Review
HJOG 2021, 20 (3), 117-130 | doi: 10.33574/hjog.0202
Charilaos Ioannidis
IASO General Hospital, Athens, Greece
Correspondence: Charilaos Ioannidis, Consultant P/R Surgeon UCL Hospitals NHS Trust, Assoc. Professor of Surgery, University of Leuven (B), 18, Ioannou Gennadiou str., Athens 11521, Greece. Tel.: +30 210 7242109, e-mail : ioannidc@otenet.gr
Abstract
Breast Implant – Associated Anaplastic Large Cell Lymphoma is a newly recognized malignant neoplasm presenting in breasts of women who have had breast implants for cosmetic or reconstructive purposes. A review of the literature showed thatit is an uncommon, slow growing T-cell lymphoma with morphology and immunophenotype similar to anaplastic lymphoma kinase (ALK)-negative anaplastic large cell lymphoma. Its clinicopathologic features and treatment, however, are unique. It usually follows an indolent clinical course, but it has the potential to form a mass, to invade locally through the periimplant breast capsule into the breast parenchyma or soft tissues and/or to spread to regional lymph nodes. Surgical removal of the implant en bloc with the whole of the capsule (explantation plus complete capsulectomy) is the treatment of choice and confers an excellent disease free and overall survival. In the few cases with metastatic disease, chemotherapy is used as an adjuvant therapy. Early detection and management convey the best prognosis; therefore clinicians, gynecologists among others, ought to be aware of this new entity and refer suspicious cases for further evaluation and treatment. Change in attitudes towards implant based surgery does not seem necessary, as long as patients are properly informed about the risk of breast implant –associated anaplastic large cell lymphoma.
Keywords: Breast implants, surgery, anaplastic large cell lymphoma, anaplastic lymphoma kinase, CD30, explantation, capsulectomy
Introduction
Breast implants are medical devices used for breast enlargement (augmentation mammoplasty) or breast reconstruction after total mastectomy. The first silicone breast implants (filled with silicone gel) were developed by plastic surgeons T. Cronin and F.Gerow and the Dow Corning Corporation in 1961. The first augmentation mammoplasty using the Cronin-Gerow implant was performed in 19621. In 1964, the French company Laboratoires Arion developed and manufactured the saline breast implant (filled with saline solution), and introduced it as a medical device that same year2. Specific concerns of carcinogenicity, autoimmune diseases, product failure and impaired mammographic evaluation led to a moratorium on the use of all silicone gel implants in 19923,4. In 1997, the United States Department of Health and Human Services (HHS) appointed the Institute of Medicine (IOM) of the U.S. National Academy of Sciences to investigate the potential risks of operative and postoperative complications from the emplacement of silicone breast implants. The IOM’s review reported that “the evidence suggests diseases or conditions, such as cancer, connective tissue diseases, neurological diseases, or other systemic complaints or conditions are no more common in women with breast implants, than in women without implants”.Subsequent studies and systemic reviews found no causal link between silicone breast implants and disease2. In 1999, the IOM published the Safety of Silicone Breast Implants study that reported no evidence that saline – filled and silicone gel -filled breast implant devicescaused systemic health problems2. In 2006, the U.S. Food and Drug Ad-ministration lifted its restrictions against silicone –gel breast implants for breast reconstruction and augmentation mammoplasty.
Breast implant – associated anaplastic large cell lymphoma (BIA-ALCL) is a newly recognized provisional entity in the 2017 revision of the World Health Organiza-tion Classification of Tumors of Hematopoietic and Lymphoid Tissues5. It is a rare type of non-Hodgkin lymphoma presenting in women with breast implants, that is likely under-recognized and under-reported. Aim of the present is to review the recent literature on the epidemiology, the clinicopathologicfeatures, the therapeutic approach, the outcomes, and the prognosis of BIA-ALCL. Factorspossibly supporting a causal relationship between breast implants and BIA-ALCL are also analyzed. The role of the gynecologist in early detection of this disease is emphasized.
Overview on breast implants
Approximately 410,000 breast implants are placed annually for cosmetic or reconstructive purposes(299,715 cosmetic -107,238 reconstructive procedures in 2019 according to the Statistics Report of the American Society of Plastic Surgery) in the United States. Most women are between30 and 39 years and the average patient age at the time of implant placement is 34 years5. In 1992, the FDA announced that the breast implants filled with silicone gel would be available only for reconstructive surgery through controlled clinical trials, due to insufficient evidence demonstrating the safety of these devices3. In 1998, the FDA issued its first release on the risk of breast implants, stating that a pathogenetic relationship between these devices and breast cancer or rheumatic disease had been excluded3. However, this release did not address any potential asso-ciation of breastimplants with other malignancies (e.g. lymphoma). A mounting number of case reports of anaplastic large cell lymphoma (ALCL) in women with breast implants led the FDA to issue a safety warning on the possible association between breast implants and ALCL in 20116.
Breast implants consist of an outer silicone shell that is filled with either saline or silicone gel. The outer shell of an implant can be either smooth or textured; furthermore, there are differences in the implant make and synthesis according to the various manufacturers. The first implants (early 60s) had a smooth outer surface, whereas textured silicone-surface implants were introduced in 1987. The latter have a rough and irregular surface which is designed to minimize implant movement within the breast pocket, as well as potentially reduce capsular contracture, a tightening of the fibrous capsule (Fig. 1 and 2) formed around the implant, causing the breast to feel indurated and /or painful along with change of the shape (Fig. 1) and cosmesis of the breast7,8. The fibrous capsule is usually firmly attached to the implant surface; occasionally, there is a virtual space, in which a minimal amount of fluid is present. In the latter circumstance, the capsule develops a synovium-like lining layer (Fig. 2)5. Uncomplicated fibrous capsules are usually less than 500 μm in thickness and are mostly devoid of inflammatory cells5.
