Abstract
Among mollusks, the octopods stand out due to the almost entire absence of a stabilizing shell, potentially rendering these organisms susceptible to deformation caused by fixation and preservation. Such artifacts have previously been shown to occur especially in specimens of the deep-sea taxon Cirrata, the finned octopods. As an adaptation to their extreme habitat, many cirrates are composed of (semi-)gelatinous tissues, which are particularly prone to distortion, shrinkage, and deformation following fixation and preservation. Using one or more species from each of the eight currently recognized cirrate genera, the present study provides pre- and post-fixation color imagery of entire specimens as well as measurements of taxonomically relevant characters. These novel data illustrate the sometimes drastic effects that short- and long-term chemical treatment entails for this cephalopod taxon. Both structural and color changes may occur when finned octopods are fixed and preserved using routine protocols. However, shrinkage does not occur uniformly in all taxonomically relevant structures. The consequences of such a treatment for species descriptions and identification are discussed and existing anaesthetization, fixation and preservation protocols are summarized.
Similar content being viewed by others
Introduction
To preserve extant zoological material for future reference as well as further study, collected specimens are conventionally treated using a variety of chemical protocols. These lead to the fixation of tissues in order to prevent decay post-mortem and additionally permit preservation of the specimen by preventing microbial degradation during long-term storage. While fixation and preservation form the basis for creation and maintenance of natural history collections, all chemicals routinely employed are known to have one or more side effects (Russell 1963; Steedman 1976; Lincoln and Sheals 1979; Williams and Van Syoc 2007; Simmons 2014). Due to an almost entire absence of stabilizing hard parts, soft-bodied organisms such as cephalopods (Mollusca: Cephalopoda) are particularly prone to deformation, distortion, and shrinkage caused by chemical treatment for fixation and preservation purposes (Boyde and Barber 1969; Cohen 1976; Roper and Sweeney 1983; Andriguetto and Haimovici 1988; Voss and Pearcy 1990; Voight 1991; O’Shea 1997, 1999; Gleadall et al. 2010; Jereb et al. 2014). As a rule of thumb, those cephalopod specimens with more gelatinous tissues suffer increased structural alteration during fixation and preservation than their more muscular peers (Robson 1932; Voight 2001).
Semi-gelatinous to gelatinous species are particularly common among deep-sea cephalopod taxa such as the finned octopods (Octopoda: Cirrata), sister group to the much better-known, predominantly shallow-water finless octopods (Octopoda: Incirrata). In fact, cirrates are the deepest known cephalopods, occurring down to a bathymetric depth of at least 6957 m (Jamieson and Vecchione 2020). In cirrates, life in an extreme habitat has led to a number of structural adaptations, e.g. the presence of elastic, fluid-rich, gelatinous tissues that are supposed to aid in maintaining neutral buoyancy (Aldred et al. 1978, 1983; Roper and Voss 1983). However, these structural properties render cirrates difficult candidates for fixation and long-term preservation (Collins et al. 2001; Collins and Villanueva 2006). For example, a typical artifact known to occur in cirrate museum specimens is that the eyes and fins appear relatively much larger following fixation and preservation due to significant shrinkage of the surrounding mantle tissues (Young and Vecchione 2016). Given that morphometric characters and relational measurements still constitute important, if not essential arguments for cephalopod species description and identification, deformations may have significant taxonomic consequences.
Although the often quite drastic effects of fixation and preservation on cirrate specimens have been known for a long time (e.g. Eschricht 1836; Reinhardt and Prosch 1846; Meyer 1906; Ebersbach 1915), no study has so far systematically documented the degree of deformation, distortion, or shrinkage found in fixed material of this group of cephalopods. By taking advantage of the fact that digital color imagery of freshly caught cirrate specimens has become increasingly available over the past 2 decades (e.g. Piatkowski and Diekmann 2005; Allcock 2014; Shea et al. 2018; Golikov et al. 2020; Ziegler and Sagorny 2021; Verhoeff 2022), we here present a systematic visual and morphometric overview of adult specimens incorporating at least one species per extant cirrate genus. In addition, comparable imagery for selected cirrate juveniles as well as a direct comparison between a cirrate and an incirrate deep-sea specimen treated identically and documented over known time intervals are presented. The combined data provide an overview of structural and color changes exhibited by cirrates chemically treated from about 1 month to almost 20 years. The present article permits a better evaluation of the suitability of certain morphometric and relational characters for cirrate species description and identification. In addition, the study provides an overview of established protocols for the anaesthetization, fixation and preservation of finned octopods.
Materials and methods
Specimens
Selection of specimens for this study was conducted based on several minimum requirements, in particular the availability of (1) high-resolution color photography pre- and post-fixation, (2) scaled imagery, (3) photographs with comparable specimen orientation, and (4) documented archival history. In addition, adult as well as juvenile specimens were selected to cover a large spectrum of ontogenetic stages. The resulting dataset comprises specimens mentioned in previous publications as well as unpublished material. However, all specimens incorporated into the present study (Table 1) are deposited in one of the following museum collections for future reference: BMNH = Natural History Museum, London, United Kingdom; LACM = Natural History Museum of Los Angeles County, Los Angeles, CA, USA; MNHNCL = Museo Nacional de Historia Natural de Chile, Santiago de Chile, Chile; NIWA = National Institute of Water and Atmospheric Research, Wellington, New Zealand; NMNS = National Museum of Natural Science, Taichung City, Taiwan; NMV = Museums Victoria, Melbourne, Victoria, Australia; USNM = Smithsonian National Museum of Natural History, Washington, DC, USA; YPM = Peabody Museum of Natural History, New Haven, CT, USA; ZMB = Museum für Naturkunde, Berlin, Germany; ZMH = Museum der Natur, Hamburg, Germany.
Photography
The photographs shown here were made over the past 4 decades using a broad range of classical and digital cameras with different lighting setups. Wherever possible, specimens were placed into liquid-filled containers and photographed with a uniform background. Where applicable, the photographer of a given image is mentioned in the figure legend. Due to the heterogeneity of the imagery shown here, color standardization using color charts was not implemented. Therefore, numerical color comparisons are not possible using the present dataset.
Measurements and indices
Where possible, morphological characters were measured on the physical specimen itself using calipers or rulers both pre- and post-fixation. In addition, standard measurements were obtained from the literature. However, in multiple cases, measurement data were gathered based on calibrated imagery. These virtual measurements were conducted using the free software Fiji/ImageJ 1.54b (Schindelin et al. 2012). Designation of body measurements and indices follows Collins (2003) and Verhoeff and O’Shea (2022): AL = arm length (here: of longest arm), ALI = arm length index, CLI = cirrus length index, ED = eye diameter, EDI = eye diameter index, FL = fin length, FLI = fin length index, FS = fin span, FuL = funnel length, FuLI = funnel length index, FW = fin width, FWI = fin width index, HW = head width, HWI = head width index, MCL = maximum cirrus length, ML = mantle length (here: dorsal ML, where applicable), MSD = maximum sucker diameter, MW = mantle width, MWI = mantle width index, PA = pallial aperture gape, PAI = pallial aperture gape index, SDI = sucker diameter index, TL = total length. The entire set of morphometric data is provided in Table 1.
