Abstract
Coral-eating crown-of-thorns seastars (CoTS, Acanthaster spp.) are major contributors to the coral reef crises across the Indo-Pacific region. Until recently, CoTS throughout the Indo-Pacific were regarded to be a single species, Acanthaster planci. However, genetic and morphological analyses demonstrated that there are at least four distinct species: Acanthaster benziei in the Red Sea, Acanthaster mauritiensis and A. planci in the Indian Ocean, and Acanthaster cf. solaris in the western Pacific. Acanthaster cf. ellisii in the eastern Pacific needs more taxonomic attention. Here, we review the biological knowledge for each species adapting a pragmatic geographical species definition and using a systematic literature review complemented with more focused searches for individual species. The vast majority of CoTS research (88%) was conducted on A. cf. solaris, with much of this research undertaken on the Great Barrier Reef or in Japan. Many studies of A. cf. solaris are focused on monitoring or documenting incidences of outbreaks, though there is a solid base of knowledge on larval, juvenile and adult ecology derived from field and laboratory experiments. By contrast, most of the published studies on the four remaining species simply document cases of population outbreaks. The major taxonomic bias in CoTS research constitutes a significant limitation for understanding and managing these species for two reasons. First, even for A. cf. solaris, which is the most studied species, limited fundamental knowledge of their biology and ecology constrains understanding of the drivers of outbreaks and hinders corresponding management actions for prevention and control of these events. Second, understanding and management of other species are predicated on the assumption that all CoTS species have similar biology and behaviour, an unsatisfying assumption for ecosystem management.
Similar content being viewed by others
Introduction
Acanthaster spp., referred to as Crown-of-Thorns Seastars (CoTS) inhabit coral reefs throughout the Indo-Pacific and are among the most notorious corallivorous invertebrates (Glynn and Enochs 2010). CoTS usually occur in low numbers (< 0.001 ind. 100 m−2) (Pratchett et al. 2017a), but periodically exhibit major population outbreaks (Uthicke et al. 2009; Pratchett and Cumming 2019), which have attracted attention since the 1960s. On many reefs throughout the Indo-Pacific, population outbreaks of CoTS represent one of the major causes of coral mortality and reef degradation (Pratchett et al. 2014; Castro-Sanguino et al. 2021). New or reoccurring CoTS outbreaks have recently been reported from the Maldives, French Polynesia, and Baja California (Kayal et al. 2012; Baird et al. 2013; Nakamura et al. 2014; Roche et al. 2015; Saponari et al. 2015; Yasuda 2018). On Australia’s Great Barrier Reef, it has recently become apparent that CoTS densities are increasing in the northern sector, likely representing the initiation of the 5th outbreak event since the 1960s (Uthicke et al. 2022).
Acanthaster spp. (NB: for the purpose of this review we omit A. brevispinus, generally a non-coral eating [but see Keesing et al. (2023) and Yuasa et al. (2017)] sister species to the CoTS species complex) occur throughout the tropical Indo-Pacific and were formerly regarded as a single, widely distributed species (A. planci). DNA barcoding in the 2000s, however, suggested that there are at least four species with generally non-overlapping distributions (Vogler et al. 2008, 2012, 2013). It has since been realised that there are at least five species (Haszprunar and Spies 2014; Haszprunar et al. 2017) and that Acanthaster planci (Linnaeus, 1758) (which was originally named based on specimen(s) collected in Goa, India) is restricted to the Northern Indian Ocean. In the same publications, it was also realised that the (mainly) Southern Indian Ocean species is correctly referred to as Acanthaster mauritiensis (Loriol 1895), whereas the species in the Red Sea was recently named Acanthaster benziei (Wörheide et al. 2022). Thus, all Indian-Ocean and Red Sea species now have accepted species names. Most of the Western Pacific Ocean and the coral triangle appear to be inhabited by a single species, Acanthaster cf. solaris (Vogler et al. 2008, 2013), thus covering a large range and occurring on both hemispheres. Although some debate exists regarding the correct species name of the latter due to the absence of holotypes, Haszprunar and Spies (2014) and Haszprunar et al. (2017) suggested A. solaris (Schreber, 1795) for that species, and many publications currently use the name A. cf. solaris. An exception to the homogeneity in the Pacific Ocean appears in the East Pacific (e.g., Gulf of California, Panama, Costa Rica), and Haszprunar et al. (2017) suggested Acanthaster ellisii (Gray, 1850) as the correct name for that species, but acknowledged that clarification is required. Although A. ellisii is a valid species, it cannot easily be distinguished by COI barcoding from A. cf. solaris, although genetic substructure was detected in mtDNA control region sequence analyses (Vogler et al. 2008, 2013). A recent genomic study (Yuasa et al. 2021) also suggested that additional undescribed species in the Pacific are likely because Hawaiian CoTS clustered into a clade different from many other A. cf. solaris. For the purpose of this review, CoTS in Hawai’i are considered to be part of the A. cf. solaris complex.
The cause(s) or driver(s) of CoTS population outbreaks are still unresolved (e.g., Pratchett et al. 2014; Babcock et al. 2016a, b) and may vary among species and regions. It was proposed that outbreaks can be natural or anthropogenic driven and alter in frequency (summarised in (Pratchett et al. 2014). One of the main hypotheses debated is that overfishing of adult CoTS predators such as Triton snails (Endean 1969) or fish (McCallum 1987) promotes outbreaks. Conversely, the “nutrient limitation hypothesis” poses that increased runoff from agriculture promotes phytoplankton growth which leads to faster growth and higher survivorship of the planktotrophic CoTS larvae (Birkeland 1982; Brodie et al. 2005; Fabricius et al. 2010). Understanding the causes and drivers of outbreaks in a regional context is vital for informed management. For instance, an imbalance in predator abundance can be addressed through fisheries management or areas closed to fishing (Marine Protected Areas), whereas nutrient runoff needs to be managed terrestrially through improved land-management practices (Babcock et al. 2020).
As outlined above, ecological knowledge of the respective species is essential for understanding outbreak dynamics and deciding on appropriate management actions. Although several recent summaries and reviews on CoTS ecology exist (Pratchett et al. 2014, 2017a, 2021a), these mostly focus on research conducted within the West Pacific and are therefore mostly relevant for only Acanthaster cf. solaris. Here, we aim to (1) review research efforts on the five individual CoTS species and (2) summarise current knowledge and identify knowledge gaps for each individual species.
Materials and methods
Working definition of species based on geography
To compare research and knowledge among distinct species of Acanthaster spp., we utilise currently recognised geographical distributions of the five nominal species (Haszprunar and Spies 2014; Haszprunar et al. 2017; Fig. 1). We refer to the Red Sea species as A. benziei and the two Indian Ocean species as A. planci and A. mauritiensis. We use A. cf. solaris for the widely distributed western and central Pacific species following Haszprunar et al. (2017), which suggests that the most appropriate type species is likely Acanthaster solaris (Schreber, 1795). To acknowledge the fact that Acanthaster ellisii needs more taxonomic and molecular work to be confirmed as a separate species (see Haszprunar et al. 2017), we refer here for the Eastern Pacific species to A. cf. ellisii. This allowed us to classify most publications with high confidence based on their geographic location. Uncertainty exists in some areas of Indonesia, Malaysia, and Thailand (see details in Fig. 1), because in that region A. planci and A. cf. solaris occur sympatrically. Similarly, in Oman and the United Arab Emirates (UAE), A. planci and A. mauritiensis co-exist (Vogler et al. 2008, 2012; Haszprunar et al. 2017). To ensure we were considering individual species, we omitted reports from these areas (N = 3) from downstream analyses.
Map of over 5000 observations of Acanthaster species retrieved from the Ocean Biodiversity Information System (OBIS) databases. The majority of reports (99.8%) were recorded as Acanthaster planci. Colouring denotes species under our current working definition based on geography (Haszprunar and Spies 2014; Haszprunar et al. 2017). Zones of sympatry exist between A. planci and A. mauritiensis in Oman (white dots) and A. planci and A. cf. solaris in West Indonesia (grey dots). Reports from those regions were not considered for this review. We manually added one location of A. cf. solaris in Vietnam based on Yasuda et al. (2022), and three locations in the South China Sea and Taiwan based on Kimura et al. (2022). Similarly, based on our review of A. cf. ellisii several locations were manually added for that species. The OBIS download and species designations as applied here are included in Supplementary Table 1
To illustrate the species distributions and names as applied here, we downloaded all observations for Acanthaster from the Ocean Biodiversity Information System (OBIS, https://obis.org) and allocated species names by geography as outlined above (Fig. 1, download and our annotation of the species in Supplementary Table 1). This database contained 5031 observations, most of which (with the exception of 12 observations only labelled to Genus name) were entered as A. planci. Observations in some countries (e.g., Vietnam, China) are sparse or absent, despite that several research papers from that area exist. Specimens from the latter countries are also classified as A. cf. solaris for the purpose of this review.
General literature review
To document research efforts on the different Acanthaster species over the last 35 years (the period comprising detailed information available on the Web of Science Core Collection), we conducted a Web of Science Topic Search (TI = Acanthaster, date range = 1987 to January 2023, Web of Science Core Collection). We screened each of the resulting publications and scored whether these were relevant to research on Acanthaster. For the relevant studies, we identified the study region or location of the source material and assigned species according to our geographic definition. We omitted three studies conducted in the geographic areas of uncertainty highlighted above. In addition, we grouped each of the relevant studies into one of three research topics (a) species ecology, biology; (b) populations and outbreaks; (c) management, general and 17 traits or subtopics studied (see Table 1, details on how each study was classified in Supplementary Table 2).
Results
General literature review
We reviewed a total of 784 publications, 309 of which were excluded because while they made reference to Acanthaster, they were not relevant to discerning the biology or behaviour of one or more distinct species of CoTS. Thus, 475 publications were included in the final general literature review.
