations) in the F2 gene. There are two types of inherited prothrombin deficiency. Type 1 or hypoprothrombinemia and type 2 or dysprothrombinemia. Type 1 prothrombin deficiency (hypoprothrombinemia) is the result of decreased prothrombin production. Factor levels of 4-10% have been reported. Levels of factor II activity are also low. Type 2 prothrombin deficiency (dysprothrombinemia) is due to poor function of the prothrombin protein. Prothrombin antigen levels may be normal or low-normal, but activity is depressed. Inheritance of both types is autosomal recessive. Diagnosis is based on laboratory test results that are consistent with the deficiency.
The treatment of hypoprothrombinemia depends on the underlying cause. Plasma-derived products that contain factor II are available. Vitamin K-1 (phytonadione) is used to treat vitamin K deficiency as well as warfarin overdose. In autoimmune disease, treatment is not entirely straightforward, and immunosuppressive therapy is used in severe cases.
Hypoprothrombinemia causes
Hypoprothrombinemia may be acquired or inherited 2.
Acquired hypoprothrombinemia
Acquired hypoprothrombinemia (prothrombin deficiency) is caused by several factors including long-term use of antibiotics, bile obstruction, impaired absorption of vitamin K from the intestines and severe liver disease. It is more common than the inherited hypoprothrombinemia.
Acquired hypoprothrombinemia may be secondary to decreased production or increased consumption. Acquired isolated hypoprothrombinemia is usually autoimmune and associated with the lupus anticoagulant. A relatively common form of acquired hypoprothrombinemia is vitamin K deficiency. Levels of other vitamin K–dependent procoagulant factors (factors VII, IX, and X) and anticoagulant factors (protein C and protein S) are also decreased in vitamin K deficiency.
When prothrombin (factor II) deficiency is acquired, it is not caused by genetic changes in the F2 gene. In these cases, hypoprothrombinemia (prothrombin deficiency) typically does not run in families, unless the underlying cause of the associated disease is also passed from parents to children 3.
Inherited hypoprothrombinemia
Inherited hypoprothrombinemia is very rare 4. Inherited hypoprothrombinemia is estimated to affect 1 in 2 million people in the general population 1. The prothrombin (F2) gene is found on chromosome 11. Mutations in the F2 gene cause inherited hypoprothrombinemia (prothrombin deficiency). The F2 gene provides instructions for making the prothrombin protein (coagulation factor II), which plays a critical role in the formation of blood clots in response to injury. Prothrombin is the precursor to thrombin, a protein that initiates a series of chemical reactions to form a blood clot. After an injury, clots protect the body by sealing off damaged blood vessels and preventing further blood loss.
F2 gene mutations reduce the production of prothrombin in cells, which prevents clots from forming properly in response to injury. Problems with blood clotting can lead to excessive bleeding. Some mutations drastically reduce the activity of prothrombin and can lead to severe bleeding episodes. Other F2 gene mutations allow for a moderate amount of prothrombin activity, typically resulting in mild bleeding episodes.
There are two types of inherited hypoprothrombinemia (prothrombin deficiency), type 1 and type 2. Type 1 or hypoprothrombinemia is more severe, and it is characterized by a decreased level of normally functioning protein, and therefore, by a decrease in protein activity. Type 2 or dysprothrombinemia is characterized by normal or low-normal levels of an abnormal (dysfunctional) protein. Bleeding symptoms vary depending on the amount of residual functional activity 5.
