Abstract
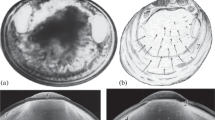
A comparative analysis of the early life history of two common gastropod forms living on the leaves of the Mediterranean seagrass Posidonia oceanica has been carried out, taking into account timing and phases of development. The forms have a different distribution along a depth transect at Ischia Island (Gulf of Naples, Italy), the more squat and ribbed being mainly present between 1 and 15 m depth, with a maximum density at 3 m, the other, between 10 and 30 m. They were distinguished as sibling species, Rissoa italiensis (Verduin, 1985) and Rissoa auriscalpium (L., 1758), on the basis of protoconch size. However, to date, the systematic relationship between them is still unclear. Electrophoretic studies showed lower genetic distance between the two forms along the transect, than among populations from other locations in the Mediterranean Sea. R. auriscalpium have planktonic development, with a higher number of smaller eggs, while R. italiensis have an intracapsular veliger, with hatching juveniles. As for the patterns of development, while the same stages are recognizable in the two gastropod forms up to the formation of the veliger, several differences in timing occur, along with differences in growth and death rates of the embryos. R. auriscalpium hatches as a veliger at the first phase of torsion, about 10 days after spawning, while R. italiensis completes its torsion and metamorphosis within the capsule, and then hatches as a juvenile about 18 days after spawning. The time of encapsulation is what seems to lead to similar developmental sequences with different reproductive patterns and ecological roles, depending on the organization of the elements in the capsule.






Similar content being viewed by others
References
Aartsen JJ van (1982) Synoptic tables of Mediterranean and European conchology. Genus Alvania. Shell 14:156–165
Bouchet P (1989) A review of poecilogony in gastropods. J Mollusc Stud 55:67–78
Bucquoy E, Dautzemberg P, Dollfus G (1882) Les mollusques marins du Roussillon, tome I. Baillière, Paris
Burger JW, Thornton CS (1935) A correlation between the food eggs of Fasciolaria tulipa and the apyrene spermatozoa of prosobranch mollusks. Biol Bull (Woods Hole) 68:253–257
Cadée GC (1998) Rissoa membranacea (J. Adams, 1800) (Gastropoda, Prosobranchia) from the Dutch Wadden Sea. Basteria 61:89–98
Colognola R, Masturzo P, Russo GF, Scardi M, Vinci D, Fresi E (1986) Biometric and genetic analysis of the marine rissoid Rissoa auriscalpium and its ecological implications. Mar Ecol 7:265–285
Gostan G (1966) Aspects cycliques de la morphogénèse de la coquille de Rissoa parva Da Costa (Gasteropoda, Prosobranchia). Vie Milieu 17:9–107
Hickman CS (1992) Reproduction and development of trochacean gastropods. Veliger 35:245–272
Hoagland KE, Robertson R (1988) An assessment of poecilogony in marine invertebrates: Phenomenon or fantasy? Biol Bull (Woods Hole) 174:109–125
Jablonsky D, Lutz RA (1980) Molluscan larval shell morphology. Ecological and paleontological applications. In: Rhoads DC, Lutz RA (eds) Skeletal growth of aquatic organisms. Plenum, New York, pp 323–377
Lebour M (1934) Rissoid larvae as food of the young herring. The eggs and larvae of the Plymouth Rissoidae. J Mar Biol Assoc UK 19:523–539
Levin LA, Zhu J, Creed E (1991) The genetic basis of life-history characters in a polychaete exhibiting planktotrophy and lecithotrophy. Evolution 45:380–397
Munksgaard C (1991) Electrophoretic separation of morphologically similar species of the genus Rissoa (Gastropoda: Prosobranchia). Ophelia 13:97–104
Nordsieck F (1972) Die Europäischen Meeresschnecken (Opisthobranchia mit Pyramidellidae; Rissoacea). Fischer, Stuttgart
Oliverio M (1994) Development vs genetic variation in two Mediterranean rissoid gastropod complexes. J Mollusc Stud 60:461–465
Palmer AR (1985) Quantum changes in gastropod shell morphology need not reflect speciation. Evolution 39:699–705
Pechenik JA (1979) Role of encapsulation in invertebrate life histories. Am Nat 114:859–870
