If you have ever come to our lab blog you almost certainly have come across a post where we have mentioned “laminins”. This page is a work in progress as sort of introduction to the most important protein in the body (in my opinion!). I’m trying to make this accessible to all, so if you are reading this and it doesn’t make sense can you let me know so I can change it (@lantsandlaminin). If you scroll to the bottom you can see some of these details in video form. You could also check out the wiki page too, but hopefully this one is more enjoyable!
Let’s start with a picture. In the image below, I have stained a recently closed wound with antibodies that recognise one laminin subtype, laminin alpha3, in magenta, and for another protein, fibronectin, in green. The blue stuff in the middle is what is left of the clot that filled the wound.

What you’ll notice is that the laminin makes a nice line running across the tissue. That line is the boundary between the sheets of epithelial cells (above the line) and more amorphous structural region below it; this is called the dermis in skin, or more generally the stroma. The boundary line is a really well-ordered structure that we call either the basement membrane or the basal lamina.
Laminin-associated diseases
All sheets of epithelial, nerves, muscles and vessels have basement membranes. Having a functional basement membrane is critical to holding tissues together.
Laminins are one of two families of structural proteins that are present in every basement membrane, the other being type iv collagens. It is because of this role that a lot of the early research into laminins took place. Whenever the basement membrane of the skin goes wrong either because of an inherited genetic mutation or due to autoimmune disease you get extensive skin blistering in conditions such as junctional epidermolysis bullosa and mucous membrane pemphigoid.
The charity DEBRA has extensive information on this group of inherited disorder and do some great work fundraising to support research toward finding a cure #fightEB. These are truly horrible conditions but research has led to development of new treatment options; my blog post here talks about one such development.

In similar ways, mutations in laminins that are expressed in other tissues leads to different phenotypes. One of the PhD students in the Hamill lab (Liam) works on a disorder termed Pierson syndrome (NIH description of the disorder) which presents with kidney problems and a variety of lens and other eye abnormalities ultimately leading to blindness. Similarly mutations to a laminin that is enriched in muscle tissue gives rise to a muscular dystrophy (MDC1A).
It’s not just inherited disorders that affect laminin function, there is a growing list of other conditions where changes to laminin abundance/expression patterns are associated with the disease. Examples include lung fibrosis, the corneal thinning disease “keratoconus”, chronic non-healing wounds and reductions of specific laminins are associated with ageing.
Laminins are also really important in keeping cells in the appropriate place and can be used as a road for cells to migrate upon. Therefore it’s probably no suprise to hear that changes to laminin expression is associated with different cancer subtypes. One of the focuses of the Hamill lab is squamous cell carcinoma and in particular head and neck squamous cell carcinoma, where, with funding from the British Skin Foundation and North West Cancer Research, we are investigating a potential new diagnostic/prognostic marker and hopefully a new target for therapy. You can read about that project here.
Controlling cell behaviour