Inherited hypoprothrombinemia inheritance pattern
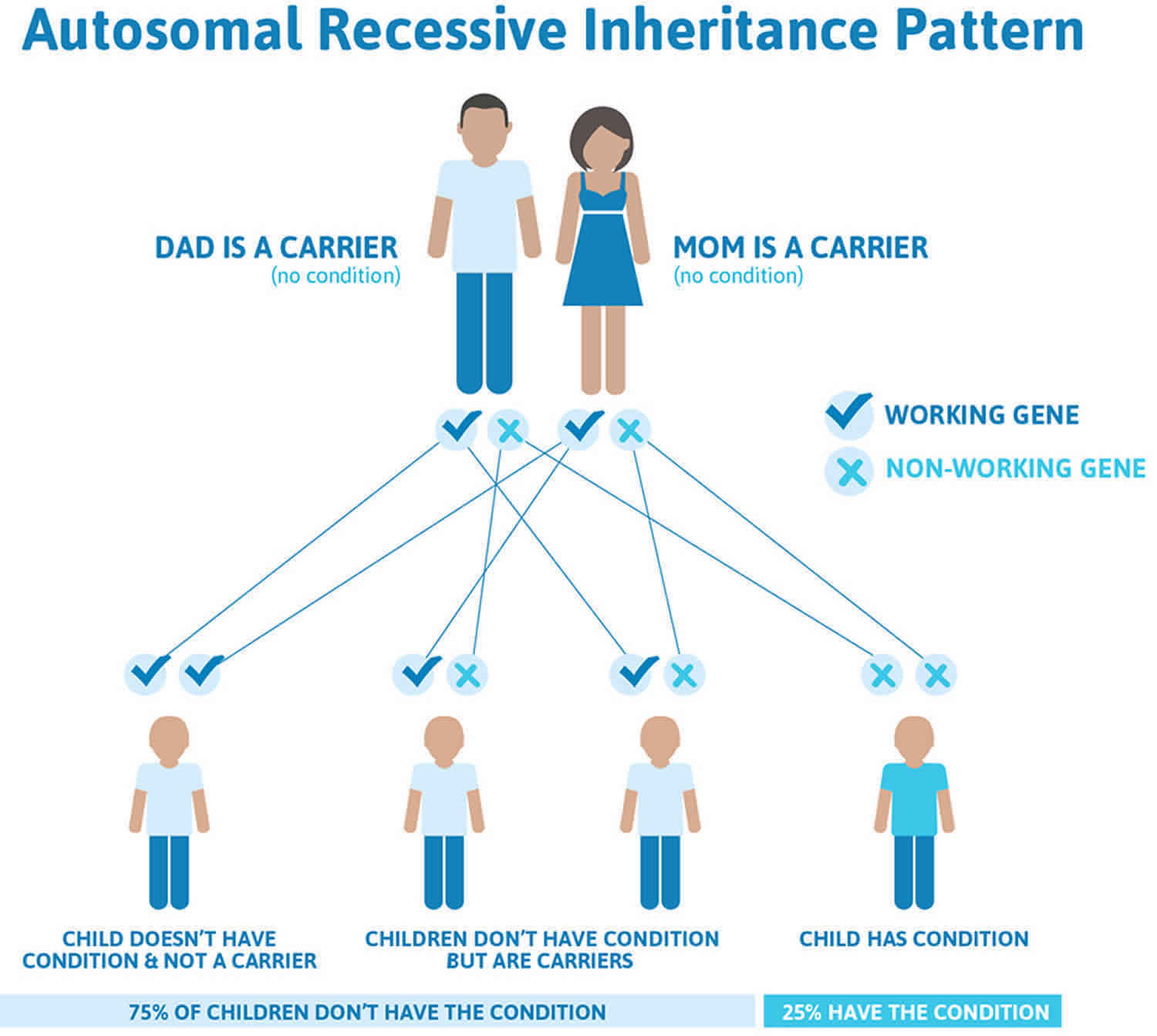
Inherited hypoprothrombinemia is inherited in an autosomal recessive pattern, which means both copies of the gene in each cell have mutations. The parents of an individual with an autosomal recessive condition each carry one copy of the mutated gene, but they typically do not show signs and symptoms of the condition.
Heterozygotes for prothrombin deficiency have factor 2 levels of 30-60% of the reference range. Heterozygotes are usually asymptomatic, although a study by Girolami et al 6 found that mean prothrombin activity was lower in patients who were heterozygous for prothrombin deficiencies than in controls (0.49 IU/dL vs 0.91 IU/dL, respectively), with bleeding manifestations found in 31.8% of the heterozygous individuals, compared with 6.8% of controls.