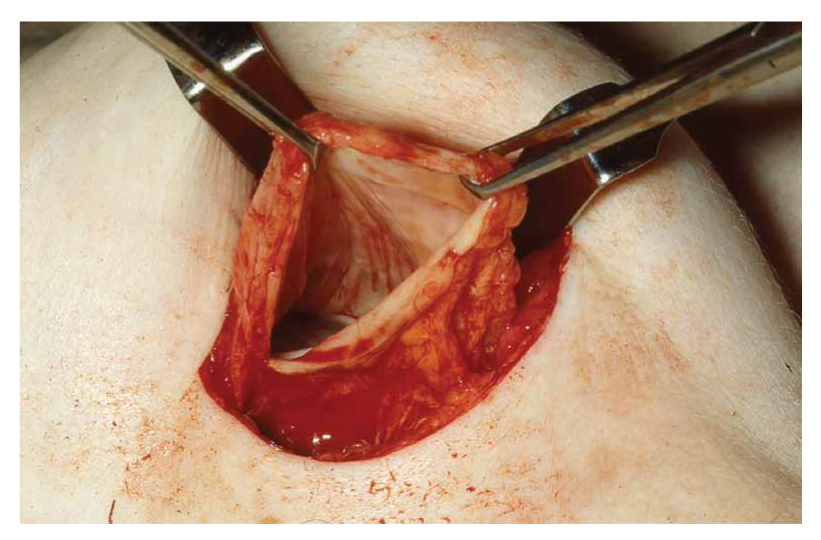
Figure 1. The periimplant fibrous capsule is clearly visible. The breast implant has already been removed (explanted) because of rupture.
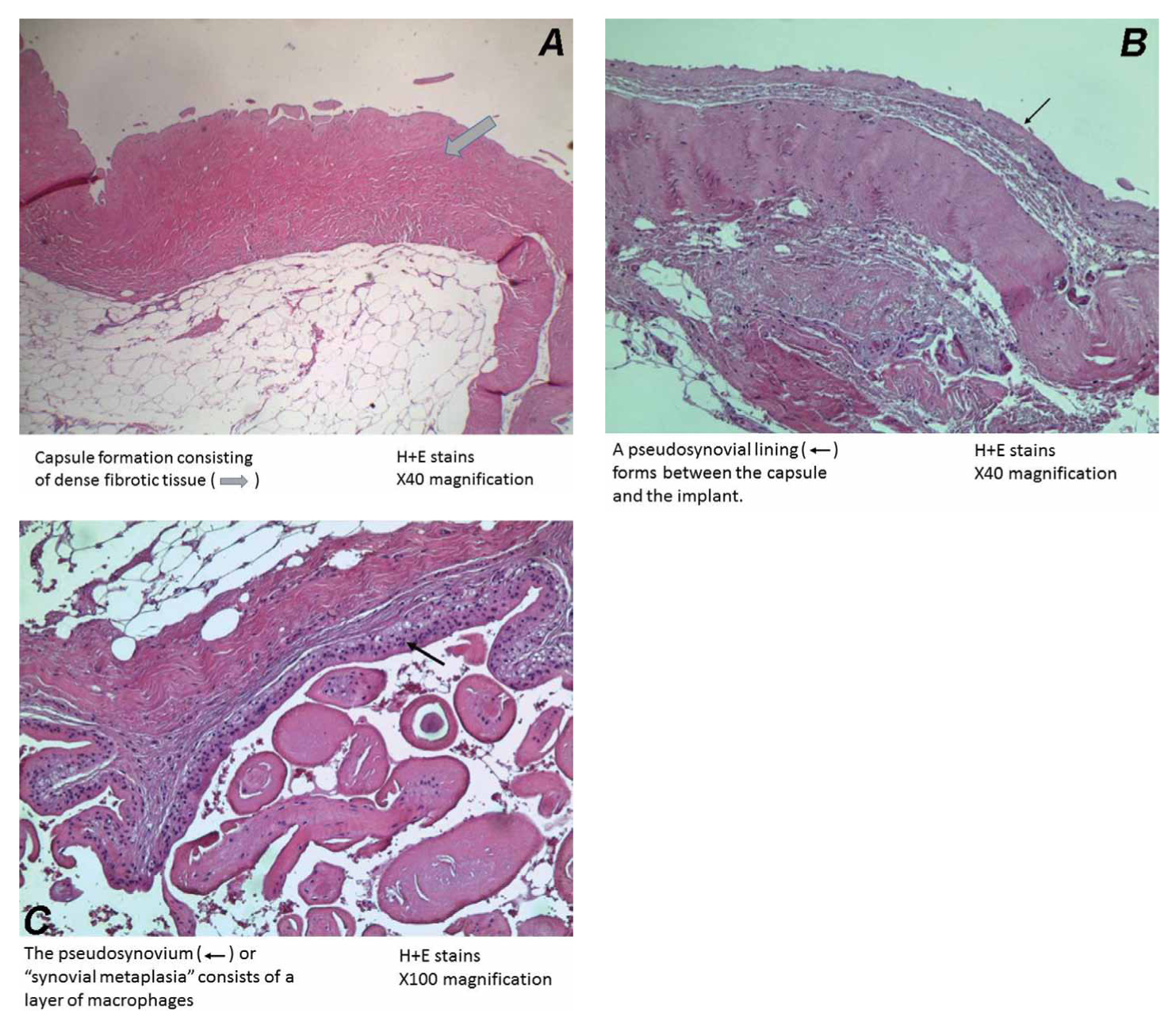
Figure 2. A. Capsule formation consisting of dense fibrotic tissue around a breast implant of a 56-year old patient, who had her implants removed because of development of rheumatoid arthritis (H&E, x 40). B. A pseudosynovial lining (<—) forms between the capsule and the implant (H&E, x 40). C. The pseudosynovium (<—) or “synovial metaplasia “consists of a layer of macrophages (H&E, x 100).