Since 2017, an increasing number of publications acknowledged the existence of multiple CoTS species and utilise species names relevant to the specific geographic location of the respective research. Most of the CoTS research (Fig. 2), however, was conducted in the Indo-West- Pacific, the most appropriate name for the Western Pacific CoTS is likely Acanthaster solaris (Schreber, 1795), for which the type locality is likely the Philippines (Haszprunar and Spies 2014; Haszprunar et al. 2017), but given persistent uncertainty, it is largely referred to A. cf. solaris. While there have been 416 publications on A. cf. solaris since 1987, there have been less than 20 publications for all other species, with no publication in our literature search focussing solely on A. mauritiensis. Nevertheless, the species A. mauritiensis was mentioned in several review papers, publications discussing CoTS species complex, and some molecular genetic studies (see the section on A. mauritiensis below).
By far, most publications (255) were conducted on Australia’s Great Barrier Reef (GBR), followed by studies conducted in Japan (mainly Okinawa: 49). Fourteen relatively recent publications focus on CoTS in China, whereas French Polynesia (15), New Caledonia (7), and Taiwan (6), are other centres of CoTS research. The majority of publications focused on chemistry, toxicity and medical applications, adult ecology, physiology, behaviour, and the ecological effects of CoTS outbreaks in coral reef ecosystems (Table 1). Because this review focuses on the ecology and biology of these species “Chemistry, toxicity, medical applications” are not further considered.
Summary of knowledge on individual species
In the following section, we summarise knowledge on each species based on the WoS literature review. However, given the limited data available for most species apart from A. cf. solaris, we complemented the WoS search with additional targeted searches and included “grey literature” reports and information available from the period prior to 1987. Figure 3 shows photographs of the five species discussed.

Credit: a—Tom Shlesinger; b—Omri Bronstein; c—Morgan Pratchett; d—Morgan Pratchett; e—Juan José Alvarado
The five nominal CoTS species recognised based on geographical distributions. a A. benziei from the Red Sea (Eilat, Gulf of Aqaba). b A. planci from the northern Indian Ocean (Maldives). c A. mauritiensis from the southern Indian Ocean (Zanzibar, Tanzania). d A. cf. solaris from the western and central Pacific (Great Barrier Reef, Australia). e A. cf. ellisii from the Eastern Pacific (Isla del Caño (Costa Rica). f Phylogenetic tree redrawing data from Vogler et al. (2008) and Wörheide et al. (2022).
Acanthaster benziei Wörheide & Kaltenbacher in Wörheide et al. 2022 (Fig. 3a)
Relatively few studies have specifically investigated CoTS within the Red Sea, especially since A. benziei was formally described (Wörheide et al. 2022). However, several seminal studies on Acanthaster spp. (e.g., Goreau 1962; Polunin 1974) were based on research conducted in the Red Sea. In all, a total of 11 studies were based on research conducted in the Red Sea over the past 35 years, though only one study explicitly references A. benziei.
Species ecology and biology
Original insights into the feeding ecology of Acanthaster spp. were based on research conducted in the Red Sea (e.g., Goreau 1962) and, therefore, relevant solely for A. benziei. Goreau (1962) reported on its feeding behaviour based on observations during the Israel South Red Sea Expedition in 1962. The latter author observed that A. benziei feed nocturnally, that they restrict their feeding activities to circumscribed territories, and that they spend prolonged periods immobile during the day before coming out to feed again at night. Goreau (1962) suggested that this territorial feeding behaviour may be responsible for the failure of large coral reefs to develop in the investigated area in the Dahlak Archipelago, Eritrea. Campbell and Ormond (1970) then reported on the Cambridge Expedition conducted in 1968 and 1969 to the area around Port Sudan, Sudan, and confirmed CoTS nocturnal feeding behaviour as suggested by Goreau (1962). They also suggested that due to its feeding on living corals and a lack of predators of adults (the Giant Triton snail, Charonia tritonis, a predator of adult CoTS, is not common in the Red Sea, so is less likely to control their population density) causes extensive damage and leaves reefs vulnerable to erosion. They conducted extensive surveys by snorkelling and SCUBA diving and found that CoTS were present in relatively low densities, but more commonly found on sheltered sides of barrier reefs and exposed sides of fringing reefs. The size distribution of individual CoTS in the southern Red Sea forms two main groups, with overall diameters averaging 22.5 and 32.5 cm (Campbell and Ormond 1970). The latter authors suggested these groups correspond to ages of 1.5–2.5 and 2.5–3.5 years, respectively.
Generally, CoTS were found in parts of the reef that were rather sheltered. The corals they preyed upon were predominantly species of Goniastrea, with Pocillopora, Acropora, Favia and Porites also being consumed frequently (Campbell and Ormond 1970). It was suggested that the ease with which the seastar can maintain its grip on corals while feeding, seemed to determine which prey species they target. In very exposed areas, A. benziei appeared to prefer branching forms like Acropora and Pocillopora; in less exposed sites, smaller rounded forms like species of Goniastrea and Goniopora were the more common prey; and in increasingly protected sites, larger rounded Porites species were targeted. In addition, based on observations by divers stung in the Red Sea, Campbell and Ormond (1970) suggested that CoTS in the Red Sea are less poisonous than CoTS from the Pacific (i.e., A. cf. solaris). Similar to Goreau (1962), the latter authors reported low densities of triton snails in the area, but observed damaged arms in 23% of the examined CoTS, presumably caused by predation attempts.
Populations and outbreaks
Off the Sudanese coast, A. benziei densities were reported between as low as 5–20 ind. × km−1 in the early 1970s, but on some reefs, aggregations of several hundred individuals were reported within a few hundred meters (Roads and Ormond 1971), likely representing the first report of a CoTS outbreak in the Red Sea.
In 1998, outbreaks of A. benziei were first documented off the mainland Egyptian Red Sea coast, at Hurghada's Shabror Umm Gam'ar reef. To assess the impact of this event, Ammar (2005, 2007) monitored coral recovery in the aftermath of the outbreak. Their results showed that stony corals had an estimated recovery time (RT) of 64.9 years with a recovery rate of 0.67% cover/year following CoTS infestation. In contrast, soft corals had both fast RT (0.76 years) and recovery rate (1.31%). Diversity had an estimated fast RT of 4.3 years indicating that the space cleared by A. benziei improves the diversity faster than improving the percent cover. The slow recovery time for stony corals was largely attributed to ongoing CoTS predation. Soft corals were able to adapt quickly and out-compete hard corals after CoTS infestation. These studies by Ammar (2005; 2007) highlighted how CoTS can have a significant impact on coral reefs of the Red Sea, but also, how reefs can be resilient if given enough time to recover from such events.
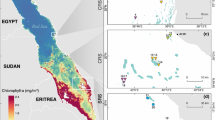
Riegl et al. (2013) found that one-third of the reefs in Saudi Arabia between the Farasan Islands and Al Wajh Banks exhibited degradation due to CoTS outbreaks or bleaching (during 1998 and 2010), with bleaching increasing towards the south. Their ecological models suggested community resilience at a disturbance frequency of 20 years or less, but degradation at higher frequencies. CoTS impacts and bleaching were found to be key drivers of coral degradation along the sampled sites in the Saudi Arabian Red Sea.
Riegl et al. (2013) concluded that active management of water quality is necessary to reduce coral population decline. Following increased runoff, elevated chlorophyll-a concentrations (indicative phytoplankton biomass) were observed at Wajh Banks, Saudi Arabia, six months prior to CoTS outbreaks in the region. The latter finding can be interpreted as support for the nutrient limitation hypothesis.
Management, general
In the northern Gulf of Aqaba, CoTS have been actively removed from Eilat Coral Beach Nature Reserve by rangers of the Nature and Parks Authority. This practice was adopted likely as a precaution inspired by the reports of CoTS outbreaks in the Pacific. However, since 2010, CoTS removal from the wild was completely halted and no significant increase in population size or outbreak occurred. Following the identification of A. benziei as a novel species, a research program was established in January 2023 to evaluate and regularly monitor CoTS populations on the coral reefs of Eilat and develop eDNA-based procedures to facilitate effective management of the species. In Egypt, CoTS monitoring has been carried out as part of the Ras Mohammed National Park monitoring program (Smith and McMellor 2006) reporting zero observations at the monitored sites and only occasional solitary individuals throughout the region. To the north of the Sinai Peninsula, adjacent to the town of Dahab, CoTS monitoring was included in an 11-year long coral community monitoring program that similarly reported remarkably low CoTS abundances, with a single individual observed over the 824 transects surveyed (Reverter et al. 2020). Nevertheless, the severity of threats to the marine biodiversity of Jordanian coral reefs from CoTS outbreaks is classified as ‘very high’ in the latest Aqaba Marine Reserve management plan for 2022–2026.
Acanthaster planci (Linnaeus, 1758) (Fig. 3b)
A total of 347 (out of 475) publications in the dataset from 1987 to 2023 referred to A. planci, but only 10 of these studies included sampling within the northern Indian Ocean, which is the recognised range of A. planci (Haszprunar and Spies 2014; Haszprunar et al. 2017) (Fig. 2). Acanthaster planci is readily distinguished from other species of CoTS based on distinctive colouration (often vibrant blue) along the aboral distal portion of the arms where there is also a conspicuous lack of spines (Fig. 3). However, only eight publications in the WoS survey specifically relate to the true A. planci. Moreover, most of these publications are descriptive accounts of outbreaks within the northern Indian Ocean [e.g., Maldives (Morri et al. 2010; Saponari et al. 2015, 2018)] and do not advance understanding of the biology and ecology of this species.
Species ecology and biology
An intensive study of the biology and ecology of A. planci was undertaken in the Maldives (Burn et al. 2020), which showed that this species (at least in this location) is rarely exposed during the day and generally active and feeding only at night. A. planci has been recorded feeding extensively on Acropora spp. when and where available in high abundance (James et al. 1990; Saponari et al. 2015). Conversely, A. planci has been observed feeding on a variety of prey, including soft corals, where Acropora spp. are scarce (Chansang et al. 1987).