Results
Effects of fixation and long-term preservation on adult cirrate specimens
Cirroteuthidae Keferstein, 1866
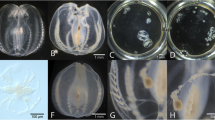
A medium-sized female specimen of Cirrothauma magna (Hoyle, 1855) with 190 mm ML was collected from the Mid-Atlantic Ridge in the Atlantic Ocean at 2995–3071 m depth (Richards and Vecchione 2020). The specimen was photographed following its collection on 04 July 2009 (Fig. 1A, left), subsequently placed in 10% formalin for fixation and later in 50% isopropyl alcohol for preservation. 13 years and 293 days later (28 February 2023), the museum specimen (USNM 1502928) was retrieved from the preservative and photographed again (Fig. 1A, right). Fixation and preservation resulted in significant shrinkage of the posterior-most part of the mantle and a tightening of the primary web around the arms. However, no significant changes in coloration were observed, although the violet hue of the arms seemed to have faded to a uniform pinkish coloration of the entire body. Shrinkage was most pronounced for ML (33%), MW (32%), and AL (29%), while TL shrank by 23% (Table 1).
Effects of the fixation and long-term preservation on selected adult cirrate specimens of the genera Cirrothauma, Cirroteuthis, Stauroteuthis, and Luteuthis—left image depicts fresh and right image shows preserved specimen. A Cirrothauma magna, USNM 1502928, formalin-fixed and preserved in isopropyl alcohol, interval 13 years and 293 days, 190 mm vs. 128 mm ML, ventral views (courtesy Michael Vecchione). B Cirroteuthis cf. muelleri, NMV F245713, formalin-fixed and ethanol-preserved, interval 3 years and 197 days, 116 mm vs. 56 mm ML, dorsal views (courtesy Karen Gowlett-Holmes and Tristan J. Verhoeff, respectively). C Stauroteuthis gilchristi, NIWA 41297, formalin-fixed and ethanol-preserved, interval 14 years and 278 days, 66 mm vs. 33 mm ML, dorsal views (courtesy Darren Stevens and Sadie Mills, respectively). D Luteuthis shuishi, NMNS 002157–00088, formalin-fixed and ethanol-preserved, interval 5–6 years, 185 mm vs. 88 mm ML, dorsal views (courtesy Ping-Ho Ho and Wen-Sung Chung, respectively). Note that color in these images has not been standardized
The small female specimen of Cirroteuthis cf. muelleri Eschricht, 1836 with 116 mm ML shown here (Fig. 1B, left) was collected off East Gippsland in the Tasman Sea at 2338–2581 m depth (Verhoeff 2022). This specimen was photographed fresh on 25 May 2017 and was then placed in 10% formalin for fixation, followed by preservation in 70% ethanol in the museum collection (NMV F245713). After 3 years and 197 days, the specimen was photographed again on 08 December 2020 (Fig. 1B, right). Treatment with fixative and preservative resulted in substantial deformation of the specimen’s body, in particular of the primary web, the fins, and the posterior-most part of the mantle. The overall color of the specimen changed from red-violet to grey-brown and the semi-transparent aspect in certain parts of the body was almost entirely lost. The most pronounced shrinkage was observed for FW (54%) and ML as well as MW (both: 52%), TL shrank by 42% (Table 1).
A medium-sized unsexed specimen of Stauroteuthis gilchristi (Robson, 1924) with 66 mm ML was collected in the Scott seamounts area of the Southern Ocean at 855–879 m depth. The specimen was photographed following its capture on 03 March 2008 (Fig. 1C, left). It was then placed in formalin for fixation and later transferred to ethanol for long-term preservation. After 14 years and 278 days in the collection, the museum specimen (NIWA 41297) was again photographed on 07 December 2022. Chemical treatment resulted in significant changes to the specimen’s aspect with almost all body parts affected, in particular primary web, arms, fins, and mantle. The color of the specimen changed from a vivid, semi-transparent dark-red to a dull, dark-brown hue with patches of a dirty-red color in the mantle area (Fig. 1C, right). Shrinkage was most pronounced for AL (62%) and amounted to 61% for HW, ED, and TL each (Table 1).
Grimpoteuthidae O’Shea, 1999
The single known specimen of Luteuthis shuishi O’Shea & Lu, 2002 was a female collected off Pratas Islands in the South China Sea at 754–767 m depth (O’Shea and Lu 2002). This particularly gelatinous specimen with 185 mm ML was photographed on 23 April 1995 while still fresh (Fig. 1D, left). The organism was fixed using 10% formalin and transferred to 70% ethanol after several days (Lu 2010). About 5–6 years later, the museum specimen (NMNS 002157–00088) was photographed again (Fig. 1D, right). Changes to the appearance of the mantle of this individual are most obvious: due to the reduction of the mantle’s size, the fins appear significantly larger in relation to the body. Because of chemical treatment, the aspect of the specimen changed from a whitish semi-transparency to a non-transparent beige color. Shrinkage in this specimen was most pronounced for HW (55%) as well as ML and MW (both: 52%), while shrinkage of TL was 40% (Table 1).
A medium-sized specimen of Grimpoteuthis abyssicola O’Shea, 1999 with 99 mm ML was obtained off Bermagui in the Tasman Sea at 2687–2821 m depth (Verhoeff and O’Shea 2022). This male was photographed fresh on 27 May 2017 (Fig. 2A, left), subsequently was placed in 10% buffered formalin and later transferred to 70% ethanol. After 3 years and 195 days in solution, the museum specimen (NMV F245714) was again photographed on 08 December 2020 (Fig. 2A, right). Chemical treatment of this semi-gelatinous individual resulted in significant deformation of the mantle and fins in particular. Because of fixation and preservation, semi-transparency of the fins, mantle, and arms was lost, and the specimen showed a dull-brown aspect. Shrinkage in this specimen was most obvious for FuL (56%), but ML (49%) and MW (46%) were also significantly affected. In turn, TL shrank by 34% (Table 1).
Effects of the fixation and long-term preservation on selected adult cirrate specimens of the genus Grimpoteuthis—left image depicts fresh and right image shows preserved specimen. A Grimpoteuthis abyssicola, NMV F245714, formalin-fixed and ethanol-preserved, interval 3 years and 195 days, 99 mm vs. 50 mm ML, dorsal views (courtesy Karen Gowlett-Holmes and Tristan J. Verhoeff, respectively). B Grimpoteuthis angularis, NIWA 68993, formalin-fixed and ethanol-preserved, interval 9 years and 285 days, 73 mm vs. 56 mm ML, dorsal views (courtesy Darren Stevens and Tristan J. Verhoeff, respectively). C Grimpoteuthis imperator, ZMB MOLL 240160, formalin-fixed and ethanol-preserved, interval 1 year and 147 days, 95 mm vs. 81 mm ML, dorsal views. D Grimpoteuthis wuelkeri, ZMH 12244, formalin-fixed and ethanol-preserved, interval 18 years and 245 days, 128 mm vs. 55 mm ML, dorsal views (courtesy Michael Türkay and Bernhard Hausdorf, respectively). Note that color in these images has not been standardized
A medium-sized female specimen of the recently described species Grimpoteuthis angularis Verhoeff & O’Shea, 2022 was collected on the Chatham Rise in the Pacific Ocean at 627–628 m depth (Verhoeff and O’Shea 2022). This specimen with 73 mm ML was photographed fresh on 23 June 2010 (Fig. 2B, left), prior to its fixation in 5% formalin and subsequent transfer to 70% ethanol for preservation. On 03 April 2020, i.e. after 9 years and 285 days, the specimen (NIWA 68993) was removed from its jar for additional photography (Fig. 2B, right). Deformation was quite pronounced, with distortion of arms, mantle, and fins particularly visible. Chemical treatment led to an almost complete loss of transparency of the mantle and fins—the specimen showed a dull-white aspect. Externally visible morphological characters most affected by shrinkage were HW (41%), MW (36%), and PA (32%). Due to the significant shrinkage of the mantle, FL increased by 27%, while TL was reduced by 24% (Table 1).
