Malic Acid 101
Um, what’s that? I thought we were talking about wine…
We are! Malic acid is one of the main acids found in grapes. Together, malic and tartaric acid make up about 90% of the berries’ total acid. There are many characteristics that go into a wine’s flavor and taste, and malic acid is certainly one of them. It is imperative for winemakers to keep tabs on the malic acid content throughout the entirety of the winemaking process.
This is the part where Imbibe Solutions comes in to do what we do best – SCIENCE!
What is Malic Acid?
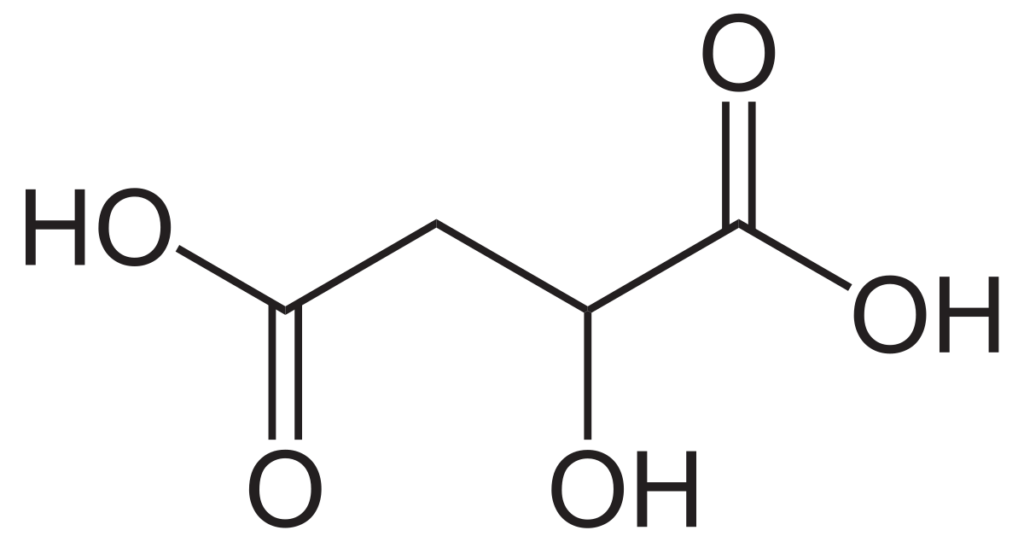
This is the chemical structure of malic acid. If you want to learn more about its chemical properties CHECK OUT THE SDS.
Malic acid is a non-volatile, diprotic, organic acid that is found in all living organisms. It is especially characteristic in unripe fruits, contributing a strong sour, green apple-like taste. The concentration is highest in grapes at the point of veraison and decreases through malic respiration and various types of fermentation.
Fun Fact
Malic comes from the Latin word malum, meaning “apple.”
Why should we care?
The amount of malic acid in the grape juice and throughout the fermentation process plays a large role in how the finished wine tastes. Wines with too much malic acid can taste aggressively sour and tart. However, wines with too little malic acid can be perceived as “flat”.
There is a large spectrum regarding the acidity of wines, and part of the challenge is finding that perfect balance. The amount of malic acid can be manipulated according to the winemaker’s preference, depending on whether they want a smoother or crisper wine. It is important to measure malic acid in your grape juice samples and throughout the fermentation process to monitor any changes that may affect the final taste, balance, and stability of your wine.
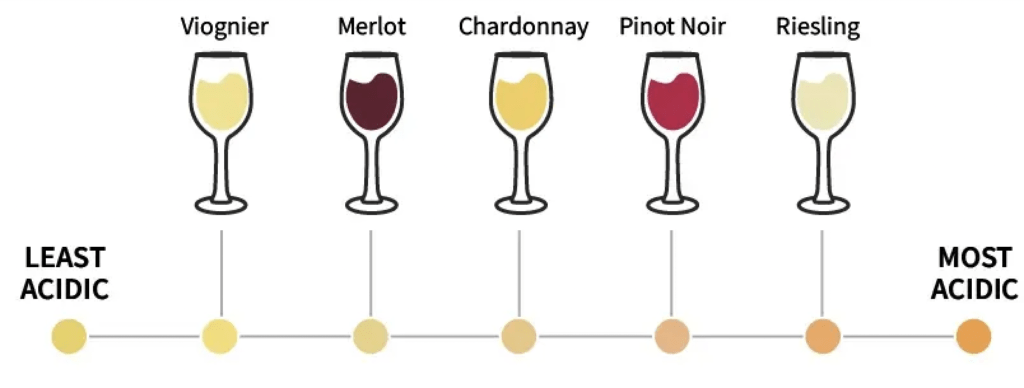
Starting in the vineyard, the concentration of malic acid in berries can be used in conjunction with Brix to test the ripeness of grapes, which can help winemakers decide when the grapes are ready for harvesting. Lower concentrations equate to riper fruit.