While there is very limited demographic information available for A. planci, the maximum recorded size is 50 cm (Burn et al. 2020). At some locations, however, only relatively small individuals occur. In the Andaman Islands, for example, CoTS sampled ranged in size from 12.5 to 15.5 cm in diameter and had 14–19 arms (James et al. 1990).
Populations and outbreaks
Outbreaks of A. planci have been reported in the Maldives (Saponari et al. 2015, 2018; Caragnano et al. 2021), Thailand (Chansang et al. 1987), India (Andaman Isl) (Ravindran et al. 1999). Furthermore, population outbreaks have been reported from Oman (NB: listed here under A. planci, but note species uncertainty in Oman, Fig. 1). Densities of A. planci recorded during outbreaks in the Maldives averaged 3.62 ind. 100 m−2 (Saponari et al. 2018; see also Burn et al. 2020), though maximum reported densities approached 25 ind. 100 m−2 at Ari Atoll (Saponari et al. 2018).
Management, general
The documented occurrence of population outbreaks of A. planci in central Ari Atoll in the Maldives (Saponari et al. 2018), which is highly isolated, makes it difficult to attribute outbreaks to anthropogenic influences. Outbreaks of A. planci contributed to extensive coral loss and shifts in coral composition recorded in Maldives from 2015 to 2018, but the effects were compounded by mass coral bleaching in 2016 (Saponari et al. 2018; Pisapia et al. 2019). There are, therefore, extensive efforts to remove A. planci from reef areas during population outbreaks (e.g., Stevens and Froman 2019).
Acanthaster mauritiensis de Loriol, 1885 (Fig. 3c)
Acanthaster mauritiensis is the least researched species of the five species reviewed here, with no publications in our systematic WoS review referring to that species alone. However, following our geographical species assignment (Fig. 1), designated searches for Acanthaster in the South Indian Ocean, revealed a number of publications (erroneously) referring to A. planci, while likely addressing A. mauritiensis. Originally described by de Loriol (1885) from Mauritius, A. mauritiensis was initially considered to be morphologically indistinguishable from Acanthaster in the North Indian Ocean (i.e., A. planci) (Döderlein 1888), but this may be attributable to comparisons of poorly preserved or dried specimens. In reality, A. mauritiensisis is distinguished based on its “light blue to rusty” coloration in contrast to the “electric blue” colour typical of North Indian Ocean A. planci (Haszprunar et al. 2017) (Fig. 3). Moreover, molecular evidence clearly defines A. mauritiensis as a distinct species based on both COI barcoding (Vogler et al. 2008, 2012) and microsatellite analysis (Yasuda et al. 2009).
Species ecology and biology
To date, no available studies focused on the ecological and biological uniqueness of A. mauritiensis with respect to the other CoTS species. Studies comparing the damage caused by A. mauritiensis coral predation show a clear preference for A. mauritiensis to Acropora species (Muhando and Lanshammar 2008). While noticeably showing a preference for hard coral prey, A. mauritiensis was occasionally reported to also prey upon soft corals, and even sponges (Celliers and Schleyer 2006).
In La Reunion Island, A. mauritiensis was more common on the outer reef slopes (83%) than on the reef flat (17%), with individuals rarely seen below 20 m depth. These populations were low in abundance (less than 4 individuals per 30 min dive), and the modal size of individuals at La Reunion was about 40 cm (Emeras et al. 2004). Following the 1992 localised outbreak on Two-mile Reef, Sodwana Bay, South Africa, CoTS size frequency distributions showed minimal body diameters of 40 cm in 1998, with a mean body diameter of 50–55 cm (Celliers and Schleyer 2006). At the same site, CoTS appeared to aggregate at the deeper fringing reef (24–27 m) where the reef shelf emerges from sediment-covered bedrock (27–30 m). Once aggregated (during spring and summer), they jointly moved to feed on the reef (Schleyer 1998). In the Comoro Islands, three size cohorts were observed in 1973, with the largest individuals reaching 44 cm in diameter (Polunin 1974).
The reproductive season of A. mauritiensis in South Africa (based on field dissections) shows that gonad development takes place in spring and summer (November–March), reaching a peak in February, followed by greatly reduced gonads in March—indicative of spawning. A number of specimens collected showed scars and signs of regeneration on the arms of A. mauritiensis, providing evidence that this species is predated in South Africa (Schleyer 1998).
Populations and outbreaks
Early reports of CoTS in the Western Indian Ocean indicate outbreaks as early as 1973 off the north coast of Grande Comore, Comoro Islands (Polunin 1974). The authors estimate between 40 and 90% mortalities of hard corals. In surveys conducted across other West Indian Ocean sites from Mohéli and Mayotte (Comoro Islands) to Aldabra and Assumption, the latter authors found no signs of CoTS. In Tanzania, CoTS outbreaks were rare events prior to 1988, when the first reported outbreak was observed on a single reef on Pange (UNEP 1989). Smaller incidences occurred in 1996–1997 on the reefs of Changuu Island (Mohammed et al. 2000), and since 2002 a series of outbreaks erupted on several reefs close to Zanzibar and quickly expanded to infest more than 60% of Tanzanian reefs by 2006 [including Tanga, Zanzibar, Pemba, Songosongo, Mafia and Mnazi Bay reefs Obura et al. 2004; Mohammed et al. 2005; Obura 2005; Ussi 2009)]. Repeated outbreaks have been reported in Zanzibar, Tanga, Mikindani Bay, and Mtwara (Muhando and Lanshammar 2008). On Changuu, as a consequence of the CoTS outbreak of 1996, the previously dominant Acropora corals were replaced by Porites and Galaxea. On the adjacent Bawe Island, Acropora cover remained stable at about 21% until the CoTS outbreak of 2003, when Acropora cover steadily declined to 1–2% in 2008 (Muhando and Lanshammar 2008), demonstrating the substantial ecological impact of A. mauritiensis on southwestern Indian Ocean coral reefs.
In the remote Chagos Archipelago, CoTS outbreaks at Danger and Eagle Islands were noticed in 2012 although outbreaks were observed on only two out of the six sites surveyed in 2013 (Roche et al 2015). On the southern boundary of its range, in the high-latitude coral communities along the east coast of South Africa, A. mauritiensis were documented since the early 1970s (Heydorn 1972) and have since been reported on all the major South African reefs (Celliers and Schleyer 2006, 2008), as well as those in southern Mozambique and the Bazaruto Archipelago (Schleyer 1998). During the 1990s, local outbreaks (termed spot outbreaks or aggregations) have been reported from Two-mile Reef (TMR) at Sodwana Bay (Celliers and Schleyer 2006). Lasting for nearly a decade, these outbreaks peaked between 1994 and 1996, although they were spatiotemporally limited to the fore-reef of TMR and disapated completely in the following years to the point that A. mauritiensis became locally extinct on these reefs (Celliers and Schleyer 2006).
CoTS affecting coral reefs in Mozambique have been documented in recent years (Wilkinson 2004; Haszprunar et al. 2017), although earlier reports of localised outbreaks exist (Bligh and Bligh 1971). In Seychelles, CoTS outbreaks have been reported in some locations in 1997 and 2014 (Obura et al. 2017). The latter report also mentions CoTS outbreaks at ‘certain sites’ in Mauritius in 2014, although more specific information is not provided.
Outbreaks were reported for reefs in the Bazaruto Archipelago and nearby coastal reefs in 1995–1996, impacting approximately 90% of corals on affected reefs (Schleyer and Celliers 2005). Later, CoTS were reported from reefs in southern Mozambique (Celliers and Schleyer 2006). Hill et al. (2009) reported that CoTS in the Quirimbas Archipelago of northern Mozambique and COTS outbreaks have also disturbed reefs in southern Tanzania near the Mozambique border (Wagner 2008).
Genetic data on A. mauritiensis includes material collected from Kenya, Mayotte and Madagascar (Gerard et al. 2008a; Yasuda et al. 2009). Despite being referred to as A. planci in the original studies, samples from all sites in the Southwestern Indian Ocean show clear genetic distinction from their Pacific, Northern Indian Ocean, and Red Sea congeners.
Management, general
Several local and regional CoTS monitoring programs have been implemented in the Southern Indian Ocean, mostly operated by local NGOs. In Zanzibar (Tanzania), annual reef benthos surveys that include data on CoTS have been performed since 1992 on the reefs of Chumbe, Bawe and Changuu Islands (Muhando and Lanshammar 2008). Their data show that following the 1997–1998 El Niño-associated bleaching event, which reduced coral cover in the Indian Ocean by up to 30%, a slow recovery in coral cover was observed which ended with the 2002–2003 CoTS outbreak in the region. Following this outbreak, live Acropora cover was reduced dramatically. However, a single site, the protected Chumbe Reef Sanctuary, showed no signs of coral decline. The authors attributed these findings to management action rapidly implemented by the Chumbe Island Coral Park (CHICOP) that initiated an active CoTS removal programme as early as 2004. More than 3000 CoTS individuals were removed from within the 0.4 km2 marine park, keeping densities close to zero at all times. Consequently, in contrast to other reefs in Tanzania (Tanga, Dar es Salaam, and on reefs off Zanzibar town), where active CoTS removal initiated much later, on the protected reefs of Chumbe Island, the percent live coral cover was back to the same level as before the El Niño bleaching event (Muhando and Lanshammar 2008). Removal programmes in Dar es Salaam (Wagner 2008) and in Tanga were similarly unsuccessful as CoTS removal was initiated well after significant coral damage occurred.
In South Africa, sodium bisulphate poisoning was recommended to control outbreaks at Bazaruto Island and Sodwana Bay (Schleyer 1998). Most CoTS were physically removed by volunteers at Bazaruto within a year but the conservation authority at Sodwana Bay, the KwaZulu-Natal Nature Conservation Services, has decided upon a policy of cosmetic control only at intensively used dive sites, and to date only limited quantities of research material have been removed (Schleyer 1998).