It is rare to see any history of autosomal recessive conditions within a family because if someone is a carrier for one of these conditions, they would have to have a child with someone who is also a carrier for the same condition. Autosomal recessive conditions are individually pretty rare, so the chance that you and your partner are carriers for the same recessive genetic condition are likely low. Even if both partners are a carrier for the same condition, there is only a 25% chance that they will both pass down the non-working copy of the gene to the baby, thus causing a genetic condition. This chance is the same with each pregnancy, no matter how many children they have with or without the condition.
- If both partners are carriers of the same abnormal gene, they may pass on either their normal gene or their abnormal gene to their child. This occurs randomly.
- Each child of parents who both carry the same abnormal gene therefore has a 25% (1 in 4) chance of inheriting a abnormal gene from both parents and being affected by the condition.
- This also means that there is a 75% ( 3 in 4) chance that a child will not be affected by the condition. This chance remains the same in every pregnancy and is the same for boys or girls.
- There is also a 50% (2 in 4) chance that the child will inherit just one copy of the abnormal gene from a parent. If this happens, then they will be healthy carriers like their parents.
- Lastly, there is a 25% (1 in 4) chance that the child will inherit both normal copies of the gene. In this case the child will not have the condition, and will not be a carrier.
These possible outcomes occur randomly. The chance remains the same in every pregnancy and is the same for boys and girls.
Figure 1 illustrates autosomal recessive inheritance. The example below shows what happens when both dad and mum is a carrier of the abnormal gene, there is only a 25% chance that they will both pass down the abnormal gene to the baby, thus causing a genetic condition.
Figure 1. Inherited hypoprothrombinemia autosomal recessive inheritance pattern
People with specific questions about genetic risks or genetic testing for themselves or family members should speak with a genetics professional.
Resources for locating a genetics professional in your community are available online:
- The National Society of Genetic Counselors (https://www.findageneticcounselor.com/) offers a searchable directory of genetic counselors in the United States and Canada. You can search by location, name, area of practice/specialization, and/or ZIP Code.
- The American Board of Genetic Counseling (https://www.abgc.net/about-genetic-counseling/find-a-certified-counselor/) provides a searchable directory of certified genetic counselors worldwide. You can search by practice area, name, organization, or location.
- The Canadian Association of Genetic Counselors (https://www.cagc-accg.ca/index.php?page=225) has a searchable directory of genetic counselors in Canada. You can search by name, distance from an address, province, or services.
- The American College of Medical Genetics and Genomics (http://www.acmg.net/ACMG/Genetic_Services_Directory_Search.aspx) has a searchable database of medical genetics clinic services in the United States.
Hypoprothrombinemia symptoms
Symptoms include excessive umbilical cord bleeding, easy bruising, frequent nosebleeds and hemorrhaging after surgery or trauma. Women with factor II deficiency experience menorrhagia, heavy menstrual bleeding, and postpartum hemorrhage after childbirth. Joint bleeding is uncommon.
Patients with a hypoprothrombinemia (factor II deficiency) may report a family history of bleeding disorders. They may also report a personal history of the following:
- Umbilical cord stump bleeding at birth
- Prolonged bleeding following circumcision
- Postpartum bleeding
- Easy bruising
- Bleeding gums
- Epistaxis
- Menorrhagia
- Prolonged postsurgical bleeding
- Melena
- Hematuria
- Hemarthroses
- Soft-tissue hemorrhages
- Intracranial bleeding
The physical examination of a patient with hypoprothrombinemia (factor II deficiency) may reveal petechiae and/or ecchymoses, which commonly develop in areas of minor trauma. Ambulatory patients may have petechiae or ecchymoses in the ankle area, whereas bedridden patients may have them on the back. Petechiae may develop following blood pressure measurements in the area beneath the cuff. Additionally, patients may ooze from venipuncture sites. Patients with active hemorrhage may also be seen in emergency departments.