The different tissues affected in these different genetic disease points to tissue specific roles for the laminins. There are at least 16 different laminin subtypes and which member of the family is expressed when and where is not only a key determinant of the mechanical properties of the basement membrane but also how the cells respond to it in terms of defining what type of cell they become. Lots of work at the moment is exploring and exploiting this context functional specificity to control how cells behave. This is most useful in tissue engineering fields, where stem cells can be driven down a specific pathway, for example to become and insulin producing pancreatic cells by exposing the cells to right type of laminin for that purpose. Are you a cell biologist? If so, consider using the best laminin for your cell type rather than generic laminin 111; biolamina sell many recombinant laminins at reasonable costs.
Each laminin molecule is made up of three subunits termed alpha, beta and gamma. Each of these subunits are derived from a separate gene of which there are 5 alpha chains, 3 beta and 3 gamma in humans. These assemble inside the cell before being deposited as either an approximately cross, anchor or T-shaped mature protein.
Indeed the critical importance of laminin proteins in holding tissues together and its cruciform structure have being increasingly appreciated by Christians (evangelical Lou Giglio video) or books ‘Laminin,’ Binding Truth of the Cross and Creation (amazon link) and you can even buy laminin themed jewellery
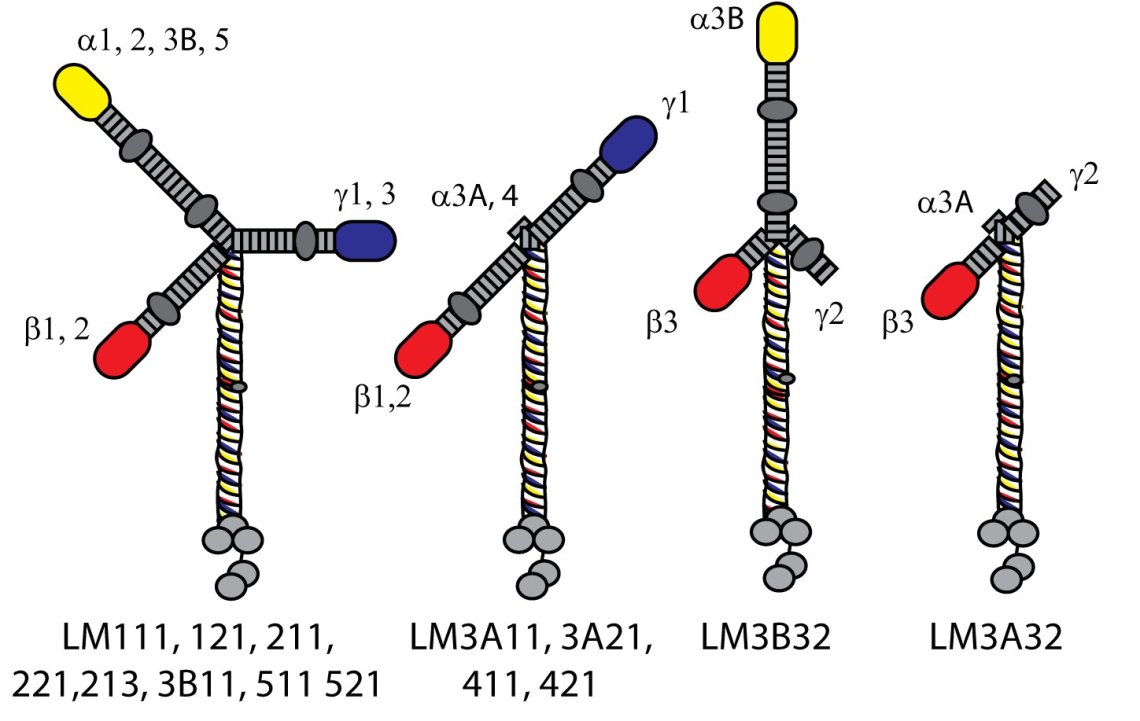
The different cellular responses to the different laminin subunits reflects differences in what other proteins can bind to them and the relative strength of those interactions. Most of this binding occurs at one end (the carboxy terminal) of the alpha subunit (the five gray circles at the bottom of the diagram). There are quite a few types of proteins that bind to laminins but the major complexes that regulate cellular function involve integrins in two complexes termed “hemidesmosomes” and “focal adhesions”.
Holding on: Hemidesmosomes and focal adhesions

To be able to use laminins to hold tissues together or to use them as a substrate for cell migration during processes like wound repair, cells need to assemble complexes of proteins that can link the scaffolding like proteins within to cell to the laminins beneath it. As you could imagine, holding on tight requires slightly different proteins than moving so the cell assembles different type of complexes for each activity (its slightly more complex than this… the two complexes talk to each other and it is probably more to do with the balance between these complexes than one doing one thing and one doing another; you can read about this crosstalk here).
For strong, stable attachment the major protein complex is called a hemidesmosome. These look and act like spot welding points that button down the sheets of cells onto the laminins in the basement membrane. They do this by alpha6beta4 integrin and type XVII collagen binding to the laminin outside the cell and recruiting two other proteins, plectin and BPAG1e on the inside of the cell. Plectin and BPAG1e then go on to bind to the intermediate filament proteins. These intermediate filaments are what provide the structural scaffolding within the cells. Indeed, in epitheliums, the intermediate filament proteins are keratins, the same family of protein that makes hair and nails. I mentioned above the existence of a horrible blistering disorder epidermolysis bullosa. Well, mutations to any of the hemidesmosome proteins lead to different variants of this disease with the level at which the skin splits dependent on where the protein is.
Different complexes are used to drive cell migration, we call these focal contacts or focal adhesions. At its core, focal contact again have an integrin; for laminins integrin alpha3beta1 which again provides an outside to inside cell linkage. Inside the cell literally hundreds of other proteins have been shown to associate with focal adhesions depending on a variety of other states. Some of the big players are paxillin, talin and vinculin and, because there are much better tools to study these, you will often see these proteins used to indicate focal adhesions. Ultimately the focal contact protein complex links to the actin cytoskeleton. Whereas the intermediate filaments are scaffolding proteins, the actin actually does a lot of force transmission. It is through the actin that cells can pull themselves forward and it is at the focal contacts that these forces are transmitted.