Acanthaster cf. solaris (Schreber, 1793) (Fig. 3d)
As stated previously, most of the scientific research and monitoring on Acanthaster was undertaken in the Western Pacific (Table 3), for which the most appropriate species name is likely Acanthaster solaris (although further work is required to confirm the nomenclature). Over 380 of the publications identified in the WoS review focused exclusively on this species. In addition, several major reviews summarised the biology and ecology of this species (Moran 1986; Caballes and Pratchett 2014; Pratchett et al. 2014, 2017a). Hence, we only briefly summarise knowledge on this species herein.
Species ecology and biology
About half of all publications identified for A. cf. solaris fall into the biology and ecology category, with most publications focussing on adults (Table 3). Age and longevity of CoTS are difficult to assess due to the plasticity of their weight and size. The spines of CoTS have pigmented bands which may serve as annual growth markers (Stump and Lucas 1990), but this is not universally accepted (Souter et al. 1997). Nevertheless, individuals sampled during population outbreaks hardly possess more than 8 bands (Souter et al. 1997; MacNeil et al. 2017), which can be taken as evidence that few individuals get older than 8–10 years. Maximum diameter (L∞) modelled in the latter study varies from 28 to 41 cm (depending on locality), but individuals over 60 cm in diameter have been recorded (Pratchett 2005). Recently, telomer length has been tested as a marker for CoTS age (Kwong et al. 2023).
Adult A. cf. solaris can consume 161–678 cm2 of coral per day (Keesing and Lucas 1992). Based on this and coral growth rates, the latter authors suggested that densities > 10 CoTS ha−1 (0.1 ind. 100 m−2) would lead to coral depletion. A variety of coral species are consumed, but a hierarchy of preference exists (De'ath and Moran 1998b; Pratchett 2007; Pratchett et al. 2009). Acropora spp. are usually the preferred coral prey, but once these are depleted also other species are readily consumed (Pratchett 2007). CoTS were documented to move 10.3 m day−1 on average (Keesing and Lucas 1992), but maxima up to 520 m day−1 were proposed (Pratchett et al. 2017b) and this species exhibits high site fidelity in areas with abundant coral prey (Ling et al. 2020).
A. cf. solaris are most active and tend to feed mostly at night (Burn et al. 2020; Ling et al. 2020), especially smaller (< 20 cm) individuals (De'ath and Moran 1998a).
Adult A. cf. solaris can tolerate a wide range of temperatures but show metabolic depression at temperatures higher than 33 °C (Lang et al. 2021). No reduced oxygen uptake was detected in sub-adults until 36 °C.
Gonad maturation and spawning in A. cf. solaris occur in early summer in each hemisphere. On the GBR, gonads rapidly mature in November and December (Lucas 1973), with many spawning observations in late November to December (summarised in Pratchett et al. 2014). In the northern GBR, genetic larval identification suggested the main spawning peak occurred after mid-December, once water temperatures climbed above 28 °C (Uthicke et al. 2019). However, in the same study larvae were still found towards late February. In the northern hemisphere (Japan), spawning was first observed in May at the location nearest to the equator, and occurs later (up to July) at higher latitudes (Yasuda et al. 2010). The same study also suggested 28 °C as a threshold for spawning. A. cf. solaris is a highly fecund species, with > 100 Mio eggs observed in individual females (Babcock et al. 2016b).
Experiments on larval ecology by raising larvae under varying conditions have been conducted in laboratories in Guam (Caballes et al. 2016) and Japan (Fabricius et al. 2010). In addition, several laboratories have raised larvae from CoTS of the GBR (Lucas 1973; Keesing et al. 1997; Fabricius et al. 2010; Wolfe et al. 2015; Pratchett et al. 2017c; Uthicke et al. 2018b). Larvae undergo a classical life cycle for feeding asteroid larvae, from early embryonal stages (Gastrula, Blastula) via Bipinnaria and Brachiolaria stages (Keesing et al. 1997).
Under optimum conditions, larvae can reach late Brachiolaria and settle after 9–11 days (Lucas 1982) but can survive > 40 days under limiting food conditions (Lucas 1982; Pratchett et al. 2017c). Larvae can develop to the Bipinnaria stage and survive for two weeks without food (Lucas 1982). With the exception of extreme food densities (i.e., orders of magnitude above naturally occurring cell densities), which can limit development (Pratchett et al. 2017c), the developmental time of the larvae decreases with increasing food density, as has now been studied at three separate outbreaks on the GBR (Lucas 1982; Fabricius et al. 2010; Pratchett et al. 2017c; Uthicke et al. 2018b). Increased developmental time for A. cf. solaris larvae will lead to increased mortality in the plankton due to high predation rates (Uthicke et al. 2018b).
Settlement and metamorphosis of larvae are induced by cues from crustose coralline algae (CCA) or associated microbes (Johnson et al. 1991), with induction ranging from 2 to > 90% between CCA species (Doll et al. 2023). Settlement success and survival can also depend on temperature, with some indications that climate change may reduce recruitment success (Lang et al. 2022).
Settled larvae rapidly metamorphose to juvenile seastars, which appear to initially feed on biofilms (own observation) but after a few weeks switch to crustose coralline algae feeding. However, juveniles can be kept on biofilms for many months, resulting in stunted development (Deaker et al. 2020b). When suitable corals are present, juveniles start switching to a coral diet after 140 days, with 50% having transitions after 175–191 days (Neil et al. 2022). However, if no coral is present, transition can be (reversibly) delayed for many years resulting in small individuals (Deaker et al. 2020a). Once juveniles switched to coral diets, they showed a similar preference towards Acropora species as adults (Johansson et al. 2016). When corals are available, CoTS can reach maturity and first spawn after about two years (Zann et al. 1987).
A review on CoTS predation [NB: possibly including some references to Acanthaster species other than cf. solaris] identified 80 species consuming CoTS at different adult and sub-adult developmental stages, mainly fishes and invertebrates (Cowan et al. 2016). Several additional predators were detected using eDNA analysis of fish gut contents (Kroon et al. 2020). A detailed experimental study found a variety of crustaceans as predators of juvenile CoTS, with one species of decorator crab consuming more than five specimens per day (Desbiens et al. 2023).
Many publications (N = 36, Table 3) investigated the genetics of A. cf. solaris. Most of these were population genetic studies investigating population structure and connectivity. Throughout the last four decades, these have used several genetic methods available at the time. Early studies by John Benzie and colleagues on the GBR and other Australian locations used allozyme variation (Benzie and Stoddart 1992; Benzie and Wakeford 1997) and showed high connectivity within the GBR but some separation from West Australian and Lord Howe Island populations. Later studies used mitochondrial DNA and found more phylogeographic structure in larger geographic areas (Williams and Benzie 1998). These studies ultimately led to work across the entire geographic range of the genus, resulting in the delineation of species as we now understand it (Vogler et al. 2008, 2012, 2013). Generally, mitochondrial markers also failed to detect differences within the Pacific archipelagos, despite highlighting some regional structuring (Timmers et al. 2011). Studies using microsatellites found no genetic structure within the GBR (Harrison et al. 2017). However, microsatellite markers and analysis of complete mitochondrial genomes were able to separate populations in larger geographic areas across the Pacific Ocean (Yasuda et al. 2009, 2022; Tusso et al. 2016).
Recently, eDNA has been used to detect A. cf. solaris post-settlement (Doll et al. 2021; Kwong et al. 2021; Uthicke et al. 2022) as well as CoTS larvae (Uthicke et al. 2015). The genome of A. cf. solaris has also been published from specimens in Australia and Japan (Hall et al. 2017), unfortunately under the wrong species name (A. planci), and no vouchers were kept for future studies.
Studies on CoTS fossil record are all concerned with sediment cores from the GBR and discuss whether these can be used to infer changes in the frequency of outbreaks (Walbran et al. 1989; Fabricius and Fabricius 1992). Publications on disease and microbiology are mainly descriptive, focusing on the microbiome in different tissues investigating potential disease (Høj et al. 2018; Wada et al. 2020) or exploring microbes as control options (Rivera-Posada et al. 2012).
Populations and outbreaks
Outbreaks of A. cf. solaris have attracted increased attention in Okinawa (Japan) and the GBR (Australia) since the 1960s, but there is evidence that these have occurred in both regions prior to that (Walbran et al. 1989; Yasuda 2018). To date, outbreaks of this species have been reported throughout the entire range of this species, with reports of outbreaks in over 20 countries.
Most publications in this category (50) document the ecological effects of population outbreaks. Many of these document the obvious, primary effects of the CoTS outbreak, namely a decline in overall coral cover (Lourey et al. 2000; Wakeford et al. 2008; Baird et al. 2013). Selective feeding on Acropora species can lead to species shift among hard corals on coral reefs (Pratchett et al. 2009), and the open space created can promote the proliferation of other taxa such as soft coral (Fabricius 1997). Loss of corals as habitat can have consequences on fish communities (Kayal et al. 2012) or fish communities may be affected by changes in the availability of food (Hart et al. 1996). In addition, the recruitment of fishes to reefs may be altered due to olfactory responses (Coppock et al. 2016). Other ecological effects measured after CoTS outbreaks are increases in nitrogen fixation (Larkum 1988), promotion of coral disease (Nugues and Bak 2009; Katz et al. 2014) and impeding the potential of reefs to recover following other disturbances such as coral bleaching (Haywood et al. 2019).
Most modelling studies reviewed here investigate the effects of outbreaks or the propagation of outbreaks among reefs. These include general population models attempting to elucidate causes for population fluctuations (Seymour 1990; McCallum 1992; Matthews et al. 2020).
Other modelling studies are testing the importance of CoTS as one of the multiple stressors to coral reefs, including modelling under future climate scenarios (Wolff et al. 2018). The latter study demonstrated that even under climate change scenarios CoTS and other local pressures will have a significant impact on coral reefs. Castro-Sanguino et al. (2021) showed that, among other pressures, CoTS have a strong negative influence on the state and performance of GBR coral reefs.