Epidemiology
Lymphomas involving the breast account for approximately 2% of all extra nodal lymphomas and less than 1% of all non-Hodgkin lymphomas9. Most lymphomas involving the breasts are of B-cell lineage9. Anaplastic large cell lymphoma (ALCL) is an uncommon T-cell neoplasm that accounts for approximately 3% of non-Hodgkin lymphomas. The first case of a female patient with ALCL linked to breast implants was reported by Keech and Creech in 199710. Roden et al11, in 2011 suggested that breast implant ALCL is a unique entity with indolent clinical course. The same year, De Jong et al12 in a case control study cautioned for an increased risk of ALCL in patients with breast implants. In the study of Carty et al13, the first death attributed to breast implant ALCL was reported. In a series of 106 cases, Talwakar et al9 reported that cases of ALCL accounted for 6% of all lymphomas involving the breast, and three cases in that study were associated with breast implants.
De Jong et al12 conducted a case control study in the Netherlands that included 389 female patients with non-Hodgkin lymphoma in the breast over a 16-year period; five cases of ALCL were identified in patients with breast implants and 6 others with ALCL with no implants. The authors selected for each of these 11 patients, 1-5 controls with other lymphomas of the breast, matched to age and year of diagnosis. The calculated odds ratio was 18.2 (95% CI, 2.1-156.8) indi- cating that patients with breast ALCL are significantly more likely to have breast implants12. In another study from the Netherlands, the cumulative risks of BIA-ALCL in women with implants were reported 29 per million at 50 years and 82 per million at 70 years. The number of women with implants needed to cause one breast ALCL case before age 75 was 692014.
Lipworth et al15 reviewed the evidence from five long-term follow-up studies comprising over 43,000 women with cosmetic breast implants followed for up to 37 years, which reported results specifically regarding the incidence of non-Hodgkin’s lymphoma, among other cancers. Overall, there were 48 observed incident cases of non-Hodgkin’s lymphoma compared with 53.9 cases expected, yielding a summary standardized incidence ratio of 0.89 (95% CI, 0.67 to 1.18).None of the epidemiological cohort studies reported a primary lymphoma originating in the breast. The authors con-cluded that till 2009 there was no credible evidence of an increase of non-Hodgkin’s lymphoma regardless of site or specifically originating in the breast among women with cosmetic implants15.
In a more recent study, Wang et al16 evaluated the association between breast implants and incident T-cell lymphomas in the California Teachers Study (CTS) co-hort. Of the 123,392 eligible participants, 2,990 women reported having a breast implant (1,715 silicone, 712 saline, 361 both, 202 unknown). Eighty-nine incident T-cell lymphomas were diagnosed during follow-up (average 14 years). Of the 10 women diagnosed with incident ALCL, two reported having breast implants at study entry. Calculation of HRs yielded no association between breast implant and T-cell lymphomas overall or the subgroup of peripheral T-cell lymphomas; a statistically significant association was observed for ALCL (HR=10.9, 95% CI, 2.18-54.0). The time lag from age at first implant to age at ALCL diagnosis was ca.20 years and both women reported using both saline and silicone implants. One ALCL was identified as primary site at the breast and the other at multiple lymph nodes.The authors’ data supported a positive association between breast implants and ALCL risk, but the occurrence of ALCL among women with breast implants remains extremely low16.
Doren et al17, in a retrospective review of documented cases of BIA-ALCL in the U.S. from 1996 to 2015, identified one hundred pathologically confirmed BIA-ALCL cases. Mean age at diagnosis was 53.2±12.3 years. Mean interval from implant placement to diagnosis was 10.7±4.6 years. Forty-nine patients had implants placed for cosmetic reasons, 44 for mastectomy reconstruction and seven for unknown reasons. Assuming that ALCL occurs only in textured breast implants, the incidence rate is 2.03 per 1 million (203 per 100 million person-years), which is 67.6 times higher than that of primary ALCL of the breast in the general population (three per 100 million per year; p<0.001).Lifetime prevalence was 33 per 1 million persons with textured breast implants17.
An increased proportion of BIA-ALCL was reported among women with textured implants14. A report of the U.S. Food and Drug Administration (March 2018) mentioned that out of 272 cases with details on the implant surface 242 were textured, while only 30 were smooth18. According to the same report, the lifetime risk for breast implant ALCL is between 1 in 3,817 and 1 in 30,000 women patients received multiple implant replacements before a diagnosis of breast BIA-ALCL was rendered, and retrieval of information on the surface of the implant was not definitive5.
As of July 1, 2018, 561 cases of BIA-ALCL across 29 countries worldwide have been reported5. Quesada et al, however, believe that the frequency of this neoplasm is likely to be underreported and presently limited mainly to cases in the United States, Europe, and Australia5.
Clinical features
The most common clinical presentation of BIA-ALCL is an effusion around the implant (Stage IA, Table 1) in about two thirds of patients5. Shah et al20 re-ported that 35.6% of their patients presented with an effusion only (IA). The latter typically manifests clinically as unilateral breast enlargement resulting in asymmetry (Fig. 3), and discomfort, approximately 7 to 10 years after implantation although cases occurring at intervals as short as one to four months after repeat implantation have been reported21. Capsule thickening and capsular contracture characteristically contribute to the unilateral breast enlargement. Capsule infiltration is less frequently encountered (T2, T3); however, the disease is still considered localized (StageIB and IC- Table 1). In a series of 23 patients from the UK, fifteen patients (ca 60%) presented with stage I (capsule confined –Table 1)22. The periimplant effusion is often referred to as a ‘seroma’. Quesada et al5, however, support the notion that the above designation is scientifically incorrect. A seroma should be a transudate, with a low cell count and low protein content. In contrast, in patients with BIA-ALCL the effusion around the implant contains liquefied and necrotic lymphoma cells with high protein content19.