The single known specimen of the recently described Grimpoteuthis imperator Ziegler & Sagorny, 2021 was collected on Tenji Seamount in the Pacific Ocean at 3912–4417 m depth (Werner et al. 2016; Ziegler 2021; Ziegler and Sagorny 2021). This medium-sized male with 95 mm ML was photographed fresh on 05 July 2016 (Fig. 2C, left) before being placed in a fixative composed of 10% buffered formalin and later transferred to 70% ethanol—please see the cirrate-incirrate comparison below for the precise sequence of events. The museum specimen (ZMB MOLL 240160) was removed from the preservative for photography after 1 year and 147 days on 29 November 2017 (Fig. 2C, right). Changes to the overall aspect were notable for the mantle and primary web in particular: the somewhat inflated mantle appeared reduced in size, while the primary web had retracted. The overall color of the animal had changed from white-purple to white-beige, but with semi-transparency largely preserved. The most prominent shrinkage occurred in MW (26%), FS (16%), and ML (15%), while TL was reduced by 2% (Table 1).
A single, unsexed specimen of Grimpoteuthis wuelkeri (Grimpe, 1920) with 128 mm ML was collected in the Angola Basin area of the Atlantic Ocean at or above 5430 m depth (Piatkowski and Diekmann 2005). The specimen was photographed upon arrival on deck on 30 July 2000 (Fig. 2D, left) and was then fixed using 4% buffered formalin. On land, the museum specimen (ZMH 12244) was transferred to 70% ethanol on 21 March 2001 and photographed again on 01 April 2019, i.e. 18 years and 245 days following its capture (Fig. 2D, right). The most obvious changes to the appearance of the organism following chemical treatment were reductions in the relative size of mantle, fins, and primary web. Due to the deformation of the mantle, the fins appeared much larger in the fixed specimen (Fig. 2D, right). The color of the mantle had changed from a vivid purple primary web and semi-transparent whitish hue of the mantle to a dull purple web and a beige, almost non-transparent aspect. Shrinkage was most pronounced for ML (57%) and MW as well as HW (both: 56%), while TL shrank by 48% (Table 1).
The single known specimen of Cryptoteuthis brevibrachiata Collins, 2004 was obtained from the Porcupine Seabight in the Atlantic Ocean at 2274–2300 m depth (Collins 2004). This medium-sized female with 41 mm ML was photographed fresh on 25 April 2001 (Fig. 3A, left), prior to its fixation in formalin and subsequent long-term preservation in 80% industrial methylated spirit for storage as a museum specimen (BMNH 20040614). After 19 years and 285 days following capture, the animal was photographed again on 04 February 2021 (Fig. 3A, right). Fixation and preservation led to pronounced changes in the overall aspect of the specimen: the arms were bent in an almost rectangular angle toward the dorsal mantle and the fins appeared relatively much larger than in the fresh specimen. The color had changed from light purple, semi-transparent to a dull-beige hue. Shrinkage was most obvious for MW (68%), HW (61%), and FS (41%). As in G. abyssicola and G. angularis (see above), shrinkage of the mantle resulted in an increase in FL (here: by 14%). TL was reduced by 33% (Table 1).
Effects of the fixation and long-term preservation on selected adult cirrate specimens of the genera Cryptoteuthis and Cirroctopus—left image depicts fresh and right image shows preserved specimen. A Cryptoteuthis brevibrachiata, BMNH 20040614, formalin-fixed and preserved in industrial methylated spirit, interval 19 years and 285 days, 41 mm vs. 32 mm ML, dorsal views (courtesy Martin A. Collins and Jon Ablett, respectively). B Cirroctopus cf. glacialis, YPM IZ 049350, formalin-fixed and ethanol-preserved, interval 8 years and 276 days, 85 mm vs. 71 mm ML, dorsal views (courtesy Eric A. Lazo-Wasem). Note that color in these images has not been standardized
Cirroctopodidae Collins & Villanueva, 2006
An unsexed specimen tentatively assigned to Cirroctopus cf. glacialis (Robson, 1930) based on its place of origin was collected off the Antarctic Peninsula in the Southern Ocean at 627–628 m depth (Allcock 2014). The specimen with 85 mm ML was photographed fresh on 04 March 2009 prior to its fixation in formalin and subsequent preservation in 70% ethanol (Fig. 3B, left). The museum specimen (YPM IZ 049350) was photographed again on 05 December 2017, i.e. after 8 years and 276 days following capture. Due to the more muscular habitus of cirroctopodids in general (Vecchione et al. 1998), the deformation of this specimen was not as pronounced as in the more gelatinous cirrate species (Fig. 3B, right). However, the animal appeared more bent toward the ventral side, the arms were somewhat contracted against the upper body, and the fins no longer were oriented perpendicular to the mantle. The overall color scheme had changed from a vivid dark-purple to a dull, pinkish-brown. Shrinkage was less pronounced than in other cirrates, but was most obvious for MW (28%) as well as FW (22%) and ML (16%), while TL shrank by 22% (Table 1).
Opisthoteuthidae Verrill, 1896
A medium-sized female of Opisthoteuthis bruuni (Voss, 1982) with 64 mm ML was found off the Algarrobo coast in the Pacific Ocean at 512 m depth (Ibañez et al. 2011; Pardo-Gandarillas et al. 2021). It was photographed fresh on 04 September 2008 (Fig. 4A, left) before being placed directly in 96% ethanol for fixation and long-term preservation. 14 years and 82 days later, the museum specimen (MNHNCL 300139) was removed from its jar on 25 November 2022 and photographed again (Fig. 4A, right). The pictures illustrate the drastic changes in body structure and color that took place due to chemical treatment. Mantle, primary web, fin, and arm tissues contracted significantly, and the entire animal appeared compressed. The color of the specimen changed from a vivid, sprinkled, bright-red semi-transparency to a dull black-to-beige aspect without any transparent tissues. Shrinkage was most pronounced for FW (64%) and ML as well as AL (both: 63%). Correspondingly, shrinkage of TL was observed to be 57% (Table 1).
