Mullite
(With friendly support & Copyright by Thomas Witzke/Stollentroll)
Mineralogical definition "natural mullite"
For the sake of completeness, the mineralogical definition of mullite should also be mentioned here - the "natural" mullite - a very rarely occurring mineral from the mineral class of "silicates and germanates" and which is designated with the chemical formula Al[6]Al1+x[4][O|Si1-xO4-x/2]. Mullite crystallizes in the orthorhombic crystal system and usually develops small, prismatic to needle-like crystals with a glass-like luster on the surfaces. In pure form, mullite is colorless and transparent. However, due to multiple refraction caused by lattice defects or polycrystalline formation, it can also appear white and can take on a gray, light pink to red or violet color due to foreign additions, whereby the transparency decreases accordingly..
Mullite is formed by metamorphosis from kaolinite at normal pressure and about 1200 °C or as a decay product of sillimanite or its polymorphic disthene or andalusite at over 1000 °C (Sillimanit→Mullit+SiO2). Since these conditions correspond to the metamorphic sanidinite facies, which is only rarely reached in nature and in limited rock volumes (for example in the host rock of basaltic volcanic vents), mullite usually occurs there only in very small quantities.
Mineralogical definition "synthetic mullite"
Technically, mullite is produced by melting a mixture of kaolinite and alumina in an electric arc furnace or by sintering a briquetted mixture of kaolinite, aluminum hydroxide and water in a tunnel furnace.
The starting mineral kaolinite produces mullite as an essential component in the manufacture of porcelain and bricks as well as fireclay bricks. Mullite is also used to manufacture filter elements for hot gas filtration. It is also used as an inert carrier material for coated catalysts. Another field of application for mullite is high-temperature thermal insulation.
Mullite as part of the porcelain
In porcelain production, "mullite" is created by melting kaolin, quartz and feldspar at a temperature above 1,320 °C, so to speak as an automatic process through hard firing. Depending on the temperature and composition of the raw materials mentioned above
- Scaly mullite
- Needle mullite
Scaly mullite - primary mullite
If one looks at a mixture of kaolin and feldspar at first, the first mullite formation (primary mullite) can be observed from about 1000°C. This occurs first in the kaolinite relics, because the most easily mobile ion, the potassium ion, penetrates into these relics, where it forms a small proportion of the melt phase and thus mediates mullite formation. However, this causes a reduction of the K2O content in the feldspar melt, whereby the composition in the phase diagram moves in the direction of the mullite field, so that mullite crystallization also takes place in the feldspar melt.
Needle mullite - secondary mullite
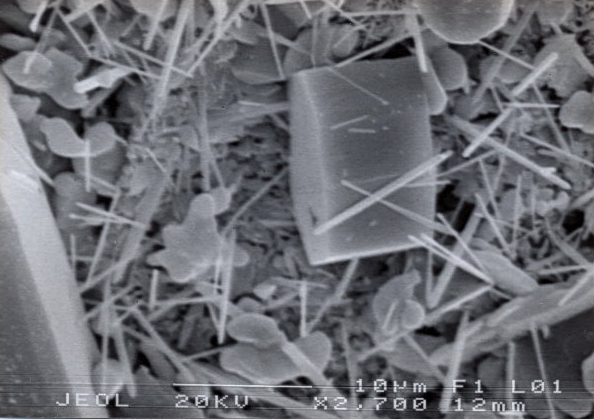
The needle mullite as secondary mullite is created in the "glass phase" of the porcelain firing from the flaky mullite by higher melting temperatures or longer firing times. During this process the grain size of the flake mullite decreases and thousands of small mullite needles are formed at the contact surface of the body and glaze. These interlocking and interlocking "microneedles" (see picture above) act as a stabilizer and increase the strength of the porcelain.
Conclusion: Mullite must always be detectable as an indicator of "real porcelain". Unfortunately, this proof can only be provided by "detection" - as a result of a mineral phase analysis and a chemical laboratory test.