Figure 3. A and B. Enlarged right breast of a 38-year old female patient, who had an augmentation mammoplasty 6 years previously, resulting in breast asymmetry. Echography (U/S) showed a right periimplant effusion. Cytological examination of the aspirate showed no signs of malignancy. The patient requested explantation of both breast implants and capsulectomy. Histopathological examination of the capsules showed no signs of malignancy.
Approximately 30% of patients with BIA-ALCL present with a tumor mass, with or without effusion, usually discovered by the patient as an indurated area along the medial or lateral surfaces of the implant5. If the mass is confined to the capsule, the disease is still stage I. When the mass infiltrates beyond the capsule (extracapsular mass), the stage becomes IIA (Table 1). Sixteen per cent of the UK series22 presented with Stage IIA disease. Approximately 20% of patients present with associated regional, usually axillary, lymphadenopathy (Stage IIB- III). Infraclavicular or supraclavicular lymph nodes are less frequently involved (< 10%)23. A small subset of patients complains of mastodynia (painful breast) and rare-ly patients complain of a skin rash or pruritus on the chest or breast area5. In very few patients, BIA-ALCL was discovered incidentally, at the time of surgery for unrelated causes or in the contralateral breast of patients with BIA-ALCL under-going implant removal; in these patients, the effusion is minimal or absent and no gross tumor is identified5,24. Systemic symptoms, such as fevers, weight loss or night-sweats are rare but have been reported25. Distant metastases (Stage IV disease) are rare5. Three patients with bilateral disease were reported by Quesada et al5 as Stage IV disease. The authors, however, question the correctness of the above, commenting that bilateral BIA-ALCL, if confined to the luminal side of the capsule, should better be considered as Stage I with two independent primary neoplasms, each with its own prognostic characteristics5. If one side is invasive through the capsule, the contralateral side may represent disseminated disease. If bilateral axillary lymph nodes are involved, Stage IV disease seems likely26. While largely indolent, BIA-ALCL may rarely be aggres-sive and lead to death, as has been reported for 33 women so far27. When using the traditional Ann Arbor/Lugano staging system, 83% of patients with BIA-ALCL have clinical Stage I, 10% Stage II, and 7% Stage IV at initial diagnosis24. Using the proposed clinical and pathological staging for BIA-ALCL, which follows the MD Anderson Solid Tumor Staging System modelled after the American Joint Committee on Cancer TNM system and now advocated by the National Comprehensive Cancer Network (Table 1), patients have a spectrum of disease from Stage IA (35-70%, effusion only), IB (3-11%), IC (8-13%), IIA (8-25%), IIB (3-5%), III (3-9%) to Stage IV (1-2%,distant metastasis)20.
Among patients who develop BIA-ALCL, approximately 60% had implants for cosmetic reasons and 40% for reconstruction after mastectomy19. Patients who have had implants after mastectomy are more likely to have regular follow-up post therapy, whereas patients with implants for cosmetic reasons are less likely to have regular follow-up beyond the early postoperative period. Therefore, Quesada et al5 expressed the hypothesis that BIA-ALCL may be detected at an earlier stage in reconstructed breast cancer patients compared with patients who had a cosmetic augmentation mammoplasty. This hypothesis, however, is still to be tested.
The time interval from implantation to diagnosis of BIA-ALCL varies in different series. The median interval is 8-11 years with a range from 2 years to as late as 32 years5,19,21.
Both saline and silicone- filled implants have been reported in association with BIA- ALCL without a statistical difference in frequency19. Cases of BIA-ALCL, however, are almost exclusively associated with textured implants5,24,27,28,29. In a recent literature review, Collett et al29 stated that high-textured high-surface area implants (grade 4 surface) carry the highest risk of BIA-ALCL (1/2,832), which is in accordance with the findings of Groth and Graf27 (texture grades 3 and 4 seem to pose a higher risk than grades 2 and 1). Regulating agencies in several countries, including all of Europe and Canada, have banned macro textured implants. In the U.S., the FDA on 2 May 2019, despite patients’ requests, decided against a ban on textured breast implants30. However, several American colleagues strongly support the notion that it is time for professional societies to recognize that the device is the problem rather than the surgical technique30.
Imaging studies
Various imaging studies have been used in patients with BIA-ALCL. Abrada et al31 conducted a retrospective review of the findings of 44 patients in order to determine the sensitivity and specificity of various imaging modalities in the detection of the presence of an effusion or a mass related to BIA- ALCL. The sen-sitivity for detecting an effusion was 84, 55, 82, and 38% and for detecting a mass it was 46, 50, 50 and 64%, by U/S, CT, MRI, and PET, respectively. The sensitivity of mammography in the detection of an abnormality without distinction of effusion or mass was 73%, and specificity 50%.
Sutton et al32 postulated that MRI is the most sensitive imaging modality for detecting a peri-implant fluid collection when compared with mammogram and ultrasound. In a more recent study, the above authors32 studied routine MRIs performed for silicone implant rupture screening in 1070 women with silicone implants and identified late peri-implant fluid and/or masses post breast reconstruction or augmentation in 1.7% of the patients (18/1070). Only one of the 15 delayed peri-implant fluid collections and/or masses with adequate follow-up was malignant BIA-ALCL, with a positive predictive value of 6.7%. The authors concluded that although rare, peri-implant fluid collections and/or masses, when detected on routine MRIs, should be followed by ultrasound-guided fine needle aspiration with CD30 immunohistochemistry and cell block cytology in order to exclude BIA-ALCL32.