During the fermentation process, malic acid content decreases by around 15 percent. However, there is still plenty remaining for it to be a key player in the crispness of the wine. If a winemaker chooses, they can start a secondary fermentation, called malolactic fermentation, to lower or eliminate the malic acid completely.
What is Malolactic Fermentation?
Malolactic fermentation is the conversion of malic acid into lactic acid by bacteria. Lactic acid is less harsh and acidic than malic acid which softens the profile of the wine. This process is done to most reds, as white wine generally wants to maintain that crisper edge – though some white wines benefit too!
In addition to changing the perceived acidity of the wine, malolactic fermentation also contributes new aromas and flavors. One of these flavors is diacetyl. It presents as popcorn butter or butterscotch and gives the famous California chardonnay its characteristic butter flavor and creamy mouthfeel.
For wines that go through full malolactic fermentation, there is an added benefit of bacterial stability. By allowing the lactic acid bacteria to consume and convert all the malic acid, it prevents further growth later in the process.
Usually, malolactic fermentation takes about four weeks to reach completion. There are multiple factors that are present that can either inhibit or stimulate the lactic acid bacteria. A few examples include pH, temperature, oxygen, sulfur dioxide, and alcohol levels.
Fun Fact
A type of bacteria called Oenococcus oeni is most often responsible for malolactic fermentation. It may be naturally present on the fruit or in used barrels, or it may be added by the winemaker.
Check out this video from GuildSomm about malolactic fermentation.
How Do We Measure Malic Acid?
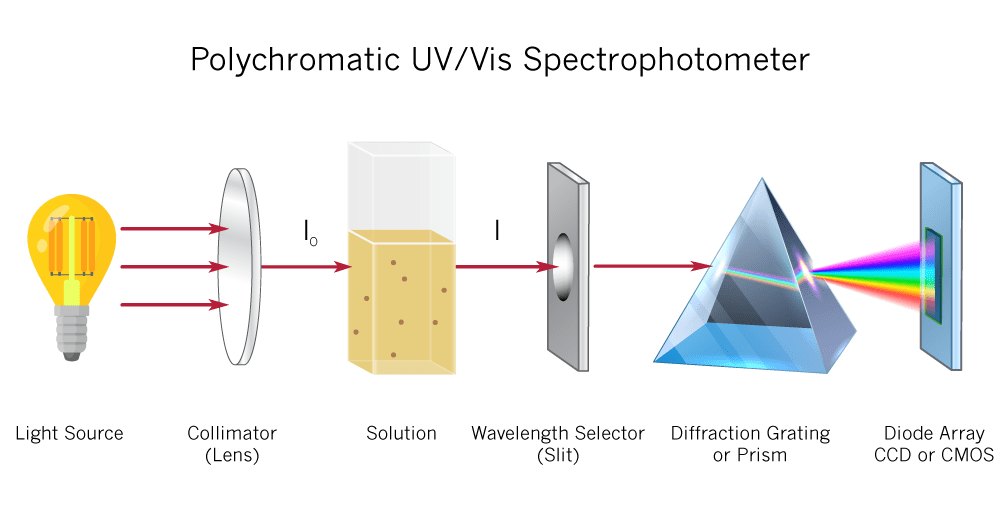
Malic acid is measured in grams per liter. At Imbibe Solutions, we measure malic acid enzymatically using a device called a UV-Vis spectrometer. When a certain enzyme reacts with malic acid, NADH is formed. On the UV-Vis light scale, NADH has the highest absorbance at 340 nm. By measuring the absorbance at 340 nm after this reaction, we can calculate the amount of NADH produced and thereby give an accurate measurement of the sample’s malic acid concentration.

Key Word Review
science words to smarten up your next convo
Diprotic
The physical characteristic of a compound with two hydrogen atoms that can be donated to other compounds. They are more acidic (though not necessarily stronger) than their monoprotic counterparts.
Malolactic Fermentation
aka secondary fermentation
The metabolism of malic acid into lactic acid and CO2 by bacteria. Other byproducts, like diacetyl, are also created in the process.
NADH
Nicotinamide Adenine Dinucleotide + Hydrogen
A coenzyme that transfers H+ ions from one compound to another. It is basically an energy transporter. (Ok, that is an oversimplification, but hopefully you get the idea.)
Resources:
Eisenman, Lum. “Malic Acid.” The Home Winemakers Manual, Del Mar, 1998, pg. 26.
Measurement of Malic Acid in Wine.” The Australian Wine Research Institute, 4 July 2011, https://www.awri.com.au/industry_support/winemaking_resources/laboratory_methods/chemical/malic_acid/
Mooney, Alyson. “Why Is Testing for L-Malic Acid Important in Winemaking?” Randox Food, 27 Sept. 2019, https://www.randoxfood.com/why-is-testing-for-l-malic-acid-important-in-winemaking/
J. Robinson (ed). “Malic Acid.” The Oxford Companion to Wine. Third Edition, Oxford University Press, 2006, pg 421-422.