Studies using hydrographic models generally focus on the connectivity of larvae and to some extent attempt to explain outbreaks and spread of CoTS on the GBR (Dight et al. 1990; Black et al. 1995).
Management, general
A variety of monitoring techniques have been used to survey CoTS populations. One issue is that densities between outbreaks and non-outbreaks are vastly different. Outside of outbreaks, CoTS are rare and highly cryptic making it very difficult to effectively quantify densities. As a compromise, many surveys use the manta tow technique, a method covering large areas but with low detectability (Fernandes et al. 1990; Miller and Death 1996). Other techniques used include SCUBA swim surveys or underwater transects (Moran and Death 1992; Pratchett 2005). More recently, eDNA methods were developed to monitor and detect low densities of CoTS (Uthicke et al. 2018a, 2022).
A variety of theories have been put forward to explain the initiation of population outbreaks of A. cf. solaris, including the possibility that these are natural phenomena. Predator removal of adult CoTS (e.g., the Triton shell, Charonia tritonis) was first put forward as a plausible cause in the late 1960s (Endean 1969). Since then, a variety of predators of all life history stages of CoTS have been identified (Cowan et al. 2016, 2017). Some crustaceans can eat several juvenile CoTS in a day, suggesting that predation on juvenile CoTS may be a more important population-regulating mechanism than that of adults (Desbiens et al. 2023). Although CoTS sub-lethal damage was frequently observed, marine park zones did not seem to affect this damage in juveniles (Wilmes et al. 2019) or adults (Messmer et al. 2017). Terrestrial runoff increasing the phytoplankton food for larvae is the second major hypothesis for CoTS outbreaks (nutrient limitation hypothesis), initially proposed for Pacific Island groups (Birkeland 1982). Experimental results suggesting that larvae are indeed limited by food (Lucas 1982; Fabricius et al. 2010) and other evidence (e.g., correlations with runoff events) led to the suggestion that this may also be the cause for outbreaks on the GBR (Brodie et al. 2005). Wooldridge and Brodie (2015) proposed that strong larval retention around reefs in conjunction with enhanced runoffs may trigger outbreaks. Hence, outbreak causes are still not resolved, and may differ under different circumstances. In fact, Babcock et al. (2016a) proposed the best explanation for outbreaks is a combination of nutrient enhancement and overfishing.
Understanding the cause(s) of outbreaks is important to focus management decisions. For example: is it more important to protect predators, e.g., through MPAs, or address land runoff? Current management on the GBR (and elsewhere) mainly focuses on culling once outbreaks occur. A threshold of 10–15 ind. ha−1 (0.1–0.15 ind. 100 m−2) was used as a threshold to define outbreaks (‘ecological threshold’) (Keesing and Lucas 1992; Moran and De'ath 1992). More recently, Rogers et al. (2017) modelled that reproductive output disproportionally increases at densities > 3 ind. ha−1 (0.03 ind. 100 m−2), which is referred to as the reproductive (or Allele) threshold. These values are currently used on the GBR to manage and report outbreaks. Culling of CoTS is usually achieved through under water injection of household vinegar, lime juice or bile salts (Rivera-Posada et al. 2013; Moutardier et al. 2015; Bostrom-Einarsson and Rivera-Posada 2016). In some countries, such as Japan, CoTS are also removed manually from the reef (Nakamura et al. 2016). Increasingly, culling effort is accompanied by modelling best culling strategies, e.g., in Australia (Plaganyi et al. 2020) or Guam, Hawaii and Samoa (Plaganyi et al. 2020).
Acanthaster cf. ellisi Gray, 1840 (Fig. 3e)
With 14 studies on the putative species A. cf. ellisii identified in our WoS search, this species is the second most studied species of the species complex. Several additional studies not listed on WoS or published prior to 1987 were identified.
Acanthaster cf. ellisii is distributed throughout the Eastern Tropical Pacific (ETP), from the Gulf of California to the Galapágos Islands (Solís-Marín et al. 2012). In Mexico there are reports of the species in several locations in Baja California (Ziesenhenne 1937; Holguin Quinones et al. 2000; Hermosillo-Nuñez et al. 2015; Solís-Marín et al. 2016) (Fig. 1).
In Costa Rica, the species has been reported for the Cocos Islands (Guzman and Cortes 1992), and two continental islands (Guzmán 1988; Chacón-Monge et al. 2021). In Panama it has been reported only for the Gulf of Chiriquí (Glynn 1973), while in Colombia it is found in the Malpelo (Narváez and Zapata 2010) and Gorgona islands (Zapata et al. 2017). For Ecuador, the only reports of the species come from the Galapagos archipelago, exclusively for the islands of Darwin and Wolf (Feingold and Glynn 2013; Sonnenholzner et al. 2013). The species has also been seen on Clipperton Atoll at low densities (Glynn et al. 1996). The first record of this species in the ETP was in 1869 (Verrill 1869) in the Gulf of California. The presence of the species in the ETP has been documented to occur from almost 4000 years before present by describing skeletal elements in sediment cores (Enochs and Glynn 2017).
In the first systematic studies, two taxa of Acanthaster were recognised in the ETP, Acanthaster cf. ellisii (Gray, 1850) and the subspecies Acanthaster ellisii pseudoplanci (Caso 1962), indicating that they differ with respect to buccal plates, pedicelaria, spines and other structures (Caso 1962, 1970). However, on the basis of raw morphology and meristic data, Glynn (Glynn 1974) suggested that the Eastern Pacific populations of Acanthaster should be assigned to species planci, which, by today’s knowledge, is an incorrect taxonomic assignment (Haszprunar and Spies 2014; Haszprunar et al. 2017). However, additional comparative morphological as well as genetic work is needed to investigate the species sub-species status of Acanthaster cf. ellisii (Gray, 1850) and Acanthaster ellisii pseudoplanci (Caso 1962), respectively.
Species ecology and biology
Only very few morphological studies for A. cf. ellisii exist. (Glynn 1982b) reported that the specimens from Panama are smaller (average 14.8 cm in diameter) than specimens from Guam (i.e., A. cf. solaris, 17.1 cm), while the number of arms, anuses and madreporites were very similar between those locations. The same study found a lower number of crustacean lesions in the individuals studied in Panama compared to Guam. The shrimp Hymenocera picta and the polychaete Pherecardia striata frequently attack and kill A. cf. ellisii (Glynn 1984). Several other species were reported to prey on dead or moribund specimens, namely the sea urchin Diadema mexicanun, and the fish species Stegastes acapulcoensis, Thalassoma lucasanum, Holocanthus passer, Sufflamen verres, Arothron mealeagris and A. hispidus. Complete decay of the dead A. cf. ellisii takes about 4 days (Glynn 1984).
Undoubtedly, the El Niño-Southern Oscillation (ENSO) phenomenon has been a modulator of the dynamics of the ETP coral reefs, which is why it was vital to understand how these events interact with the presence of the seastar (Glynn 1985). Pocillopora represents 85% of A. cf. ellisii’s diet (Glynn et al. 1972; Glynn 1973), and Pocillopora verrucosa is the preferred prey of CoTS along the Pacific coast of Mexico (Rodríguez-Villalobos et al. 2015). However, the presence of the guardian crustaceans Trapezia and Alpheus in Pocillopora may repel attacks from the seastar (Glynn 1976, 1980). When pocilloporid corals deteriorate due to bleaching during El Niño, the density of the crabs decreases, allowing the seastar to advance and prey on them (Glynn 1985). As the Pocillopora protective barrier is lost, other corals enclosed by it, such as Gardineroseris planulata, Pavona clavus, Pavona gigantea and Pavona varians (Glynn 1976, 1985, 1984), became susceptible to Acanthaster predation. Thus, although ENSO did not directly trigger an increase in the populations of the seastar, it created opportunities for more intense predation (Glynn and Colgan 1992).
In addition to the above-mentioned genera, Acanthaster cf. ellisii was also observed to prey on corals of the genus Porites, Psammocora and Millepora (Maté 2003; Rodriguez-Villalobos and Ayala-Bocos 2021). Small seastars feed on Porites panamensis, coralline algae and turf, while larger stars feed mainly on Pocillopora and very few on macroalgae. Medium-sized stars have a greater diversity in their diet, feeding on all mentioned food types (Hernández-Morales et al. 2021). Rodriguez-Villalobos and Ayala-Bocos (2021) measured coral consumption rates of up to 0.31 m2 day−1.
Feeding of A. cf. ellisi on gorgonians has also been observed (Dana and Wolfson 1970).
Hardly any research exists on the reproduction of A. cf. ellisii, but it was suggested that this species has a protracted, if not continuous, spawning season in spring (Dana and Wolfson 1970).
Populations and outbreaks
Glynn (1973) estimated that densities greater than 2.60 ind 100 m−2 would cause rapid destruction of reefs in Panama, considering the growth of coral colonies and predation by sea stars. In that region, CoTS populations remained largely stable between 0.07 and 0.3 ind 100 m−2 (Glynn 1982a). Until 2018, along the ETP the populations of this species had not experienced population outbreaks (Guzmán 1988; Reyes-Bonilla and Calderon-Aguilera 1999; Narváez and Zapata 2010; Rodríguez-Villalobos et al. 2015; Enochs and Glynn 2017).
CoTS densities in the Gulf of California can locally reach between 2.20 and 15.00 ind 100 m−2 (Herrero-Pérezrul 2008; Rodríguez-Vilalobos and Ayala-Bocos 2018; Rodriguez-Villalobos and Ayala-Bocos 2021). Average CoTS densities in that region reached 6.07 ind 100 m−2 in 2018/2019 (Rodriguez-Villalobos and Ayala-Bocos 2021). Consequently, damage to corals and deterioration of the reef infrastructure has been evident with coral mortalities associated with A. cf. ellisii predation greater than 50% and with acute injuries, indicating active predation (Rodriguez-Villalobos and Ayala-Bocos 2021). In contrast, between 2005 and 2016, when the average CoTS densities were 0.7 ind 100 m−2, no CoTS-associated coral decline was observed (Rodríguez-Villalobos et al. 2020). The 2018/2019 CoTS population explosion also affected coral restoration projects, where the mortality of the outplanted fragments of Pocillopora was reduced between 39 and 88%, probably due to the size of the fragments and the lack of symbiont crustaceans that could defend the colonies (Martinez-Sarabia and Reyes-Bonilla 2021).