Histopathologic features
Fluid collections shortly after placement of a breast implant are common and often represent a hematoma or an effusion related to the surgical procedure, or infection. The most common clinical presentation of BIA-ALCL is a “late seroma“, an effusion ≥ 1 year after initial surgery, which can exceed 500 ml19. Therefore, a “late seroma” requires further investigation5,33. Fine needle aspiration (FNA) with cytological examination provides a fast, safe, and effective method for evalu- ation of the effusion in cases of BIA-ALCL5. Wright-Giemsa stained slides show highly cellular specimens composed of a homogeneous population of large ana-plastic lymphoma cells with irregular nuclei, prominent nucleoli and abundant cytoplasm admixed with small lymphocytes5,34. The sensitivity of the initial cytological evaluation has been reported close to 80%5. When BIA-ALCL is successfully identified by cytological examination, immediate removal of the implants plus capsulectomy should ensue, which results in excellent patient outcomes and an overall survival of more than 95%5.
The breast implants are encased by a fibrous capsule without significant irregularities or masses (Fig. 2). Histologic examination shows synovium-like cells lining the luminal side of the capsule (Fig. 2), however, there are no or minimal inflammatory cells and no large or atypical cells5. Histologic changes seem to be dynamic in nature and implantation duration and shell type play a significant role35. Beyond 5 years, synovial-like metaplasia, a foreign body type reaction, and foreign material are often observed especially in capsules around textured implants35.
In cases of BIA-ALCL, the capsule shows a pink luminal surface, occasionally with fibrinoid strands or detached fragments of pseudomembranous tissue and often no distinct mass. Upon microscopic examination, most of the surface is covered by a layer, a few cells in thickness, of anaplastic large cells or necrotic cells that appear as a fibrinoid or granular material and containing ghost cells5. The lymphoma cells of BIA-ALCL resemble systemic ALCL at nodal or extra nodal sites5,13,19,33,34. The cell nuclei are large, oval or multilobulated, with vesicular dense chromatin, and usually have prominent nucleoli and frequent mitoses. In 70% of the cases, so-called hallmark cells with a horseshoe-, kidney-, or wreath-shaped nucleus are formed5. Immunohistochemistry using CD30 highlights al-most all the identified lymphoma cells on hematoxylin and eosin as well as the outlines of ghost cells or the necrotic debris that is distinctly granular with anti-CD305. Other markers frequently expressed in BIA-ALCL are CD43 (ca 80%), CD4 (ca 80%), TIA-1 (ca 69%), granzyme B (ca 68%), epithelial membrane antigen (ca 60%), CD3 (ca 33%), and CD8 (ca 10%). Most cases of BIA-ALCL do not express a T-cell receptor (TCR), however, TCRαβ(βF1) and TCRγδ have been reported in 11.1% and 10.2% of cases, respectively5. Almost all cases are negative for anaplastic lymphoma kinase (ALK). Some positive cases reported may represent systemic disease presenting initially in the breast near an implant36. Further-more, BIA-ALCL cases are negative for CD1a, TdT, and cyclin D1. The majority of tested cases of BIA-ALCL carry monoclonal TRG or TRB rearrangements19. Translocations (ALK translocations, translocations involving DUSP22 or TP63) having been identified in other well-known types of ALCL, have not been identified in BIA-ALCL, highlighting the distinctive biologic features for breast implant ALCL37.
The pathogenesis of BIA-ALCL is not well defined. From the mechanisms pro- posed, a chronic immune-mediated inflammatory response to the silicone shell surface of implants seems to be more plausible. It has been suggested that the shell of the implant degrades over time resulting in leakage of antigens that elicit a host immune response38. Textured implants were reported to elicit a more marked response to T cells than smooth implants, and showed statistically significant higher percentages of CD3 positive cells than smooth implants39. Cytokine expression profiling of BIA-ALCL cell lines and clinical specimens reveals a predominantly type 17 helper T- cells (Th17)/Th1 signature, implicating this as its cell of origin. However, a Th2 allergic inflammatory response is suggested by the presence of IL-13 with infiltration of eosinophils and IgE-coated mast cells in clinical specimens of BIA- ALCL. These divergent results may be explained by the microenvironment-induced T-cell plasticity40. In a small number of cases, mutations resulting in constitutive Janus kinase (JAK)-STAT activation has been detected and associated with BIA-ALCL pathogenesis40.
Treatment
Initial work-up of an enlarged breast should include ultrasound (U/S) evaluation for fluid collection, breast masses and enlarged regional lymph nodes. The sensitivity and specificity of U/S for detecting an effusion or mass have been reported 84% and 75%, and 46% and 100%, respectively31. In cases where U/S is equivocal, magnetic resonance imaging (MRI) is recommended for further diagnostic work-up31. The best method to sample a periprosthetic fluid collection is U/S guided fine needle aspiration (FNA). A minimum of 50 ml of aspirate is advocated41. A suspicious mass requires tissue biopsy and evaluation. Specimens should be sent for cell morphology by cytology, CD30 immunohistochemistry, and flow cytometry for evaluation, quantification and characterization of T-cells within the specimen41. CD30, by itself, is not pathognomonic because it can be expressed on benign inflammatory cells. Scant or rare CD30 positive lymphocytes with normal morphology are considered a normal finding and do not require further investigation42. The diagnosis of BIA-ALCL requires careful clinicopathologic correlation. The importance of excluding other malignancies or benign processes that mimic BIA-ALCL has been emphasized by Quesada et al5.
Following exclusion of BIA-ALCL, benign seromas may be managed as appropriate by a plastic surgeon. The FDA recommends that all patients meeting the pathologic criteria for BIA-ALCL should be reported to the PROFILE registry of the American Society of Plastic Surgery (www.thepsf.org/PROFILE)18. A bone marrow biopsy is suggested for patients for whom there is a high suspicion of systemic ALCL such as patients with aggressive local invasion or lymph node metastasis41. Suggested laboratory testing includes a full blood count, comprehensive metabolic panel, LDH, and HepB testing (if adjuvant chemo-therapy is being considered). A preoperative PET/CT scan is optimal for demon-strating associated capsular masses, chest wall involvement, lymphadenopathy, organ metastases, and it will serve as a guide to surgical excision43.