Effects of fixation and long-term preservation on selected adult cirrates of the genus Opisthoteuthis—left image depicts fresh and right image shows preserved specimen. A Opisthoteuthis bruuni, MNHNCL 300139, ethanol-fixed and ethanol-preserved, interval 14 years and 82 days, 64 mm vs. 24 mm ML, dorsal and left lateral view (courtesy Diana Párraga and Catalina Amanda Merino Yunnissi, respectively). B Opisthoteuthis chathamensis, NIWA 68989, formalin-fixed and ethanol-preserved, interval 12 years and 171 days, 55 mm vs. 32 mm ML, dorsal views (courtesy Darren Stevens and Sadie Mills, respectively). C Opisthoteuthis pluto, NMV F245704, formalin-fixed and ethanol-preserved, interval 5 years and 190 days, 104 mm vs. 61 mm ML, dorsal views (courtesy Robert Zugaro and Julian K. Finn, respectively). D Opisthoteuthis robsoni, NIWA 68987, formalin-fixed and ethanol-preserved, interval 12 years and 171 days, 78 mm vs. 56 mm ML, dorsal views (courtesy Darren Stevens and Sadie Mills, respectively). Note that color in these images has not been standardized
An almost globose, unsexed specimen of Opisthoteuthis chathamensis O’Shea, 1999 was collected on the Chatham Rise in the Pacific Ocean at 1176–1178 m depth. Following its capture on 19 June 2010, the animal with 55 mm ML was photographed while still fresh (Fig. 4B, left). Fixation and preservation most likely consisted of formalin and subsequent ethanol treatment of the museum specimen (NIWA 68989). On 07 December 2022, i.e. 12 years and 171 days later, the specimen was again photographed (Fig. 4B, right). The images document significant changes to primary web, mantle, and fin shape. The color of the initially not very transparent specimen changed from a vivid purple to a dull red-brown aspect. Shrinkage was most pronounced for HW (44%), MW (43%), and ML (42%), while TL shrank by 63% (Table 1).
The medium-sized, unsexed specimen of Opisthoteuthis pluto Berry, 1918 with 104 mm ML obtained from the Bass Strait in the Pacific Ocean at 2692–2760 m depth was photographed fresh on 22 May 2017 (Fig. 4C, left) and was then fixed using 10% formalin and later preserved in 70% ethanol. After an interval of 5 years and 190 days, the museum specimen (NMV F245704) was once again photographed (28 November 2022). Immediately notable was the overall reduction in mantle size as well as the tightening of the primary web around the arms (Fig. 4C, right). In addition, the eyes appeared much larger in the fixed specimen. Surprisingly, the overall color scheme did not change much, although part of the semi-transparency had waned. Nonetheless, shrinkage was quite pronounced, most so for HW (53%), MW (50%), and ML (41%), while TL shrank by 30% (Table 1).
A small-sized, unsexed specimen of Opisthoteuthis robsoni O’Shea, 1999 with 78 mm ML was obtained from the Chatham Rise in the Pacific Ocean at 1,599–1,619 m depth. The animal was photographed fresh on 19 June 2010 (Fig. 4D, left) prior to a likely treatment using formalin and later 70% ethanol. After an interval of 12 years and 171 days, the museum specimen (NIWA 68987) was photographed on 07 December 2022 (Fig. 4D, right). Predominantly mantle and primary web tissues appeared to be affected by chemical treatment. The fins of the fixed specimen seemingly were larger compared to the fresh specimen. The color did not change much, although the more vivid appearance of the fresh specimen was replaced by a rather dull hue. Shrinkage was most pronounced for HW (43%), MW (30%), and ML (28%), while TL shrank by 22% (Table 1).
Effects of fixation and long-term preservation on premature cirrate specimens
A single hatchling of Grimpoteuthis sp. emerged from a cirrate egg capsule following collection of its deep-sea octocoral host organism (Anthozoa: Octocorallia) on Kelvin Seamount in the Atlantic Ocean at 1965 m depth (Shea et al. 2018; Ziegler 2018, 2021; Ziegler et al. 2021; Ziegler and Miller 2024a). Immediately afterwards, the hatchling with 13 mm ML was photographed fresh on 31 August 2005 (Fig. 5A, left). Following its direct observation on-board ship, the cirrate juvenile specimen was fixed in 4% formalin and later transferred to 70% ethanol. 10 years and 59 days later, the museum specimen (USNM 1460905) was photographed again on 29 October 2015 (Fig. 5A, center and right). Chemical treatment resulted in deformation of the fins and the mantle tissues in particular. The color of the specimen changed from a vivid dark-red with semi-transparent fins to a dull beige with pronounced transparency of mantle and fins, likely caused by the almost entire loss of the original pigmentation (Fig. 5A, center and right). Shrinkage was most pronounced for FW and MSD (both: 25%) as well as FS (15%), while TL shrank by 6% (Tab. 1). It is noteworthy to mention that this premature hatchling was characterized by the presence of a large internal yolk sac (Shea et al. 2018; Ziegler et al. 2021), potentially restricting a more pronounced shrinkage of the surrounding body parts.
Effects of the fixation and long-term preservation on selected premature cirrate specimens of the genera Grimpoteuthis and Opisthoteuthis—left image depicts fresh and right images show preserved specimen. A Grimpoteuthis sp., USNM 1460905, formalin-fixed and ethanol-preserved, interval 10 years and 59 days, 13 mm vs. 12 mm ML, dorsal and ventral views (courtesy Timothy M. Shank and Elizabeth K. Shea, respectively). B Grimpoteuthis sp., USNM 1460906, formalin-fixed and ethanol-preserved, interval 10 years and 73 days, 10 mm vs. 10 mm ML, dorsal and ventral views (courtesy Timothy M. Shank and Elizabeth K. Shea, respectively). C Opisthoteuthis sp., LACM DISCO 4111, ethanol-fixed and ethanol-preserved, interval 7 years and 282 days, 8 mm vs. 7 mm ML, dorsal and ventral views (courtesy Cheryl A. Brantley and Regina Wetzer, respectively). Note that color in these images has not been standardized
Another single, but less mature hatchling of Grimpoteuthis sp. with 10 mm ML was obtained from the Corner Rise Seamounts in the Atlantic Ocean at 2068 m depth (Ziegler 2021; Ziegler et al. 2021; Ziegler and Miller 2024a). Following photography immediately after hatching on 21 August 2005 (Fig. 5B, left), the specimen was fixed using 4% formalin and then transferred to 70% ethanol for long-term preservation as a museum specimen (USNM 1460906). The fixed individual was photographed again after 10 years and 73 days on 02 November 2015 (Fig. 5B). All external organ systems were affected by deformation: arms, primary web, mantle, and fins. The color of the specimen changed from a vivid orange with semi-transparent fins and arms to a dull-beige aspect with pronounced transparency of mantle and fins, again likely caused by the almost entire loss of the original pigmentation (Fig. 5B, center and right). Shrinkage was most obvious for AL and MSD (both: 45%) as well as FW (33%), while TL shrank by 24% (Table 1). As in the previous specimen, this premature hatchling was characterized by the presence of a large internal yolk sac (Ziegler et al. 2021).
An early juvenile of Opisthoteuthis sp. was dredged off Palos Verdes in the Pacific Ocean at 458–459 m depth (Ziegler and Miller 2024b). Prior to its direct fixation in 95% ethanol, this individual with 8 mm ML was photographed fresh on 12 November 2009 (Fig. 5C, left). After 7 years and 282 days in the fixative, the museum specimen (LACM DISCO 4111) was once again photographed on 21 August 2017 (Fig. 5C, center and right). Fixation led to a significant degree of deformation in all body parts, in particular of the mantle tissues. The color of the specimen changed from a vivid orange-red to a dull beige with underlying reddish tones (Fig. 5C, center and right). Shrinkage was most pronounced for AL (46%), HW (25%), and MW (20%), while TL shrank by 14% (Table 1). The absence or presence of an internal yolk sac could not be ascertained.