Management, general
Due to the concern about the impact that CoTS may cause throughout the region, continuous monitoring of their populations have been undertaken. In Mexico, CoTS have been culled, despite having no official strategy for doing so. Nonetheless, individual actions and continuous monitoring of its populations in the region do exist, to evaluate the impact it is causing.
The species is listed as a non-native invasive species in the Galapagos Islands (Keith et al. 2016), but due to its low densities no eradication actions have been carried out. In Costa Rica, eradication actions have been carried out by diving companies without scientific support or permits. On the island of Caño, they have extracted organisms for fear of what these seastars may cause on the reefs. In addition, the belief that it is an invasive species has led to decisions without scientific basis for its eradication, highlighting the lack of substantiated knowledge and calling for rapid action to bridge these gaps.
Discussion
The principle aim of this review was to summarise knowledge available for the five distinct species of Crown of Thorns Seastars (CoTS) and assess inter-specific variation in the ecology of these important coral predators. While we recognised a priori that most of the research on CoTS has been conducted on Acanthaster cf. solaris in the central West Pacific, the magnitude of this bias was surprising; 418 out of the 444 (94%) publications in the WoS search that focused on a single species investigated A. cf. solaris. Moreover, most of this research was undertaken in Australia, Japan, New Caledonia and French Polynesia. There is, therefore, extensive knowledge on the biology and ecology of A. cf. solaris across a broad range of different topics (Table 2), but only based on a limited number of study locations. Critically, this biased research effort towards A. cf. solaris has shaped the widely held assumptions about the biology and ecology of other Acanthaster spp., and greatly influenced management responses throughout the Indo-Pacific.
Acanthaster cf. solaris has a complex life cycle, with planktotrophic larvae that preferentially feed on certain microalgal types and densities (Mellin et al. 2017; Uthicke et al. 2018b). Larvae preferentially settle in response to cues from specific species of CCA (Doll et al. 2023) and develop into algal-feeding juveniles, which switch to coral predation at around 5–6 months (Neil et al. 2022). After switching diets, juvenile A. cf. solaris grows rapidly (Wilmes et al. 2020) and can become reproductive within two years, at 10–15 cm in diameter (Pratchett et al. 2021b). Adult A. cf. solaris tend to exhibit strong and consistent feeding preferences, such that outbreaks (which have been documented across > 20 different countries throughout the Pacific, Table 3) not only cause extensive coral loss (Chesher 1969a; Kayal et al. 2012) but disproportionally impact Acropora spp. (Pratchett et al. 2009). While the developmental progression and timing are likely to be highly conserved among distinct species of Acanthaster spp. including A. brevispinus (Lucas and Jones 1976), it is quite possible that their behavioural ecology fundamentally differs, which could have major ramifications for their population dynamics and impacts on local reef environments.
While there is a definite need for increased research on the biology of all Acanthaster spp., explicitly testing for interspecific variation in key life processes, the remainder of this discussion will focus on assessing behavioural differences and corresponding impacts on local reef environments. Most notably, we will explore variation in (1) diurnal activity patterns, (2) feeding behaviour (especially feeding preferences) of adult seastars and (3) an apparent difference in the propensity of outbreaks between the different species. These important aspects of the behaviour of Acanthaster spp. have been studied across multiple distinct species (Burn et al. 2020) and will provide critical tests of the prevailing assumption that distinct species of Acanthaster sp. are ostensibly similar in their ecology and function.
Diurnal activity patterns
Early studies on the behavioural ecology of CoTS in the Red Sea (i.e., A. benziei) reported that adult seastars feed almost exclusively at night (Goreau 1962; Haszprunar et al. 2017). There are further anecdotal observations that CoTS are observed feeding much more often at night than during the day, for A. cf. solaris at various locations throughout the Pacific (e.g., Chesher 1969a, b; Branham et al. 1971). Given their tendency to feed mostly at night, CoTS are also seen to be most exposed and moving mainly at night. Notably, Ormond and Campbell (1974) described a very similar pattern of activity for A. benziei to that formally characterised by Ling et al. (2020) for A. cf. solaris, whereby individuals became active at dusk when searching for coral prey. They then fed (often being exposed, but stationary) through much of the night before seeking shelter within the reef matrix before daylight (Ling et al. 2020). Diurnal variation in exposure and feeding is also often most pronounced in smaller individuals, at least for A. cf. solaris (e.g., Keesing 1995). This diurnal variation in behaviour is commonly attributed to the risk of predation from diurnally active predators (Cowan et al. 2017), and if so, might be influenced more by the local abundance of predatory species than interspecific differences in behaviour. Whether differences in toxicity and thus protection from predators affect diurnal behaviour as suggested by Haszprunar et al. (2017) requires further investigation.
Burn et al. (2020) explicitly compared the diurnal activity patterns of A. planci in the Maldives with that of A. cf. solaris on Australia’s GBR. The extent to which CoTS were exposed (i.e., not concealed within or beneath coral substrates) was higher (63.14%) for A. planci in the Maldives, compared to 28.55% for A. cf. solaris at Rib Reef (GBR). Moreover, > 97% of A. planci were completely exposed during surveys conducted at night, compared to only 52% for A. cf. solaris. Geographical differences were not necessarily attributable to inherent behavioural differences among species but appeared to be largely explained by apparent differences in coral cover and habitat structure between the particular study locations (Burn et al. 2020).
In general, it appears that the tendency to feed mostly at night and mainly shelter during the day is common among different Acanthaster spp., including A. benziei, A. planci and A. cf. solaris, though individual differences in diurnal activity patterns vary with the size of the individuals and habitat structure.
Food preferences
The ecological effects of CoTS on local coral assemblages and reef ecosystems are strongly influenced by the relative predilection for different coral species, or feeding preferences (e.g., De'ath and Moran 1998a; Pratchett et al. 2009). Most studies that have explicitly considered feeding preferences [which needs to take account of the availability and accessibility of different coral prey (Pratchett 2007; Tokeshi and Daud 2011)] have been undertaken in the Western Pacific for A. cf. solaris. However, the limited studies conducted elsewhere, such as in the Red Sea (Campbell and Ormond 1970), suggest that other Acanthaster spp. have similarly strong and consistent preference for Acropora spp. if available. A possible exception is A. cf. ellisii because Acropora does not exist in the ETP, thus it is unclear if that species would still prefer Acropora if present. These inherent feeding preferences are further reflected in the numerous anecdotal observations of CoTS feeding predominantly on Acropora spp. (e.g., A. mauritiensis, Roche et al. 2015), and disproportionate declines in Acropora spp. during CoTS population outbreaks (e.g., A. planci, Glynn 1973; Saponari et al. 2018). Nevertheless, in areas where Acropora spp. are scarce (or already depleted), CoTS are observed to feed extensively on alternative prey. In the eastern Pacific, for example, A. cf. ellisii feeds predominantly on Pocillopora spp. and has also frequently been observed feeding on non-hard coral prey, such as macro–, turf–, and coralline algae, as well as gorgonians (Hernández-Morales et al. 2021). This dietary flexibility, apparent for A. cf. ellisii, may represent an adaptation necessary to persist in marginal reef environments in the eastern Pacific, such as the Gulf of California (Rodríguez-Villalobos et al. 2015). It is unknown whether other Acanthaster spp. possess similar dietary flexibility, though their feeding physiology does allow for exceptional flexibility in diet (Goreau 1962), and A. mauritiensis has been observed to feed on sponges (Celliers and Schleyer 2006).
Given their strong preference for Acropora spp., severe or recurrent outbreaks of CoTS are likely to cause localised depletion of these corals (e.g., Maldives, Saponari et al. 2018; Pisapiai et al. 2019) and may contribute to persistent shifts in coral composition (e.g., French Polynesia, Pratchett et al. 2011). More importantly, however, these feeding preferences likely reflect the nutritional benefit that CoTS derive from feeding on these corals (Caballes et al. 2016), such that limited availability or accessibility to Acropora spp. may constrain their individual fitness and population viability. It is apparent for example, that CoTS will feed on an increasing variety of coral prey once Acropora spp. are locally depleted. During the latter stages of population outbreaks in Maldives, for example, A. planci were reported to feed predominantly on Porites, Favites and Pavona (Saponari et al. 2018; Burn et al. 2020). Increasing intake of these least preferred, and presumably sub-optimal coral prey, may impose physiological constraints that limit the persistence or initiation of population irruptions.
Propensity for outbreaks
Reported outbreaks of A. cf. solaris are widespread, often reoccurring and lead to significant loss of coral. This is not necessarily the case in other species. Reported outbreaks of A. benziei are relatively localised in most places where they occur, except for the Saudi Arabian Red Sea where high densities were observed on several reefs and significant coral loss reported (Riegl et al. 2013). Similarly, several outbreaks of A. mauritiensis were localised or characterised as ‘aggregations’ (Bligh and Bligh 1971; Celliers and Schleyer 2006), whereas several other outbreaks of that species were only observed once since the 1970s (Mohammed et al. 2000; Gerard et al. 2008b). However, the remoteness of most locations in the West Indian Ocean and few monitoring programmes makes it difficult to judge if additional outbreaks were overlooked. Outbreaks of A. planci in some locations also appear local, but outbreaks in several Atolls of the Maldives (Saponari et al. 2018) for example, or in Sri Lanka (Dissanayak and Terney Pradeep Kumara 2015) were also severe. On the eastern end of the Acanthaster distribution, A. cf. ellisii also appears less prone to outbreaks, with a significant outbreak only reported in 2018 in Mexico (Rodriguez-Villalobos and Ayala-Bocos 2021). Thus, although data are sparse and outbreak definitions vary between authors, it appears the species at the eastern (A. cf. ellisii) and western (A. mauritiensis, A. benziei) margin of the Genus’ distribution are less prone to severe, repeated and destructive outbreaks compared to the ‘central’ species A. planci and A. cf. solaris. It is currently unresolved whether the reasons for this are intrinsic differences in the biology of the species or driven by extrinsic processes, such as the availability of Acropora coral species.