Timely diagnosis and complete surgical excision are essential to treatment of BIA-ALCL44. The goal of surgery should be to remove the implant with the surrounding fibrous capsule and any associated fibrous mass41. Complete surgical excision prolongs event-free survival and overall survival compared with all other therapeutic interventions21,41. Proper orientation and marking of the specimen is mandatory to allow for anatomic location of the disease, tumor surveillance and, if indicated, reexcision. Co et al45, in a recent systematic review found that a mastectomy had been performed in only 2% of patients (8/395). At present, there is no clear role for mastectomy or sentinel node biopsy41. An estimated 2-4% of women develop bilateral disease; therefore surgeons may consider removal of the contralateral implant and its capsule41. Plastic surgeons, unaccustomed to optimal surgical resection of a malignancy, might benefit from a surgical oncology consultation. Involved lymph nodes, axillary in the majority of cases, are excised (axillary lymphadenectomy)23,41. Surgery alone suffices for the majority of cases (Lugano IE; MD Anderson Cancer Center IA-IIA), though a slightly higher rate of recurrence is noted with invasive disease41. The recurrence rate following complete surgical resection is 14.3% for patients with T1-3 disease (p=0.001)44. Local recurrence is most common following incomplete resection or partial capsulectomy.
Radiation therapy (24-36 Gy) is suggested for patients with residual disease, positive margins, or unresectable disease with chest wall invasion. Collins et al46 used radiotherapy in 15/39 patients (51.7%) with advanced BIA-ALCL; the authors, however, concluded that the indications for radiation in BIA-ALCL patients with advanced features are not yet clearly defined.
Patients with Lugano Stage II-IV or MD Anderson Stage IIB-IV warrant systemic therapy. Medical oncologists can consider either a standard approach utilized for systemic ALCL, such as combination anthracycline-based chemotherapy (National Comprehensive Cancer Network guidelines for first-line therapy of a peripheral T-cell lymphoma), or alternatively, a combination of brentuximab vedotin (an antibody-drug conjugate which combines a CD30 monoclonal antibody with the microtubule-disrupting agent monomethylauristatin E). The latter agent, either alone47, or in combination with cyclophosphamide, doxorubicin, and prednisone (A+CHP)48 was found to have superior results to physicians’ choice (methotrexate or bexarotene) or cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP), respectively, when used for the treatment of CD30-positive peripheral T-cell lymphomas47,48. Based on these results, the addition of brentuximab vedotin is currently considered “preferred” first line therapy for BIA-ALCL. Johnson et al reviewed the United Kingdom experience and found three patients with BIA-ALCL having extra-capsular masses21. In addition to surgery, all three of them received neo/adjuvant chemotherapy with CHOP as first line therapy. One patient progressed on CHOP but achieved pathological complete response (pCR) with brentuximab vedotin. After a mean follow-up of 23 months (range 1-56 months), all patients included in the above study remained disease –free21. A few patients have undergone ablative chemotherapy followed by hematopoietic stem cell transplant. The experience, however, is still limited5,45,46.
Patients showing a complete response after treatment can be monitored with history and clinical examination every three to six months for two years and then as clinically indicated. The role of routine imaging is unclear, but either a chest/abdominal/pelvic CT scan with contrast or PET scan could be considered every six months for two years, and then only as clinically indicated41.
Clinical outcomes and prognosis
BIA-ALCL patients with confinement of the disease within the capsule have a 5-year overall survival (OS) of nearly 100%, compared to 72.4% when the neo- plasma extends beyond the capsule (p=0.0002)5. Complete capsulectomy can confer a 5-year OS of 98.8% as compared to 57.2% in patients who did not undergo complete capsulectomy (p<0.0001)5. Patients who present with a tumor mass, as opposed to only effusion, tend to have a more aggressive disease, including regional node involvement5,24. Complete remission was seen in 93% of patients with disease confined to the fibrous capsule compared with 72% in patients with a tumor mass. The lack of lymph node involvement (LNI) at presen-tation is a favorable prognostic marker. Ferrufino-Schmidt et al reported a 5-year OS of 97.9% for patients without LNI at presentation and 75% for those with LNI (p=0.003)23. Collins et al, in a reviewof 39 patients with advanced BIA-ALCL reported that the rate of complete remission for patients with lymphadenopathy was 67% (16/24, p=0.128)46.
Bilateral disease is another unfavorable prognostic marker. The rate of complete remission after treatment in patients with bilateral disease was reported 57% (p< 0.001)46.
Miranda et al reported a median overall survival of 12 years (median follow-up, 2 years; range 0-14 years), the 3-year OS was 97% and the 5-year OS was 92% in 60 reviewed patients24.
Patients with BIA-ALCL have an excellent prognosis overall, clearly better than that of patients with systemic ALCL, both ALK+ and ALK-, and similar to primary cutaneous ALCL5.
Discussion
Breast implant placement is one of the most frequently performed operations. Worldwide, approximately 1.7 million breast implant surgeries are carried out each year49. Breast implants are well tolerated by patients with very few postoperative sequelae. In 1997, BIA-ALCL was first reported10; since then much research has led to acceptance of BIA-ALCL as a specific clinicopathologic entity. As mentioned before, when timely detected, this disease is curable. It is evident that early diagnosis is of the utmost importance.
Women with implants should perform regular self-breast exams, get routine mammography and ultrasound imaging, and visit a clinician if changes occur. As mentioned before, cosmetic patients, who are the majorityof patients with breast implants, are not likely to comply with the above and therefore have no regular long-term follow-up5. Most of these women, however, will pay regular visits to their gynecologist. The latter should be informed of the patient’s breast surgery and aware of the possible differential diagnosis of BIA-ALCL in case of late postoperative breast symptoms (e.g. mastodynia) or changes (e.g. breast enlargement, asymmetry).It should be stressed once more that most times these symptoms are of benign nature (capsular contracture, benign seroma etc.). However, referral of the patient to the plastic surgeon or to a multidisciplinary team for further investigation and treatment, as indicated, can be lifesaving.
