
Contributions
Abstract: PB2460
Type: Publication Only
Background
Hepatic veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS) is a potentially life-threatening complication of hematopoietic stem cell transplant (HSCT); VOD/SOS with multi-organ dysfunction (MOD) may be associated with >80% mortality. Defibrotide is approved for treatment of severe VOD/SOS post-HSCT in the European Union in patients aged >1 month and for VOD/SOS with renal or pulmonary dysfunction post-HSCT in the United States and Canada, but no drug is approved for VOD/SOS prevention. VOD/SOS risk factors include receiving myeloablative conditioning (MAC) or calicheamicin antibody conjugates. Defibrotide as prophylaxis reduced VOD/SOS incidence in high-risk pediatric patients (Corbacioglu et al, Lancet, 2012); data are more limited for adults. A new randomized, parallel group study of defibrotide for prevention of VOD/SOS in high-risk, post-HSCT patients of all ages (≥1 month) is underway (NCT02851407).
Aims
To assess the efficacy and safety of defibrotide prophylaxis plus best supportive care compared with best supportive care alone for prevention of VOD/SOS in adult and pediatric post-HSCT patients at high or very high risk for VOD/SOS.
Methods
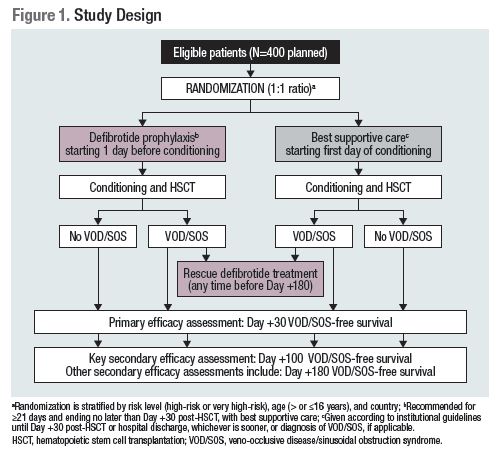
HARMONY is a phase 3, multicenter, randomized study being conducted at ~100 sites worldwide (Figure). High/Very high-risk patients receive defibrotide prophylaxis with best supportive care or best supportive care alone. VOD/SOS is diagnosed according to modified Seattle criteria, or biopsy; a blinded endpoint adjudication committee validates each diagnosis. Patients in either arm who develop VOD/SOS will receive defibrotide rescue treatment. The planned sample size of 400 provides 90% power to detect a 0.46 hazard ratio for the primary endpoint of VOD/SOS-free survival by Day +30. Study eligibility criteria include: scheduled HSCT and high/very high risk of VOD/SOS. High-risk criteria are MAC plus ≥1 additional risk factor: transaminase >2.5× upper limit of normal (ULN), serum total bilirubin >1.5× ULN, cirrhosis, hepatic fibrosis on biopsy, viral hepatitis within 1 year, prior hepatic irradiation, iron overload, or high-risk stage IV neuroblastoma. Very high-risk criteria are ≥1 of the following: osteopetrosis, familial hemophagocytic lymphohistiocytosis or predefined related disorders undergoing MAC, prior gemtuzumab (dose ≥9 mg/m2) or inotuzumab (≥1.5 mg/m2 over 28 days), or Class III high-risk thalassemia. Key exclusion criteria are hemodynamic instability, clinically significant bleeding (or from life-threatening site), or use of medication <24 hours that increases bleeding risk (heparin ≤100 U/kg/day is permitted). The recommended defibrotide dosage is 25 mg/kg/day (in 4 divided doses) for ≥21 days starting the day before conditioning and ending no later than Day +30 post-HSCT. The key secondary outcome is VOD/SOS-free survival by Day +100; other secondary assessments include VOD/SOS-free survival by Day +180, VOD/SOS incidence, disease relapse, and MOD onset and resolution.
Results
As of February 2018, 107 sites are active and 164 patients have been randomized. Enrollment is ongoing.