Conclusions
As outlined above, there are practically no data on most of the life cycle (fertilisation, larval development, settlement, CCA and coral feeding juvenile) of four of the Acanthaster species considered here. Compared to that, A. cf. solaris is extensively investigated making it one of the best studied tropical marine invertebrates. However, even for that species, knowledge is considered too limited to fully understand outbreak causes and recommend effective management action. In a recent horizon scan for that species, Pratchett et al. (2021a) identified the incidence of population irruptions, feeding ecology of larval sea stars, effects of elevated water temperature and predation on juveniles as the most important topics for further research. We urge the scientific community, nature conservation managers, and other stakeholders to support filling these research gaps for all species given that it is not appropriate to assume that the biology of five species is the same (at least not without testing) and this may result in inappropriate management responses.
Data availability
All data analysed are supplied in two electronic supplements.
References
Adjeroud M, Kayal M, Peignon C, Juncker M, Mills SC, Beldade R, Dumas P (2018) Ephemeral and localized outbreaks of the coral predator Acanthaster cf. solaris in the Southwestern Lagoon of New Caledonia. Zool Stud 57:11. https://doi.org/10.6620/zs.2018.57-04
Ammar M (2005) Recovery probabilities of coral reef communities after Acanthaster planci infestation: a case study at Shabror umm gam’ar, Hurghada, Red Sea, Egypt. Egypt J Aquat Res 31:103–112
Ammar MSA (2007) Recovery patterns of corals at Shabror Umm Gam’ar, Hurghada, Red Sea, Egypt, after the 1998 outbreak of Acanthaster planci. Zool Middle East 40:97–104
Babcock RC, Dambacher JM, Morello EB, Plaganyi EE, Hayes KR, Sweatman HPA, Pratchett MS (2016a) Assessing different causes of crown-of-thorns starfish outbreaks and appropriate responses for management on the Great Barrier Reef. PLoS One 11:20. https://doi.org/10.1371/journal.pone.0169048
Babcock RC, Milton DA, Pratchett MS (2016b) Relationships between size and reproductive output in the crown-of-thorns starfish. Mar Biol 163(234):1–7
Babcock RC, Plaganyi EE, Condie SA, Westcott DA, Fletcher CS, Bonin MC, Cameron D (2020) Suppressing the next crown-of-thorns outbreak on the Great Barrier Reef. Coral Reefs 39:1233–1244. https://doi.org/10.1007/s00338-020-01978-8
Baird A, Pratchett M, Hoey A, Herdiana Y, Campbell S (2013) Acanthaster planci is a major cause of coral mortality in Indonesia. Coral Reefs 32:1–10
Benzie JAH, Stoddart JA (1992) Genetic structure of crown-of-thorns (Acanthaster planci) in Australia. Mar Biol 112:631–639
Benzie JAH, Wakeford M (1997) Genetic structure of Crown-of-thorns starfish (Acanthaster planci) on the Great Barrier Reef, Australia: comparison of two sets of outbreak populations occurring ten years apart. Mar Biol 129:149–157
Berthe C, Chancerelle Y, Lecchini D, Hedouin L (2016) First report of a dramatic rapid loss of living coral on the north coast of Western Samoa. Vie Milieu 66:155–157
Birkeland C (1982) Terrestrial runoff as a cause of outbreaks of Acanthaster planci (Echinodermata: Asteroidea). Mar Biol 69:175–185
Black K, Moran P, Burrage D (1995) Association of low-frequency currents and crown-of-thorns starfish outbreaks. Mar Ecol Prog Ser 125:185–194
Bligh T, Bligh N (1971) Acanthaster in the Indian Ocean. Nature 229:281–281
Bostrom-Einarsson L, Rivera-Posada J (2016) Controlling outbreaks of the coral-eating crown-of-thorns starfish using a single injection of common household vinegar. Coral Reefs 35:223–228. https://doi.org/10.1007/s00338-015-1351-6
Branham J, Reed S, Bailey JH, Caperon J (1971) Coral-eating sea stars Acanthaster planci in Hawaii. Science 172(3988):1155–1157
Brodie J, Fabricius K, De’ath G, Okaji K (2005) Are increased nutrient inputs responsible for more outbreaks of crown-of-thorns starfish? An appraisal of the evidence. Mar Pollut Bull 51:266–278
Brown EK, McKenna SA, Beavers SC, Clark T, Gawel M, Raikow DF (2016) Informing coral reef management decisions at four US National Parks in the Pacific using long-term monitoring data. Ecosphere 7:18. https://doi.org/10.1002/ecs2.1463
Burn D, Matthews S, Caballes CF, Chandler JF, Pratchett MS (2020) Biogeographical variation in diurnal behaviour of Acanthaster planci versus Acanthaster cf. solaris. PLoS One 15:e0228796
Caballes CF, Pratchett MS (2014) Reproductive biology and early life history of the crown-of-thorns starfish. In: Whitmore E (ed) Echinoderms: ecology, habitats and reproductive biology. Nova publishers, New York, pp 101–146
Caballes CF, Pratchett MS, Kerr AM, Rivera-Posada JA (2016) The role of maternal nutrition on oocyte Size and quality, with respect to early earval development in the coral-eating starfish, Acanthaster planci. PLoS One 11:21. https://doi.org/10.1371/journal.pone.0158007
Campbell AC, Ormond RF (1970) The threat of the ‘crown-of-thorns’ starfish (Acanthaster planci) to coral reefs in the Indo-Pacific area: observations on a normal population in the Red Sea. Biol Conserv 2:246–251
Caragnano A, Basso D, Spezzaferri S, Hallock P, Conf Univ Suisse Occidentale S (2021) A snapshot of reef conditions in North Ari Atoll (Maldives) following the 2016 bleaching event and Acanthaster planci outbreak. Mar Freshw Res 72:987–996. https://doi.org/10.1071/mf20119
Caso ME (1962) Estudios sobre equinodermos de México. Contribución al conocimiento de los equinodermos de las islas Revillagigedo Anales del Instituto de Biología, Universidad Nacional Autónoma de México, pp 293–330
Caso ME (1970) Morfología externa de Acanthaster planci (Linnaeus). An Inst Biol UNAM Ser Cienc Mar Limnol 41:63–78
Castro-Sanguino C, Ortiz JC, Thompson A, Wolff NH, Ferrari R, Robson B, Magno-Canto MM, Puotinen M, Fabricius KE, Uthicke S (2021) Reef state and performance as indicators of cumulative impacts on coral reefs. Ecol Ind 123:107335. https://doi.org/10.1016/j.ecolind.2020.107335
Celliers L, Schleyer MH (2006) Observations on the behaviour and the character of an Acanthaster planci (L.) aggregation in a high latitude coral community in South Africa. West Indian Ocean J Mar Sci 5:105–113
Celliers L, Schleyer MH (2008) Coral community structure and risk assessment of high-latitude reefs at Sodwana Bay, South Africa. Biodivers Conserv 17:3097–3117
Chacón-Monge J-L, Azofeifa-Solano J-C, Alvarado J-J, Cortés J (2021) Área de Conservación Guanacaste Echinoderms, North Pacific of Costa Rica. Rev Biol Trop 69:487–500
Chak STC, Dumont CP, Abd Adzis KA, Yewdall K (2018) Effectiveness of the removal of coral-eating predator Acanthaster planci in Pulau Tioman Marine Park, Malaysia. J Mar Biol Assoc UK 98:183–189. https://doi.org/10.1017/s002531541600117x
Chansang H, Boonyanate P, Pongsuwan N, Charuchinda M, Wungboonkong G (1987) Infestation of Acanthaster planci in the Andaman Sea. Bull Mar Sci 41:634–634
Chesher R (1969a) Destruction of Pacific coral by Acanthaster planci. Science, NY 165:280–283
Chesher RH (1969b) Acanthaster planci: impact on Pacific Coral Reefs: final Report to [the] US Department of the Interior, Washington. Westinghouse Ocean Research Laboratory. Research Laboratories, Westinghouse Electric Corporation
Coppock AG, Gardiner NM, Jones GP (2016) Olfactory responses of coral-reef fishes to coral degradation and crown-of-thorns (Acanthaster planci). Mar Freshw Res 67:605–611
Cowan Z-L, Dworjanyn SA, Caballes CF, Pratchett MS (2016) Predation on crown-of-thorns starfish larvae by damselfishes. Coral Reefs 35:1253–1262. https://doi.org/10.1007/s00338-016-1491-3
Cowan Z-L, Pratchett M, Messmer V, Ling S (2017) Known predators of crown-of-thorns starfish (Acanthaster spp.) and their role in mitigating, if not preventing, population outbreaks. Diversity 9:7
Dana T, Wolfson AA (1970) Eastern Pacific crown-of-thorns starfish populations in the lower Gulf of California. San Diego Soc Nat Hist 16(4):83–90
de Diosi HHY, Dy DT, Sotto FB (2014) Abundance and size structure of an Acanthaster planci population (Echinodermata: Asteroidea) in Sogod Bay, Southern Leyte, Philippines. Asia Life Sci 23:65–73
Deaker DJ, Agüera A, Lin H-A, Lawson C, Budden C, Dworjanyn SA, Mos B, Byrne M (2020a) The hidden army: corallivorous crown-of-thorns seastars can spend years as herbivorous juveniles. Biol Lett 16:20190849