Direct comparison between cirrate and incirrate adult specimens
One cirrate as well as several incirrate specimens were collected in 2016 during deep-sea dredging operations in the North Pacific Ocean. These specimens were all treated in the exact same way following capture (Werner et al. 2016). This provided the opportunity to use two of these specimens as reference material for a comparative study on the effects of fixation and preservation in specimens from each of the two octopod lineages.
The cirrate specimen was the above-mentioned male of the newly described species G. imperator. This specimen with 95 mm ML was collected and photographed fresh on 05 July 2016 (Fig. 6A, left). Its mantle was filled turgescent, the fins were inflated, and mantle as well as funnel were covered by numerous red pigment spots. In addition, the ventral surface of primary web and arms as well as the posterior rim of the fins were dark red (Fig. 6A, left). Following photography, the specimen was transferred into a drum filled with 5 l of 4% buffered formalin. After 9 months and 14 days in the fixative, the museum specimen (ZMB MOLL 240160) was removed from the drum for photography on 19 April 2017 (Fig. 6A, center left). Shrinkage was most pronounced for ML (7%), while TL shrank by 1% (Table 1). After another 5 months and 3 days in the fixative, the specimen was photographed again on 22 September 2017 (Fig. 6A, center)—no further shrinkage was observed. Following this round of photography, the specimen was then transferred to 70% ethanol for long-term preservation. After 2 months and 7 days in the preservative, the specimen was photographed again on 29 November 2017 (Fig. 6A, center right). Shrinkage as compared to the fresh specimen was most pronounced for ML (15%) as well as HW and FW (both: 9%), while TL shrank by 2%. Following another year and 146 days in the preservative, the cirrate was photographed for the last time on 24 April 2019 (Fig. 6A, right). Compared to the fresh specimen collected and photographed 2 years and 293 days earlier, shrinkage was most pronounced for FW (23%), ML (16%), and HW (13%), while TL shrank by 3%—note that ED did not change at all (Table 1).
Comparison of the effects of an identical fixation and long-term preservation protocol on one adult cirrate and one adult incirrate specimen. A Grimpoteuthis imperator, ZMB MOLL 240160. Photographed fresh following collection on 05 July 2016 with 95 mm ML (left), formalin-fixed for 9 months and 14 days with 88 mm ML (center left) as well as for further 5 months and 3 days with 88 mm ML (center), then ethanol-preserved for 2 months and 7 days with 81 mm ML (center right) as well as an additional 1 year and 146 days with 80 mm ML (right), right lateral views. B Muusoctopus sp., ZMB MOLL 240161. Photographed fresh following collection on 27 July 2016 with 48 mm ML (left), formalin-fixed for 8 months and 23 days with 47 mm ML (center left) as well as further 5 months and 3 days with 46 mm ML (center), then ethanol-preserved for 2 months and 7 days with 46 mm ML (center right) as well as an additional 1 year and 146 days with 45 mm ML (right), dorsal views. Note that color in these images has not been standardized
The incirrate specimen was a female of Muusoctopus sp. collected in the Alpha Fracture Zone of the Bering Sea at 2367–2713 m depth (Werner et al. 2016; Sagorny 2017). This deep-sea finless octopod specimen (ZMB MOLL 240161) with 48 mm ML was photographed fresh on 27 July 2016 (Fig. 6B, left) and subsequently treated in the exact same way as the previous specimen. Directly following capture, the partially semi-transparent body was uniformly white, except for the light-red ventral surface of arms and primary web (Fig. 6B, left). The mantle was ovoid and the overall appearance muscular and robust. All following photographic sessions were executed on the exact same days as for the cirrate specimen mentioned above. On 19 April 2017 (Fig. 6B, center left), shrinkage was most pronounced for ML (2%), while TL shrank by 1% (Table 1). On 22 September 2017 (Fig. 6B, center), shrinkage compared to the fresh specimen was 4% for ML and 3% for HW, while TL was still reduced by 1%. Following transfer to 70% ethanol, shrinkage on 29 November 2017 (Fig. 6B, center right) was still the same as for the previous session, except for TL (2%). On the final photography session on 24 April 2019 (Fig. 6B, right) shrinkage was, compared to the fresh specimen, most pronounced for ML (6%) with the remainder of the morphological parameters unchanged, incl. ED (Table 1).
Discussion
Although common knowledge for anyone who ever observed or analyzed cirrate specimens in museum collections, the imagery shown here provides graphic evidence for the degree of alteration that such delicate material can undergo when being subjected to chemical fixation and long-term preservation. However, fixation and preservation artifacts—in particular shrinkage—are known to occur not only in cirrate, but also in deep-sea incirrate octopods (Voight 2001) as well as cephalopods in general (Cohen 1976; Andriguetto and Haimovici 1988; Voight 1991; O’Shea 1997; González et al. 1998), in addition to other metazoan taxa such as annelids (Howmiller 1972), bivalves (Mills et al. 1981), or tunicates (Nishikawa and Terazaki 1996; Mitchell et al. 2021).
Due to their often semi-gelatinous to gelatinous body composition, cirrates are particularly prone to exhibit the negative side effects of common fixation and preservation methods (Collins and Villanueva 2006). For instance, Collins and Henriques (2000) described how the appearance of specimens of Stauroteuthis gilchristi—a cirrate species with particularly gelatinous tissues—changed during preservation, in particular when treated directly with alcohol: in this scenario, the authors noted general shrinkage of arms and tissues, a significant reduction in TL and PA, suckers appearing much closer together than in specimens analyzed fresh or treated with formalin, tissues being no longer transparent with eyes and internal organs no longer visible, and fins seemingly being located much closer to the apex of the mantle. Unfortunately, such drastic changes to a specimen’s aspect can become scientifically, instead of merely aesthetically problematic, as cephalopod species description and identification rely on precise measurements and calculation of morphometric indices (Roper and Voss 1983). Cirrate taxonomists have become increasingly aware of this issue and have previously sought to quantify the changes occurring during fixation by comparing measurements carried out pre- and post-fixation. Such comparisons provided values for shrinkage of 17–32% ML (Collins et al. 2001) and 30% ML (Guerra et al. 1998) for Cirrothauma magna or 28–39% ML and 6–23% TL for S. gilchristi (Collins and Henriques 2000) in cirroteuthids. In grimpoteuthids, Luteuthis shuishi exhibited a shrinkage of 53% ML (O’Shea and Lu 2002), while Grimpoteuthis imperator showed a reduction of 16% ML (Ziegler and Sagorny 2021) and Cryptoteuthis brevibracchiata of 13% ML (Collins 2004) following chemical treatment. The present study expands these findings to selected species from all eight extant cirrate genera and underlines the significant changes that occur in members of this taxon even within relative short periods of time and even if restricted to fixation using buffered formalin. The present morphometric data (Table 1) also support the previous finding that shrinkage does not occur uniformly in all taxonomically relevant structures, thus rendering the use of morphometric indices based on ML alone problematic, at least for museum material. The comparative experiment using cirrate and incirrate adults illustrates that it is indeed the (semi-)gelatinous tissues found in most cirrates that undergo pronounced changes—and significantly more so than the muscular tissues even of deep-sea incirrates (Voight 2001).