Change in attitudes towards implant based surgery is unnecessary according to the most recent available published evidence (European Society of Breast Cancer Specialists recommendation)50.Screening and prophylactic implant removal is not recommended in asympto-matic individuals41. Patients should be properly informed, as many remain unaware of the risk for BIA-ALCL and may overlook early warning signs of the cancer51. Clemens et al have supported the notion, that difficulty with determining an accurate assessment of risk, including diagnosis, or standardized treatment regimen has led surgeons to omit preoperative discussion of this rare and frequently misunderstood cancer52. These authors suggested that BIA-ALCL should be includedwhen obtaining informed consent on the risks of breast implantation during preoperative consultation. Pertinent aspects of decision-making include disease awareness, presenting symptoms and resources for concerned patients. Education of health care professionals ensures effectiveness of the informed consent process52.
Conclusion
In conclusion, breast implant associated-anaplastic large cell lymphoma is a new, rare nosological entity presenting in patients with mainly textured breast implants. Whilst the majority of cases are localized and cured by implant removal and full capsulectomy, a small percentage requires chemotherapy; the mortality is very low. Change in attitudes towards implant based surgery, according to the most recent available published evidence, does not seem necessary, as long as patients are properly informed about the risk of BIA-ALCL.
Conflict of interest and funding
None to declare
References
1. Banks G: Invention made Houston global hub for breast implants. Chron.com/local/history/innovator-inventions/article/Local-inventio-made-Houston-international-hub-9122371.php,2016. Accessed, 3.11.2020.
2. FDA Breast Implant Consumer Handbook 2004 Archived 2008-09-17 at the Wayback Machine
3. Angel M: Breast implants-protection or paternalism? N Engl J Med 1992; 326:1695-6
4. Kessler DA: The basis of the FDA’s decision on breast implants. N Engl J Med 1992; 326: 1713-5
5. Quesada AE, Medeiros L J, Clemens MW et al: Breast implant-associated anaplastic large cell lymphoma: a review. Mod Pathol2019;32: 166-88
6. FDA. Anaplastic Large Cell Lymphoma (ALCL) in women with breast implants: Preliminary findings and analyses. http://www.fda.gov/Medical Devices/Products and Medical Procedures/Implants and Prosthetics/Breast Implants, 2011
7. Pittet B, Montandon D, Pittet D: Infection in breast implants. Lancet Infect Dis 2005; 5:94-106
8. Tarpila E, Ghassemifar R, Fagrell D et al: Capsular contracture with textured versus smooth saline-filled implants for breast augmentation: a prospective clinical study. Plast Reconstr Surg 1997; 99: 1934-9
9. Talwakar SS, Miranda RN, Valbuena JR et al: Lymphomas involving the breast: a study of 106 cases comparing localized and disseminated neoplasms. Am J Surg Pathol 2008; 32: 1299-1309
10. Keech JA, Creech BJ: anaplastic T-cell lymphoma in proximity to a saline-filled breast implant. Plast Reconstr Surg 1997; 100: 554-5
11. Roden A, Macon WR, Keeney GL et al: Seroma-associated primary anaplastic large-cell lymphoma adjacent to breast implants: an indolent T-cell lymphoproliferative disorder. Mod Pathol 2008; 21: 455-63
12. De Jong D, Vasmel WL, de Boer JP et al: Anaplastic large-cell lymphoma in women with breast implants. JAMA 2008; 300: 2030-5
13. Carty MJ, Pribaz JJ, Antin JH et al: A patient death attributable to implant-re-lated primary anaplastic large cell lymphoma of the breast. Plast Reconstr Surg 2011; 128; 112e-8e
14. De Boer M, van Leeuwen FE, Hauptman m et al: Breast implants and the risk of anaplastic large-cell lymphoma in the breast. JAMA Oncol 2018; 4: 335-41
15. Lipworth L, Tarone RE, McLaughlin JK: Breast implants and lymphoma risk:a review of the epidemiologic evidence through 2008. Plast Reconstr Surg 2009; 123: 790-3
16. Wang SS, Deapen D, Voutsinas J et al: Breast implants and anaplastic large cell lymphomas among females in the California Teachers Study cohort. Br J Haematol 2016; 174: 480-3
17. Doren EL, Miranda RN, Selber JC et al: U.S. epidemiology of Breast Implant-Associated Anaplastic Large Cell Lymphoma. Plast Reconstr Surg 2017; 139: 1042-50
18. FDA. Breast implant- associated anaplastic large cell lymphoma (BIA-ALCL). https://www.fda.gov./MedicalDevices/Productsand MedicalProcedures/Implants and Prosthetics/Breast Implants/ucm 239995.Ltm 2018
19. Miranda RN, Aladily TN, Prince HM et al: Breast implant-associated anaplastic large-cell lymphoma: long-term follow-up of 60 patients. J Clin Oncol 2014; 32; 114-20
20. Shah NM, Clemens MW, Horwitz SM: How I treat breast implant-associated anaplastic large cell lymphoma. Blood 2018; 132: 1889-98
21. Stack A, Ali N, Khan N: Breast implant-associated anaplastic large cell lymphoma: A review with emphasis on the role of brentuximab vedotin. J Cell Immunol 2020; 2: 80-9
22. Johnson L, O’Donoghue JM, McLean N et al: Breast implant associated anaplastic large cell lymphoma: The UK experience. Recommendations on its management and implications for informed consent. Eur J Surg Oncol 2017; 43: 1393-1401
23. Ferrufino-Schmidt MC, Medeiros LJ, Liu H et al: Clinicopathologic features and prognostic impact of lymph node involvement in patients with breast implant-associated anaplastic large cell lymphoma. Am J Surg Pathol 2018; 42: 293-305
