Abstract
Purpose:
Age-related macular degeneration and inherited retinal degenerations represent the leading causes of untreatable blindness in the developed world. Cell replacement therapy is one therapeutic strategy for the treatment of patients with photoreceptor loss. An essential requirement for cell-based therapies is the establishment of a robust cell manufacturing and development process that allows the derivation of large quantities of highly pure transplantable cells from renewable sources. Photoreceptors precursor cells (PRPCs) generated from human pluripotent stem cells (hPSCs) are potential cell sources for photoreceptor replacement therapy.
Methods:
Human iPS cells reprogrammed from fibroblasts with mRNA were used in the current study. Human iPS cells were transitioned from conventional 2D monolayer to 3D sphere culture in disposable spinner flasks. Retinal neuron differentiation was initiated with the supplement of small molecules and continued with 3D-sphere dissociation/reformation for up to 100 days. Retinal neuron identities at different developmental stages were analyzed and confirmed by immunocytochemistry, flow cytometry and realtime PCR.
Results:
Our well controlled 3D sphere differentiation system sequentially generated early (Rax1+) and late (Chx10+) committed retinal neuron progenitors (CRNPs), PRPCs (NR2E3+/NRL+) and photoreceptor-like cells (REC+/RHOD+) with high purity. Starting with 3 x 10-6 human iPS cells we routinely generated 3-4.5 x 10-9 PRPCs with a purity of approximately 95% from multiple iPS cell lines. Human iPS cell-derived early and late CRNPs, PRPCs and photoreceptor-like cells can be cryo-preserved for more than 2 years.
Conclusions
Our novel 3D sphere platform is amenable to the development of a GMP-compliant retinal cell manufacturing protocol from multiple renewable hPSC sources for future preclinical studies and human cell replacement therapies.
Results
Figure 1
Transition of human PSCs from 2D to 3D cultures
Capable of industrial scale production
A 10 cm 2D-cell culture dish, human iPSC monolayer colony, flow cytometry histogram of OCT4 positive human iPSCs (97.9%) and normal karyotype of human iPSCs from 2D culture. Right: A spinner flask, 3D human iPSC spheres cultured in a 30ml spinner (bioreactor), flow cytometry histogram of OCT4 positive human iPSCs (98.8%) and normal karyotype of human iPSCs from 3D spheres.

Figure 2
Retinal neuron and photoreceptor precursor differentiation under defined and 3D sphere conditions.
(A) Scheme shows 3D sphere PRPC differentiation over a period of ~140 days. The stages of retinal neuronal cells, media and components, days of differentiation, and numbers of cells generated at each time point are indicated.

(B) Retinal neuron spheres generated by continuous dissociation/reaggregation and sphere reformation regimen.

Figure 3
Characterization of iPSCs undergoing retinal neuron differentiation
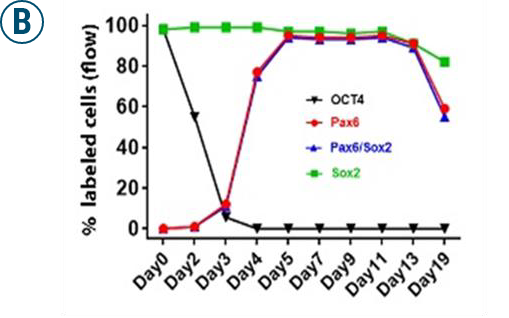
(A): Flow cytometry analyses of OCT4, PAX6, PAX6/SOX2 positive cells 13 days after retinal neuron differentiation.

(B): Graphs illustrate the % of OCT4, PAX6, PAX6/SOX2, and SOX2-positive cells of iPSC differentiation from D0 to D19 analyzed by flow cytometry.
(C): Phase contrast images and immuno-fluorescence stainings show PAX6, RAX1, NESTIN and SOX2 on cells derived from 3D neural spheres. Differentiated cells at D5 were plated and cultured for 8 days, then stained for neural specific marker PAX6 (green), EFP marker RAX1 (red), and general neural markers NESTIN (green) and SOX2 (red). A high percentage of cells are double positive for PAX6/RAX1 and NES/SOX2 indicating the acquisition of neural and early and late CRNP identity.

(D): Quantification of gene expression by qRT-PCR at different days of induced differentiations.

Figure 4
Characterization of PRPCs generated from iPSCs under 3D sphere conditions.
(A): Phase contrast images show 3D iPSC-derived 3D neural sphere morphologies prior to dissociation at D32 (top) and D82 (bottom) and dissociated single cells cultured on surface for 5 days (D37) and 18 days (D100), respectively.

(B): Immuno-fluorescence stainings show expression of CRX (green), ThRB2 (red), and NRL (red), MAP2 (green), and GFAP (red) on cells derived from 3D-PRPC spheres at D82 cultured on surface for additional 18 days. Ki67 staining (red) shows a very low percentage of mitotic cells at D100. DAPI is used for nuclear staining.

(C): Immuno-fluorescence stainings show expression of photoreceptor specific protein rhodopsin (RHOD, green) and recoverin (REC, red) in cells derived from 3D-PRPC spheres at D82 cultured on surface for additional 18 days. DAPI is used for nuclear staining.

Figure 5
3D Spheres after 120-day differentiation show integrity and express markers of photoreceptor
Immunohistochemistry stainings on sections of 3D-spheres at D120 of differentiation show expression of photoreceptor markers rhodopsin (RHOD, green) and recoverin (REC, red) without necrosis. DAPI was used to counterstain the nuclei.

Figure 6
Molecular characterization of 3D-PRPCs derived from iPSCs
Quantitative RT-PCR analyses of PSC, PRPC and photoreceptor markers on 3D sphere cells obtained from different days of retinal neural differentiation, which show a gradual acquisition of PRPC phenotype (NRL, NR2E3, ThRb2) and photoreceptor characteristics (REC, RHOD).