Fortunately, the internal anatomy seems to be less affected by shrinkage due to the histological properties of the viscera (Meyer 1906; Ebersbach 1915; Robson 1932; González et al. 1998). For example, Meyer (1906) noted that despite fixation in formalin and preservation in ethanol resulting in a suboptimal state of the internal organs in an adult specimen of Opisthoteuthis depressa Ijima & Ikeda, 1895, he was still able to obtain thin sections suitable for histological study. Consequently, internal organs as well as external structures not significantly affected by chemical treatment due to their composition (e.g. suckers, nodules) should be incorporated more consistently into future cirrate species descriptions or re-descriptions of existing taxa, as recently successfully shown for several new species (Verhoeff 2022; Verhoeff and O’Shea 2022). Apart from invasive dissections and time-consuming histological analyses, results on cephalopod internal anatomy can also be obtained using non-invasive imaging techniques such as magnetic resonance imaging or contrast-enhanced micro-computed tomography (Ziegler et al, 2011, 2018, 2021; Xavier et al. 2015; Shea et al. 2018; Ziegler and Sagorny 2021; Ziegler and Ziegler 2022).
Usually of less taxonomic or systematic, but certainly of ecological and behavioral value are descriptions of a specimen’s coloration. Unfortunately, chemical treatment of cirrates results in sometimes significant changes to their original coloration. For example, Aldred et al. (1983) noted that an adult specimen of Cirrothauma murrayi Chun, 1911 was “a red brown colour virtually all over the body and arms” when caught alive, while chemical treatment resulted in a purplish color of the preserved specimen. Furthermore, Collins and Henriques (2000) described that in preserved Stauroteuthis coloration of the animals was more opaque than in the fresh specimens. In addition, Golikov et al. (2020) noted that in Opisthoteuthis borealis Collins, 2005 a live specimen was of a “brick-orange color”, while the fixed specimen was reddish and then “brown with a dark-violet color of the oral surface of the web”. In fact, Meyer (1906) found that in his preserved specimen of O. depressa, the color was not produced by pigment cells, but by a subepithelial layer of connective tissue fibers that stored a dark-brown pigment—he concluded that the color of a living specimen cannot be inferred by observing a preserved specimen due to the structural (and chemical) changes caused by fixative and preservative. The often quite pronounced changes in specimen coloration documented here provide further evidence for the negative effects that both formalin and ethanol can have on the natural coloration of cirrates, suggesting that considerable caution should be used when employing this specific character set for scientific inferences.
Based on the protocols listed here (Table 1) as well as those previously published (Roper and Sweeney 1983, Vecchione et al. 2002), the following recommendations for fixation and preservation of cirrate specimens can be made. Although finned octopods usually arrive dead on deck due to their deep-sea provenance, some species may be caught alive. While no specific protocol on management of pain, suffering, and distress has been published for this specific cephalopod taxon, guidelines applicable to other cephalopods and, more specifically, incirrate octopods should be followed. These include the transfer of the specimen to a container filled with seawater and ethanol or magnesium chloride for anaesthesia and terminal anaesthesia (Messenger et al. 1985; Boyle 2010; Cooper 2011; Andrews et al. 2013; Gleadall 2013; Butler-Struben et al. 2018). In addition to abiding by ethical considerations and laws, this approach avoids rapid specimen contraction, thus enhancing the chances of obtaining good quality material for long-term preservation (Mandy Reid, personal communication). In addition, Vecchione et al. (1998) describe relaxing and then killing Cirroctopus by placing them in iced freshwater on-board ship.
Although shrinkage may still occur even when using low-percentage solutions of formalin buffered in seawater, the present data and previous publications indicate that formalin remains the fixative of choice, at least in the foreseeable future. However, this so-called “formalin paradigm” is being increasingly challenged—mostly due to the carcinogenicity (to the human) of the chemical itself and difficulties with subsequent DNA extraction, but also due to its negative side effects on specimen structure and coloration (Simmons 2019); consequently, new formulas that aim to replace formalin are available on the market (Cannavacciuolo et al. 2021). However, until such novel fixatives have been successfully tested on cirrates or other cephalopods, weak concentrations of formalin (4–5%) buffered in seawater or alternatively Steedman’s solution (10% propylene glycol, 2.5% formalin or 1% concentrated formaldehyde, and 0.5–1% propylene phenoxetol in seawater) should be used for fixation (Steedman 1976; Collins and Henriques 2000; Simmons 2014), and preferably also for long-term preservation (Martin A. Collins, personal communication). For fixation, the specimen should ideally be relaxed (see above) and then placed in a container filled with a liquid volume at least five times (Jereb et al. 2014), but preferentially ten times the volume of the specimen—make sure that the specimen is completely covered by the fixative, that its arms are outstretched and that it is neither bent nor folded (Mandy Reid, personal communication). In addition, check whether the fixative has entered the mantle cavity, either by injecting the fixative or by stroking the mantle to release air trapped inside the mantle cavity. If transfer to a long-term preservative on alcohol basis is unavoidable, the specimen should be kept in the formalin fixative for at least two weeks, then rinsed in tap water 2–3 times with vigorous soaking, and finally be transferred to 70% ethanol (or alternatively 40% isopropyl alcohol) using a gradual series.
As the present study illustrates, cirrate specimens in museum collections have been treated using a broad array of methods and protocols over different time scales, making a morphometric and color-based comparison between individuals even of a given species difficult. Nonetheless, certain patterns of shrinkage or loss of color in particular characters (e.g. primary web, arms) could emerge if more material is observed in the future—such data could potentially lead to the establishment of correction factors as recently introduced for specimen weight (Golikov et al. 2020, 2022). Furthermore, while previous as well as the above-mentioned suggestions regarding anaesthetization, fixation and preservation of cirrates are certainly good to know, the reality on-board a research vessel often precludes the exact application of such guidelines. We, therefore, hope that the overview of the effects of fixation and preservation on finned octopods given here will provide additional stimulus and direction to develop a standardized set of morphological characters that can be used for species description and identification independent of the sometimes drastic artifacts known to be caused by the chemical treatment of finned octopods.
Data/code availability
Morphometric data are available in Table 1. All data generated or analysed during this study are included in this published article.