Whereas hemidesmosomes are pretty stable, focal contacts are really dynamic. During migration, they rapidly form at the leading front of the cell the mature as cells move over them. The assembly and disassembley dynamics of the focal contacts are a key determinant of how fast a cell is able to move. We study these dynamics using fluorescently tagged paxillin or talin proteins as they can tell us a lot about what is going on in a cell, eg why wound healing is delayed in certain disease situations.
It’s never as simple as this , and some work I was involved in as a postdoctoral researcher showed that hemidesmosomes also contribute to wound repair by controlling the directionality of migration. So, if you think of the cell as a car, the focal contacts are the wheels, the actin cytoskeleton is the drive shaft and the hemidesmosome are part of the steering.
Forming a network
Its not just cell proteins that laminins interact with, they also interact with a variety of other proteins that are outside the cell. A few of the big players are nidogen and perlecan (also basement membrane proteins) but also proteins such as netrins and LaNts are really interesting. LaNts are one of the protein families that numerous people in the Hamill lab work on so there will be a separate page dedicated to them in the near future.

As well as other proteins, some of the laminins also can interact with themselves and each other to form a sort of polymer or higher order network. This polymerisation process enhances the structural integrity of the basement membrane, helping it resist forces such as the stretching that happens in the lung. Whether or not a laminin can form a network depends on how many of the short arms it has – the yellow/red/blue blobs in the diagram above. A lot of really elegant research has shown that for stable interaction to occur it requires interaction between one yellow, one red and one blue blob (laminin N terminal domain). Full network assembly therefore leads to hexagonal arrays in the basement membrane. LaNt and netrins can affect this process… (Specific content on LaNts coming soon!)

We think that context specificity in the laminin functional responses isn’t just about which laminin is expressed and when, it’s also how they are assembled into the matrix. For example, in this pic I have stained the laminin deposited by different epithelial cells for the same laminin; skin on the left, lung on the right. Note the difference in patterns, this reflects the biological requirement for lung cells need to resist stretching forces and so assembles fibres compared the rose like arrangement in skin where most of the forces are in the opposite direction. We actually don’t know too much about how these matrixes are assembled and one of the PhD students (Conro) in my lab is investigating this process in lung tissue.
Stuff, things and more information
Congratulations on making it this far! I’ve decided to stop for now but will likely add to this in the future. Hopefully this intro to laminins has been helpful, even if only to appreciate a little bit about why we care so much about these proteins. This is just the tip of the iceberg; there are about 700 published papers per year that mention laminins and about 24,000 in the 38 years since they were first described. Many of these publications use laminins as a tool or as a read out in disease situations, but even these studies make it clear that it is worth knowing more about this protein family. I find them fascinating and the more I learn the more I want to know more.
You’ve read about laminins, the next step is to read about LaNts or you might enjoy reading about our cancer project that puts all this stuff into real-world applications.
We don’t just work on laminins…you can read more about our lab on the lab website here, and about the people in the Hamill lab here and check out the blog for description of recent events, successes, life events etc etc. Also look out for posts on aniridia and pax6, on keratoconus, and on CTGF and glaucoma coming soon…
If you are looking for a deeper review of the laminin literature (and after reading this I am sure you will!) there are lots of excellent reviews out there; I recommend these by Erhard Hohenester and Peter Yurchenco, Karl Tryggvason, and of course one of our recent one and this one from my time with Jonathan Jones.
Video Intro
I made a video describing lots of this laminin stuff a little while ago; more aimed at uni students studying the basement membrane but it could be useful now so check it out here.
About the author
Dr Kevin Hamill is a Senior Lecturer in Cell and Molecular Biology in the Department of Eye and Vision Science, Institute of Ageing and Chronic Disease. at the University of Liverpool. His lab focuses on cell-matrix interactions; you can read about ongoing work elsewhere on this blog, the lab webpages, or published work. As well as supervising PhD, Masters in Research and Undergraduate research projects, Kevin is the academic lead for the Lab skills Module of MRes Clinical Sciences program where he teaches fundamental life scientists skills.
If you would like to support our research please get in touch, or you can donate directly via this paypal link.



Absolutely fantastic reading, please can you update me on further research.
LikeLike
Most interesting and well written.
LikeLike
thanks! I figured it was time I wrote something a bit more accessible.
LikeLike
I added a page about our cancer project “flipping the switches”, that you might like too
LikeLike