Conclusion
HARMONY will provide phase 3, randomized study data on the efficacy and safety of defibrotide for the prevention of VOD/SOS in HSCT patients at high/very high risk for VOD/SOS. These findings will extend previous pediatric study data and provide new evidence in adults.
Session topic: 23. Stem cell transplantation - Clinical
Keyword(s): Defibrotide, Hematopoietic cell transplantation, Veno-occlusive disease
Abstract: PB2460
Type: Publication Only
Background
Hepatic veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS) is a potentially life-threatening complication of hematopoietic stem cell transplant (HSCT); VOD/SOS with multi-organ dysfunction (MOD) may be associated with >80% mortality. Defibrotide is approved for treatment of severe VOD/SOS post-HSCT in the European Union in patients aged >1 month and for VOD/SOS with renal or pulmonary dysfunction post-HSCT in the United States and Canada, but no drug is approved for VOD/SOS prevention. VOD/SOS risk factors include receiving myeloablative conditioning (MAC) or calicheamicin antibody conjugates. Defibrotide as prophylaxis reduced VOD/SOS incidence in high-risk pediatric patients (Corbacioglu et al, Lancet, 2012); data are more limited for adults. A new randomized, parallel group study of defibrotide for prevention of VOD/SOS in high-risk, post-HSCT patients of all ages (≥1 month) is underway (NCT02851407).
Aims
To assess the efficacy and safety of defibrotide prophylaxis plus best supportive care compared with best supportive care alone for prevention of VOD/SOS in adult and pediatric post-HSCT patients at high or very high risk for VOD/SOS.
Methods
HARMONY is a phase 3, multicenter, randomized study being conducted at ~100 sites worldwide (Figure). High/Very high-risk patients receive defibrotide prophylaxis with best supportive care or best supportive care alone. VOD/SOS is diagnosed according to modified Seattle criteria, or biopsy; a blinded endpoint adjudication committee validates each diagnosis. Patients in either arm who develop VOD/SOS will receive defibrotide rescue treatment. The planned sample size of 400 provides 90% power to detect a 0.46 hazard ratio for the primary endpoint of VOD/SOS-free survival by Day +30. Study eligibility criteria include: scheduled HSCT and high/very high risk of VOD/SOS. High-risk criteria are MAC plus ≥1 additional risk factor: transaminase >2.5× upper limit of normal (ULN), serum total bilirubin >1.5× ULN, cirrhosis, hepatic fibrosis on biopsy, viral hepatitis within 1 year, prior hepatic irradiation, iron overload, or high-risk stage IV neuroblastoma. Very high-risk criteria are ≥1 of the following: osteopetrosis, familial hemophagocytic lymphohistiocytosis or predefined related disorders undergoing MAC, prior gemtuzumab (dose ≥9 mg/m2) or inotuzumab (≥1.5 mg/m2 over 28 days), or Class III high-risk thalassemia. Key exclusion criteria are hemodynamic instability, clinically significant bleeding (or from life-threatening site), or use of medication <24 hours that increases bleeding risk (heparin ≤100 U/kg/day is permitted). The recommended defibrotide dosage is 25 mg/kg/day (in 4 divided doses) for ≥21 days starting the day before conditioning and ending no later than Day +30 post-HSCT. The key secondary outcome is VOD/SOS-free survival by Day +100; other secondary assessments include VOD/SOS-free survival by Day +180, VOD/SOS incidence, disease relapse, and MOD onset and resolution.
Results
As of February 2018, 107 sites are active and 164 patients have been randomized. Enrollment is ongoing.

Conclusion
HARMONY will provide phase 3, randomized study data on the efficacy and safety of defibrotide for the prevention of VOD/SOS in HSCT patients at high/very high risk for VOD/SOS. These findings will extend previous pediatric study data and provide new evidence in adults.
Session topic: 23. Stem cell transplantation - Clinical
Keyword(s): Defibrotide, Hematopoietic cell transplantation, Veno-occlusive disease