References
Aldred RG, Nixon M, Young JZ (1978) The blind octopus, Cirrothauma. Nature 275:547–549
Aldred RG, Nixon M, Young JZ (1983) Cirrothauma murrayi Chun, a finned octopod. Philos Transac Royal Soc London B 301:1–54
Allcock AL (2014) Southern Ocean octopuses. In: De Broyer C, Koubbi P, Griffiths HJ, Raymond B, d’Udekem d’Acoz C, Van de Putte AP, Danis B, David B, Grant S, Gutt J, Held C, Hosie G, Huettmann F, Post A, Ropert-Coudert Y (eds) Biogeographic Atlas of the Southern Ocean. SCAR Publications, Cambridge, pp 129–133
Andrews PLR, Darmaillacq AS, Dennison N, Gleadall IG, Hawkins P, Messenger JB, Osorio D, Smith VJ, Smith JA (2013) The identification and management of pain, suffering and distress in cephalopods, including anaesthesia, analgesia and humane killing. J Exp Mar Biol Ecol 447:46–64
Andriguetto JM Jr, Haimovici M (1988) Effects of fixation and preservation methods on the morphology of a loliginid squid (Cephalopoda: Myopsida). Am Malacol Bull 6:213–217
Boyde A, Barber VC (1969) Freeze-drying methods for the scanning electron-microscopical study of the protozoon Spirostomum ambiguum and the statocyst of the cephalopod mollusc Loligo vulgaris. J Cell Sci 4:223–239
Boyle PR (2010) Cephalopods. In: Hubrecht R, Kirkwood J (eds) The UFAW handbook on the care and management of laboratory and other research animals, 8th edn. Wiley-Blackwell, Chichester, pp 794–817
Butler-Struben HM, Brophy SM, Johnson NA, Crook RJ (2018) In vivo recording of neural and behavioral correlates of anesthesia induction, reversal, and euthanasia in cephalopod molluscs. Front Physiol 9:109
Cannavacciuolo A, Chiarore A, Munari M (2021) A cold bath for a formalin-free laboratory: alternative fixative methods in early developmental stages of the sea urchin Paracentrotus lividus (Lamarck, 1816). Invertebr Surviv J 18:11–18
Cohen AC (1976) The systematics and distribution of Loligo (Cephalopoda, Myopsida) in the western North Atlantic, with descriptions of two new species. Malacologia 15:299–367
Collins MA (2003) The genus Grimpoteuthis (Octopoda: Grimpoteuthidae) in the north-east Atlantic, with description of three new species. Zool J Linn Soc 139:93–127
Collins MA (2004) Cryptoteuthis brevibracchiata: a new species and genus of cirrate octopod (Octopoda: Cirrata). J Molluscan Studies 70:263–267
Collins MA, Henriques C (2000) A revision of the family Stauroteuthidae (Octopoda: Cirrata) with redescriptions of Stauroteuthis syrtensis and S. gilchristi. J Mar Biol Assoc UK 80:685–697
Collins MA, Villanueva R (2006) Taxonomy, ecology and behaviour of the cirrate octopods. Oceanogr Mar Biol Annu Rev 4:277–322
Collins MA, O’Dea M, Henriques C (2001) A large Cirroteuthis magna (Cephalopoda: Cirroctopoda) caught on the Cape Verde Terrace (North Atlantic). J Mar Biol Assoc UK 81:357–358
Cooper JE (2011) Anesthesia, analgesia, and euthanasia of invertebrates. ILAR J 52:196–204
Ebersbach A (1915) Zur Anatomie von Cirroteuthis umbellata Fischer und Stauroteuthis sp. Z wiss Zool 113:361–483
Eschricht DF (1836) Cirroteuthis mülleri, eine neue Gattung der Cephalopoden bildend. Nova Acta Acad Caes Leop-Carol 18:626–634
Gleadall IG (2013) The effects of prospective anaesthetic substances on cephalopods: summary of original data and a brief review of studies of the last two decades. J Exp Mar Biol Ecol 447:23–30
Gleadall IG, Guerrero-Kommritz J, Hochberg FG Jr, Laptikhovsky VV (2010) The inkless octopuses (Cephalopoda: Octopodidae) of the southwest Atlantic. Zool Sci 27:528–553
Golikov AV, Blicher ME, Gudmundsson G, Manushin IE, Poulsen JY, Zakharov DV, Sabirov RM (2020) Flapjack devilfish in the northern North Atlantic: morphology, biology and ecology of Opisthoteuthis borealis (Cephalopoda, Octopoda, Cirrata). Mar Biodivers 50:108
Golikov AV, Artemev GM, Blicher ME, Gudmundsson G, Jorgensen LL, Olafsdottir SH, Walkusz W, Zakharov DV, Zimina OL, Sabirov RM (2022) Deep and cold: are Boreal and Arctic finned octopods, Stauroteuthis syrtensis and Cirroteuthis muelleri (Cephalopoda, Octopoda, Cirrata) ecological analogues? Deep-Sea Res Part I 181:103706
González AF, Guerra A, Pascual S, Briand P (1998) Vulcanoctopus hydrothermalis gen. et sp. nov. (Mollusca, Cephalopoda): an octopod from a deep-sea hydrothermal vent site. Cah Biol Mar 39:169–184
Guerra A, Villanueva R, Nesis KN, Bedoya J (1998) Redescription of the deep-sea cirrate octopod Cirroteuthis magna Hoyle, 1885, and considerations on the genus Cirroteuthis (Mollusca: Cephalopoda). Bull Mar Sci 63:51–81
Howmiller RP (1972) Effects of preservatives on weights of some common microbenthic invertebrates. Trans Am Fish Soc 4:743–746
Ibañez CM, Pardo-Gandarillas MC, Párraga D, Zilleruelo M, Sellanes J (2011) Cefalópodos recolectados en el talud continental de Chile central. Amici Molluscarum 19:37–40
Jamieson AJ, Vecchione M (2020) First in situ observation of Cephalopoda at hadal depths (Octopoda: Opisthoteuthidae: Grimpoteuthis sp). Marine Biol 167:82
Jereb P, Roper CFE, Norman MD, Finn JK (2014) Cephalopods of the world, volume 3, octopods and vampire squids. FAO Species Cat Fishery Purp 4:1–352
Lincoln RJ, Sheals JG (1979) Invertebrate animals – collection & preservation. Cambridge University Press, Cambridge
Lu CC (2010) A new species of Opisthoteuthis, O. dongshaensis sp. nov., from the South China Sea (Octopoda: Cirrata: Opisthoteuthidae). Zool Stud 49:405–420
Messenger JB, Nixon M, Ryan KP (1985) Magnesium chloride as an anaesthetic for cephalopods. Comparat Biochem Physiol Part C: Toxicol Pharmacol 82:203–205
Meyer WT (1906) Die Anatomie von Opisthoteuthis depressa (Ijima und Ikeda). Z wiss Zool 85:183–209
Mills EL, Pittman K, Munroe B (1981) Effect of preservation on the weight of marine benthic invertebrates. Canad J Fish Aquatic Sci 39:221–224
Mitchell DG, Edgar A, Martindale MQ (2021) Improved histological fixation of gelatinous marine invertebrates. Front Zool 18:29
Nishikawa J, Terazaki M (1996) Tissue shrinkage of two gelatinous zooplankton, Thalia democratica and Dolioletta gegenbauri (Tunicata: Thaliacea) in preservative. Bullet Plankt Soc Japan 43:1–7
O’Shea S (1997) A new technique for assessing fixation-induced morphological variation in octopus (Mollusca: Cephalopoda). New Zealand J Zool 24:163–166
O’Shea S (1999) The marine fauna of New Zealand: Octopoda (Mollusca: Cephalopoda). NIWA Biodivers Mem 112:1–280
O’Shea S, Lu CC (2002) A new species of Luteuthis (Mollusca: Cephalopoda: Octopoda: Cirrata) from the South China Sea. Zool Stud 41:119–126
Pardo-Gandarillas MC, Diaz-Santana-Iturrios M, Fenwick M, Villanueva R, Ibañez CM (2021) Redescription of the flapjack octopod, Opisthoteuthis bruuni (Cephalopoda: Opisthoteuthidae) from the southeastern Pacific Ocean and evolutionary relationships of cirrate octopods. Malacologia 63:155–169