24. Cordeiro PG, Ghione P, Ni A et al: Risk of breast implant associated anaplastic large cell lymphoma (BIA-ALCL) in a cohort of 3546 women prospectively followed long term after reconstruction with textured implants. J Plast Reconstr Aesthet Surg 2020; 73: 841-6
25. McCarthy CM, Loyo-Berrios N, Qureshi AA et al: Patient registry and outcomes for breast implants and anaplastic large cell lymphoma etiology and epidemiology (PROFILE): Initial report of findings 2012-2018. Plast Reconstr Surg 2019; 143: 65S-73S
26. Alobeid B, Sevilla DW, El-Tamer MB et al: Aggressive presentation of breast implant-associated ALK-1 negative anaplastic large cell lymphoma with bilateral axillary lymph node involvement. Leuk Lymphoma 2009; 50: 831-3
27. Groth AK, Graf R: Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) and the textured breast implant crisis. Aesthet Plast Surg 2020; 44: 1-12
28. Lineweaver WC: Breast implant – associated anaplastic large cell lymphoma and textured implants. Ann Plast Surg 2019; 82: 595-6
29. Collett DJ, Rakhorst H, Lennox P et al: Current risk estimate of breast implant –associated anaplastic large cell lymphoma in textured breast implants. Plast Reconstr Surg 2019; 143: 30S-40S
30. Swanson E: Plastic surgeons defend textured breast implants at 2019 U.S Food and Drug Administration hearing: Why is it time to reconsider. Plast Reconstr Surg Glob Open 2019; 7: e2410
31. Abrada BE, Miranda RN, Rauch GM et al: Breast implant-associated anaplastic large cell lymphoma: sensitivity, specificity, and findings of imaging studies in 44 patients. Breast Cancer Res Treat 2014; 147: 1-14
32. Sutton EJ, Dashevsky BZ, Watson EJ et al: Incidence of benign and malignant peri-implant fluid collections and masses on magnetic resonance imaging in women with silicone implants. Cancer Med 2020; 9: 3261-7
33. Mazzochi MDL, Corrias F, Scuderi N; A clinical study of late seroma in breast implantation surgery. Aesthet PlastSurg 2012; 36: 97-104
34. Talagas M, Uguen A, Charles-Petillon F et al: Breast implant – associated anaplastic large cell lymphoma can be a diagnostic challenge for pathologists. ActaCytol 2014; 58: 103-7
35. Wyatt Le, Sinow JD, Wollman JS et al: The influence of time on human breast capsule histology: smooth and textured silicone-surface implants. Plast Reconstr Surg 1998; 102: 1922-31
36. Taylor CR, Siddiqi IN, Brody GS: Anaplastic large cell lymphoma occurring in association with breast implants: review of pathologic and immunohistochemical features in 103 cases. ApplImmunohistochemMolMorphol 2013; 21: 13-20
37. Oishi N, Brody G, Ketterling R et al: Genetic subtyping of breast implant-associated anaplastic large cell lymphoma. Blood 2018; 132: 544-7
38. Lazzeri D, Agostini T, Bocci G et al: ALK-1 negative anaplastic large cell lymphoma associated with breast implants: a new clinical entity. Clin Breast Cancer 2011; 11: 283-96
39. Britez MEM, Llano CC, Chaux A: Periprosthetic breast capsule and immunophenotypes of inflammatory cells. Eur J Plast Surg 2012; 35: 647-51
40. Turner SD, Inghirami G, Miranda RN et al: Cell of origin and immunologic events in the pathogenesis of breast implant-associated anaplastic large-cell lymphoma. Am J Pathol 2020; 190: 2-10
41. Clemens MW, Jacobsen ED, Horwitz M: 2019 NCCN Consensus Guidelines on the Diagnosis and Treatment of Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL). Aesthet Surg J 2019; 39: S3-S13
42. Kadin ME, Deva A, Xu H et al: Biomarkers provide clues to early events in the pathogenesis of breast implant-associated anaplastic large cell lymphoma. AesthetSurg J 2016; 36: 773-81
43. Brody GS, Deapen D, Taylor CR et al: anaplastic large cell lymphoma occurring in women with breast implants: analysis of 173 cases. Plast Reconstr Surg 2015; 135: 695-705
44. Clemens MW, Medeiros LJ, Butler CE et al: Complete surgical excision is essential for the management of patients with breast implant- associated anaplastic large cell lymphoma. J Clin Oncol 2016; 34: 160-8
45. Co M, Chan TH, Ip KFS et al: Breast implant associated anaplastic large cell lymphoma-a systematic review with pooled analysis. Clin Oncol (R Coll Radiol) 2020; 32: 639-46
46. Collins MS, Miranda RN, Medeiros LJ et al: Characteristics and treatment of advanced breast implant-associated anaplastic large cell lymphoma. Plast Reconstr Surg 2019; 143: 41S-50S
47. Prince HM, Kim YH, Horwitz SM et al: Brentuximab vedotin or physician’s choice in CD30-positive cutaneous T-cell lymphoma (ALCANZA): an international, open-label, randomized, phase 3, multicenter trial. Lancet 2017; 390: 555-66
48. Horwitz S, O’Connor OA, Pro B et al: Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomized, phase 3 trial. Lancet 2019; 393: 229-40
49. Fitzal F, Turner SD, Kenner L: Is breast implant-associated anaplastic largecell lymphoma a hazard of breast implant surgery? Open Biol 2019; 9: 190006
50. Cardoso MJ, Wyld L, Rubio IT et al: EUSOMA position regarding breast implant associated anaplastic large cell lymphoma (BIA-ALCL) and the use of textured implants. Breast 2019; 44:90-3
51. Roberts JM, Carr LW, Jones A et al: A prospective approach to inform and treat 1340 patients at risk for BIA-ALCL. Plast Reconstr Surg 2019; 144: 46-54
52. Clemens MW, Miranda RN, Butler CE: Breast implant informed consent should include the risk of anaplastic large cell lymphoma. 2016; 137: 1117-22