In the case of acquired hypoprothrombinemia, physical examination may reveal signs of underlying liver disease or gastrointestinal malabsorption.
Hypoprothrombinemia diagnosis
Diagnosis is made with a prothrombin time (PT) test and an activated partial thromboplastin time (aPTT) test. Levels of prothrombin deficiency can range from 2% to 50% of normal. Patients with levels near or at 50% of normal have little to no bleeding problems. Inherited hypoprothrombinemia (factor II deficiency) must be distinguished from the acquired form.
Clotting factor assay results in patients with hypoprothrombinemia (factor II deficiency) are as follows:
- In hypoprothrombinemia, functional and antigenic levels of factor II are decreased
- In dysprothrombinemia, functional levels are decreased; antigenic levels are within reference ranges or are slightly decreased
- In isolated factor II deficiency, assays of other clotting factors should reveal normal levels
- In acquired hypoprothrombinemia due to liver disease, vitamin K deficiency, or vitamin K antagonist use, assays of other clotting factors reveal a decrease in the level of all vitamin K–dependent factors (ie, factor II, factor VII, factor IX, factor X, protein C)
Hypoprothrombinemia treatment
Treatment of hypoprothrombinemia (factor II deficiency) should be individualized and aimed at restoring circulating factor II to levels sufficient for hemostasis. Levels greater than 30% of normal are usually adequate. Additionally, in patients with acquired factor II deficiency, the underlying cause should be found and treated.
Treatment options include the following:
- Infusion of fresh frozen plasma (FFP) is usually sufficient to treat most cases of bleeding; a loading intravenous (IV) dose of 15-20 mL/kg is administered, followed by a maintenance dose of 3-6 mL/kg IV every 12-24 hour
- Plasma exchange transfusion may be used to increase factor II levels before surgery
- Prothrombin complex concentrates (PCCs) have also been used to increase factor II levels 7; prothrombin complex concentrates (PCCs) contain factors II, VII, IX, and X, along with protein C; however, prothrombin complex concentrates should be used judiciously because of the risk of thromboembolic complications
- Vitamin K administration may be useful in patients with acquired factor II deficiency
Cryoprecipitate is not an option for bleeding in prothrombin deficiency due to the lack of any prothrombin in the product.
Anti-fibrinolytic agents are often used for mild bleeding symptoms or minor surgical procedures.
Hormonal therapies containing estrogens with or without progesterone in females with menometrorrhagia and severe prothrombin deficiency (prothrombin activity level <5%) has been beneficial in reducing menstrual blood loss 8.
Consultations to consider in cases of factor II deficiency include hematologists and, in patients with congenital factor II deficiency, genetic counselors.
References- Prothrombin deficiency. https://ghr.nlm.nih.gov/condition/prothrombin-deficiency
- Hypoprothrombinemia. https://emedicine.medscape.com/article/956030-overview
- Factor II Deficiency. https://emedicine.medscape.com/article/209742-overview
- Meeks SL, Abshire TC. Abnormalities of prothrombin: a review of the pathophysiology, diagnosis, and treatment. Haemophilia. 2008 Nov. 14(6):1159-63.
- Prothrombin (Factor II) Deficiency Disease Overview. http://www.rarecoagulationdisorders.org/diseases/prothrombin-factor-ii-deficiency/disease-overview
- Girolami A, Santarossa C, Cosi E, Ferrari S, Lombardi AM, Girolami B. Bleeding manifestations in heterozygotes with prothrombin deficiency or abnormalities vs. unaffected family members as observed during a long follow-up study. Blood Coagul Fibrinolysis. 2017 Dec. 28 (8):623-6.
- Lechler E. Use of prothrombin complex concentrates for prophylaxis and treatment of bleeding episodes in patients with hereditary deficiency of prothrombin, factor VII, factor X, protein C protein S, or protein Z. Thromb Res. 1999 Aug 15. 95(4 suppl 1):S39-50.
- Lancellotti S, Basso M, De Cristofaro R. Congenital prothrombin deficiency: an update. Semin Thromb Hemost. 2013;39:596-606.