Piatkowski U, Diekmann R (2005) A short note on the cephalopods sampled in the Angola Basin during the DIVA-1 expedition. Org Divers Evol 5:227–230
Reinhardt JT, Prosch V (1846) Om Sciadephorus mülleri (Eschr.), en undersögelse. Danske Vidensk Afhandl 12:185–224
Richards J, Vecchione M (2020) Vertical and small-scale horizontal distribution of cephalopods in the Charlie-Gibbs Fracture Zone of the Mid-Atlantic Ridge. Bull Mar Sci 96:341–355
Robson GC (1932) A Monograph of the recent Cephalopoda based on the Collections in the British Museum (Natural History), Part II, the Octopoda (excluding the Octopodinae). Trustees of the British Museum, London
Roper CFE, Sweeney MJ (1983) Techniques for fixation, preservation, and curation of cephalopods. Natl Mus Victoria Mem 44:29–47
Roper CFE, Voss GL (1983) Guidelines for taxonomic descriptions of cephalopod species. Natl Mus Victoria Mem 44:49–63
Russell HD (1963) Notes on methods for the narcotization, killing, fixation, and preservation of marine organisms. Marine Biological Laboratory, Woods Hole, MA
Sagorny C (2017) Cephalopods of the R/V SONNE expedition SO-249 BERING – species descriptions of cirrate and incirrate octopods based on non-invasive imaging techniques. M. Sc. thesis, Rheinische Friedrich-Wilhelms-Universität, Bonn, Germany
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 28:676–682
Shea EK, Ziegler A, Faber C, Shank TM (2018) Dumbo octopod hatchling provides insight into early cirrate life cycle. Curr Biol 28:R144–R145
Simmons JE (2014) Fluid preservation – a comprehensive reference. Rowman & Littlefield, Lanham, MD
Simmons JE (2019) Storage in fluid preservatives. In: Elkin L, Norris CA (eds) Preventive conservation: collection storage. American Institute for Conservation, Washington, DC, pp 491–509
Steedman HF (1976) Zooplankton fixation and preservation. The Unesco Press, Paris
Vecchione M, Piatkowski U, Allcock AL (1998) Biology of the cirrate octopod Grimpoteuthis glacialis (Cephalopoda; Opisthoteuthidae) in the South Shetland Islands, Antarctica. S Afr J Mar Sci 20:421–428
Vecchione M, Collins MA, Sweeney MJ (2002) Systematics, ecology and biology of cirrate octopods: workshop report. Bull Mar Sci 71:79–94
Verhoeff TJ (2022) Finned octopus Cirroteuthis Eschricht, 1836 (Cephalopoda: Cirrata: Cirroteuthidae) confirmed from Australian waters. Molluscan Res 42:205–211
Verhoeff TJ, O’Shea S (2022) New records and two new species of Grimpoteuthis (Octopoda: Cirrata: Grimpoteuthitidae) from southern Australia and New Zealand. Molluscan Res 42:4–30
Voight JR (1991) Morphological variation in octopod specimens: reassessing the assumption of preservation-induced deformation. Malacologia 33:241–253
Voight JR (2001) Morphological deformation in preserved specimens of the deep-sea octopus Graneledone. J Molluscan Stud 67:95–102
Voss GL, Pearcy WG (1990) Deep-water octopods (Mollusca; Cephalopoda) of the Northeastern Pacific. Proc Calif Acad Sci 47:47–94
Werner R, Hoernle K, Hauff F, Portnyagin M, Yogodzinski G, Ziegler A (2016) RV SONNE Fahrtbericht / Cruise Report SO249 BERING – origin and evolution of the Bering Sea: an integrated geochronological, volcanological, petrological and geochemical approach. GEOMAR Report N Ser 30:1–451
Williams GC, Van Syoc R (2007) Methods of preservation and anesthetization of marine invertebrates. In: Carlton JT (ed) The Light and Smith Manual, 4th edn. University of Califonia Press, Berkeley, CA, pp 37–41
Xavier JC, Allcock AL, Cherel Y, Lipinski MR, Pierce GJ, Rodhouse PGK, Rosa R, Shea EK, Strugnell SM, Vidal EAG, Villanueva R, Ziegler A (2015) Future challenges in cephalopod research. J Mar Biol Assoc UK 95:999–1015
Young RE, Vecchione M (2016) Preservation. In: Grimpoteuthis Robson, 1932. TOLWEB, http://tolweb.org/Grimpoteuthis
Ziegler A (2018) Ein Dumbo-Baby aus dem Nordatlantik. GfBS Newsletter 34:32–35
Ziegler A (2021) Die Dumbo-Oktopusse der sieben Weltmeere. Naturwiss Rundschau 74:573–581
Ziegler A, Sagorny C (2021) Holistic description of new deep sea megafauna (Cephalopoda: Cirrata) using a minimally invasive approach. BMC Biol 19:81
Ziegler A, Miller A (2024a) Grimpoteuthidae O’Shea, 1999. In: Vidal EAG, Shea EK, Judkins H (eds) Early-life stages of cephalopods: an identification handbook. Springer Nature
Ziegler A, Miller A (2024b) Opisthoteuthidae Verrill, 1896. In: Vidal EAG, Shea EK, Judkins H (eds) Early-life stages of cephalopods: an identification handbook. Springer Nature
Ziegler A, Ziegler A (2022) Blaues Blut in drei Herzen. Fotopuls 3:65–69
Ziegler A, Kunth M, Mueller S, Bock C, Pohmann R, Schröder L, Faber C, Giribet G (2011) Application of magnetic resonance imaging in zoology. Zoomorphology 130:227–254
Ziegler A, Bock C, Ketten DR, Mair RW, Mueller S, Nagelmann N, Pracht ED, Schröder L (2018) Digital three-dimensional imaging techniques provide new analytical pathways for malacological research. Am Malacol Bull 36:248–273
Ziegler A, Miller A, Nagelmann N (2021) Novel insights into early life stages of finned octopods (Octopoda: Cirrata). Swiss J Paleontol 140:24
Acknowledgements
We would like to thank Jon Ablett, Andreas Allspach, Cheryl A. Brantley, Wen-Sung Chung, Martin A. Collins, Dieter Fiege, Julian K. Finn, Karen Gowlett-Holmes, Bernhard Hausdorf, Ping-Ho Ho, Eric A. Lazo-Wasem, Chung-Chen Lu, Sadie Mills, Claude Nozères, Tim O'Hara, Diana Párrage, Uwe Piatkowski, Andrea Paz Martinez Salinas, Timothy M. Shank, Elizabeth K. Shea, Darren Stevens, Michael Türkay, Michael Vecchione, Tristan J. Verhoeff, Thomas von Rintelen, Regina Wetzer, Catalina Amanda Merino Yunnissi, Christine Zorn, and Robert Zugaro for facilitating access to photographs of fresh and museum specimens. Anna Kral is thanked for discussion of previous versions of this manuscript, while comments by two anonymous reviewers additionally helped to improve the text. We are particularly grateful to the captain, chief scientist, and crew of R/V SONNE during scientific cruise SO-249 BERING for their assistance in obtaining cephalopod specimens.
Funding
Open Access funding enabled and organized by Projekt DEAL. The present study did not receive any specific funding.
Author information
Authors and Affiliations
Contributions
AZ conceived the study. AZ and CS performed measurements. AZ and CS analyzed the data. AZ wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
All applicable international, national and/or institutional guidelines for sampling, care and experimental use of organisms for the study have been followed and all necessary approvals have been obtained.
Additional information
Responsible Editor: H.-J. Hoving.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ziegler, A., Sagorny, C. Effects of fixation and long-term preservation on finned octopods (Cephalopoda: Cirrata). Mar Biol 170, 152 (2023). https://doi.org/10.1007/s00227-023-04276-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-023-04276-3