Rehfeldt N (1968) Reproductive and morphological variations in the prosobranch “Rissoa membranacea”. Ophelia 5:157–173
Richter G, Thorson G (1975) Pelagische Prosobranchier-Larven des Golfes von Neapel. Ophelia 13:109–185
Russo GF, Fresi E, Vinci D, Chessa LA (1984) Mollusk syntaxon of foliar stratum along a depth gradient in a Posidonia oceanica (L.) Delile meadow: seasonal variability. In: Boudouresque CF, Jeudy De Grissac A, Olivier J (eds) Proceedings 1st international workshop on Posidonia oceanica beds. GIS Posidonie, Marseille, pp 311–318
Russo GF, Fresi E, Vinci D (1985) The hand-towed net method for direct sampling in Posidonia oceanica beds. Rapp P-V Reun Comm Int Explor Sci Mer Mediterr Monaco 29:175–177
Russo GF, Vinci D, Scardi M, Fresi E (1991) Mollusk syntaxon of foliar stratum along a depth gradient in a Posidonia oceanica bed, vol 3. A year’s cycle at Ischia Island. Posidonia Newsl 4:15–25
Shuto T (1974) Larval ecology of prosobranch gastropods and its bearing on biogeography and paleontology. Lethaia 7:239–256
Smidt E (1938) Notes on the reproduction and rate of growth in R. membranacea (Adams) (Gastropoda Prosobranchiata) in the sound. Vidensk Medd Dan Naturhist Foren 102:169–182
Smith-Gill SJ (1983) Developmental plasticity: developmental conversion versus phenotypic modulation. Am Zool 23:47–55
Spight TM (1976a) Hatching size and the distribution of nurse eggs among prosobranch embryos. Biol Bull (Woods Hole) 150:491–499
Spight TM (1976b) Ecology of hatching size for marine snails. Oecologia 24:283–294
Staiger H (1950) Zur Determination der Nahreier bei Prosobranchiern. Rev Suisse Zool 57:496–503
Staiger H (1951) Cytologische und morphologische Untersuchungen zur Determination der Nahreier bei Prosobranchiern. Z Zellforsch Mikrosk Anat 35:496–549
Strathmann RR (1978) The evolution and loss of feeding larval stages of marine invertebrates. Evolution 32:894–906
Strathmann RR, Fenaux L, Strathmann MF (1992) Heterocronic developmental plasticity in larval sea urchins and its implication for evolution of nonfeeding larvae. Evolution 46:972–986
Thiriot-Quievreux C (1980) Protoconques et coquilles larvaires des mollusques rissoides Méditerranéens. Ann Inst Oceanogr 56:65–76
Thorson G (1950) Reproductive and larval ecology of marine bottom invertebrates. Biol Rev 25:1–45
Verduin A (1976) On the systematics of recent Rissoa of the subgenus Turboella Gray, 1847, from the Mediterranean and European Atlantic coasts. Basteria 40:21–73
Verduin A (1977) On a remarkable dimorphism of the apices in many groups of sympatric, closely related marine gastropod species. Basteria 41:91–95
Verduin A (1982a) How complete are diagnoses of coiled shells of regular build? A mathematical approach. Basteria 45:127–142
Verduin A (1982b) On the taxonomy and variability of recent European and North African marine species of the subgenus Rissostomia Sars, 1878, of the genus Rissoa Desmarest, 1814 (Mollusca, Gastropoda, Prosobranchia). Basteria 45:143–166
Verduin A (1985) On the taxonomy and variability of recent European and North African species of the subgenera Apicularia and Goniostoma of the genus Rissoa (Gastropoda, Prosobranchia). Basteria 49:105–132
Vermeij GJ (1987) Evolution and escalation. An ecological history of life. Princeton University Press, Princeton, N.J., USA
Warén A (1996) Ecology and systematics of the North European species of Rissoa and Pusillina (Prosobranchia, Rissoidae). J Mar Biol Assoc UK 76:1013–1059
Wigham GD (1975) Environmental influences upon the expression of shell form in Rissoa parva (Da Costa). (Gastropoda: Prosobranchia). J Mar Biol Assoc UK 55:425–438
Acknowledgements
We are particularly grateful to M.T. Ghiselin for very useful advice and for a critical review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Cattaneo-Vietti, Genova
Rights and permissions
About this article
Cite this article
Russo, G.F., Patti, F.P. Early life history of two closely related gastropods, Rissoa auriscalpium and Rissoa italiensis (Caenogastropoda: Rissoidae). Marine Biology 147, 429–437 (2005). https://doi.org/10.1007/s00227-005-1586-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-005-1586-9