Deaker DJ, Mos B, Lin H-A, Lawson C, Budden C, Dworjanyn SA, Byrne M (2020b) Diet flexibility and growth of the early herbivorous juvenile crown-of-thorns sea star, implications for its boom-bust population dynamics. PLoS One 15:e0236142
De’ath G, Moran PJ (1998a) Factors affecting the behaviour of crown-of-thorns starfish on the Great Barrier Reef: 1: patterns of activity. J Exp Mar Biol Ecol 220:83–106
De’ath G, Moran PJ (1998b) Factors affecting the behaviour of crown-of-thorns starfish on the Great Barrier Reef: 2: feeding preferences. J Exp Mar Biol Ecol 220:107–126
Desbiens AA, Mumby PJ, Dworjanyn S, Plagányi ÉE, Uthicke S, Wolfe K (2023) Novel rubble-dwelling predators of herbivorous juvenile crown-of-thorns starfish (Acanthaster sp.). Coral Reefs 42:579–591
Dight IJ, James MK, Bode L (1990) Modelling the larval dispersal of Acanthaster planci. II patterns of connectivity. Coral Reefs 9:125–134. https://doi.org/10.1007/bf00258224
Dissanayak KN, Terney Pradeep Kumara P (2015) Impacts of Acanthaster planci (Crown-of-Thorns Starfish) population expansion on the Coral ecosystem of Pigeon Island M arine N ational Park, Sri Lanka. In: Proceedings of the national Resource Research and Development Agency (NARA), Scientific Sessions 2015. NARA
Döderlein L (1888) Echinodermen von Ceylon. Bericht über die von den Herren Dres Sarasin gesammelten Asteroidea, Ophiuroidea und Echinoidea. Zool Jahrb Abt Für Syst Geogr Biol Der Tiere 3:821–846
Doll PC, Messmer V, Uthicke S, Doyle JR, Caballes CF, Pratchett MS (2021) DNA-based detection and patterns of larval settlement of the corallivorous crown-of-thorns sea star (Acanthaster sp.). Biol Bull 241:271–285
Doll PC, Uthicke S, Caballes CF, Diaz-Pulido G, Abdul Wahab MA, Lang BJ, Jeong SY, Pratchett MS (2023) Settlement cue selectivity by larvae of the destructive crown-of-thorns starfish. Biol Lett 19:20220399
Dumas P, Peignon C, Dumas M, Bourgeois B, Gossuin H, Fiat S (2022) Destructive outbreaks of the corallivorous starfish Acanthaster cf. solaris spare coral assemblages in the shallowest reef flat areas in New Caledonia. ICES J Mar Sci 79:350–361. https://doi.org/10.1093/icesjms/fsab262
Emeras J, Falquet M, Conand C (2004) Acanthaster planci on La Reunion Reefs (Western India Ocean). Reef Encount 32:26–27
Endean R (1969) Report on investigations made into aspects of the current Acanthaster planci (crown of thorns) infestations of certain reefs of the Great Barrier Reef. Queensland Department of Primary Industries (Fisheries Branch), Brisbane, pp 40
Enochs IC, Glynn PW (2017) Corallivory in the eastern Pacific. In: Glynn PW, Manzello DP, Enochs IC (eds) Coral reefs of the Eastern Tropical Pacific: persistence and loss in a dynamic environment. Coral Reefs of the World, vol 8. Springer, London, pp 315–337
Fabricius KE (1997) Soft coral abundance on the central Great Barrier Reef: effects of Acanthaster planci, space availability, and aspects of the physical environment. Coral Reefs [coral Reefs] 16:159–167
Fabricius KE, Fabricius FH (1992) Re-assessment of ossicle frequency patterns in sediment cores: rate of sedimentation related to Acanthaster planci. Coral Reefs 11:109–114
Fabricius KE, Okaji K, De’ath G (2010) Three lines of evidence to link outbreaks of the crown-of-thorns seastar Acanthaster planci to the release of larval food limitation. Coral Reefs 29:593–605
Feingold JS, Glynn PW (2013) Coral research in the Galápagos Islands, Ecuador The Galápagos marine reserve: a dynamic social-ecological system. Springer, Berlin, pp 3–22
Fernandes L, Marsh H, Moran P, Sinclair D (1990) Bias in manta tow surveys of Acanthaster planci. Coral Reefs 9:155–160
Gerard K, Roby C, Chevalier N, Thomassin B, Chenuil A, Feral JP (2008a) Assessment of three mitochondrial loci variability for the crown-of-thorns starfish: a first insight into Acanthaster phylogeography. C R Biol 331:137–143. https://doi.org/10.1016/j.crvi.2007.11.005
Gerard K, Chevalier N, Chenuil A, Feral J-P, Thomassin B (2008b) Etude sur l’origine génétique des étoiles demer Acanthaster planci présentes dans le lagon et lesrécifs coralliens de Mayotte [Genetic origin of thecrown-of-thorns starfish Acanthaster planci present in the lagoon and coral reefs of Mayotte]. Coll. départ.Mayotte: Direction de l’Environnement et du Développement Durable
Glynn PW (1973) Acanthaster: effect on coral reef growth in Panama. Science 180:504–506
Glynn PW (1974) The impact of Acanthaster on corals and coral reefs in the eastern Pacific. Environ Conserv 1:295–304
Glynn PW (1976) Some physical and biological determinants of coral community structure in the eastern Pacific. Ecol Monogr 46:431–456
Glynn PW (1980) Defense by symbiotic crustacea of host corals elicited by chemical cues from predator. Oecologia 47:287–290
Glynn P (1982a) Acanthaster population regulation by a shrimp and a worm. In: ED Gomez, CE Birkeland (eds) Proceedings of 4th International Coral Reef Symposium, vol 2. Manila, pp 607–612
Glynn P (1982b) Individual recognition and phenotypic variability in Acanthaster planci (Echinodermata: Asteroidea). Coral Reefs 1:89–94
Glynn PW (1984) An amphinomid worm predator of the crown-of-thorns sea star and general predation on asteroids in eastern and western Pacific coral reefs. Bull Mar Sci 35:54–71
Glynn PW (1985) El Nino-associated disturbance to coral reefs and post disturbance mortality by Acanthaster planci. Mar Ecol Prog Ser 26:295–300
Glynn P (1987) Some ecological consequences of coral-crustacean guard mutualisms in the Indian and Pacific Oceans. Symbiosis 4:301–324
Glynn PW, Colgan MW (1992) Sporadic disturbances in fluctuating coral-reef environments—El Nino and coral-reef development in the eastern Pacific. Am Zool 32:707–718
Glynn PW, Enochs IC (2010) Invertebrates and their roles in coral reef ecosystems. In: Dubinsky Z, Stambler N (eds) Coral reefs: an ecosystem in transition, vol 1. Springer, London, pp 273–325
Glynn PW, Stewart RH, McCosker JE (1972) Pacific coral reefs of Panama: structure, distribution and predators. Geol Rundsch 61:483–519
Glynn PW, Veron JEN, Wellington GM (1996) Clipperton atoll (eastern Pacific): oceanography, geomorphology, reef-building coral ecology and biogeography. Coral Reefs 15:71–99
Goreau T (1962) On the predation of coral by the spiny starfish Acanthaster planci (L.) in the southern Red Sea. Israel South Red Sea Expedition Reports. Nature 194:1134–1137
Guzmán HM (1988) Distribución y abundancia de organismos coralívoros en los arrecifes coralinos de la Isla del Caño, Costa Rica. Rev Biol Trop 36:191–207
Guzman HM, Cortes J (1992) Cocos Island (Pacific of Costa Rica) coral-reefs after the 1982–83 El Nino disturbance. Rev Biol Trop 40:309–324
Hall MR, Kocot KM, Baughman KW, Fernandez-Valverde SL, Gauthier MEA, Hatleberg WL, Krishnan A, McDougall C, Motti CA, Shoguchi E, Wang T, Xiang X, Zhao M, Bose U, Shinzato C, Hisata K, Fujie M, Kanda M, Cummins SF, Satoh N, Degnan SM, Degnan BM (2017) The crown-of-thorns starfish genome as a guide for biocontrol of this coral reef pest. Nature 544:231–234. https://doi.org/10.1038/nature22033
Harriott VJ (1995) Is the crown-of-thorns starfish a threat to the reefs of Lord-Howe-Island. Aquat Conserv Mar Freshw Ecosyst 5:179–190. https://doi.org/10.1002/aqc.3270050303
Harrison H, Pratchett M, Messmer V, Saenz-Agudelo P, Berumen M (2017) Microsatellites reveal genetic homogeneity among outbreak populations of Crown-of-Thorns Starfish (Acanthaster cf. solaris) on Australia’s Great Barrier Reef. Diversity 9:16
Hart AM, Klumpp DW, Russ GR (1996) Response of herbivorous fishes to crown-of-thorns starfish Acanthaster planci outbreaks.2. Density and biomass of selected species of herbivorous fish and fish-habitat correlations. Mar Ecol Prog Ser 132:21–30. https://doi.org/10.3354/meps132021
Haszprunar G, Spies M (2014) An integrative approach to the taxonomy of the crown-of-thorns starfish species group (Asteroidea: Acanthaster): a review of names and comparison to recent molecular data. Zootaxa 3841:271–284
Haszprunar G, Vogler C, Wörheide G (2017) Persistent gaps of knowledge for naming and distinguishing multiple species of crown-of-thorns-seastar in the Acanthaster planci species complex. Diversity 9:22
Haywood MDE, Thomson DP, Babcock RC, Pillans RD, Keesing JK, Miller M, Rochester WA, Donovan A, Evans RD, Shedrawi G, Field SN (2019) Crown-of-thorns starfish impede the recovery potential of coral reefs following bleaching. Mar Biol 166:15. https://doi.org/10.1007/s00227-019-3543-z
Hermosillo-Nuñez B, Rodríguez-Zaragoza F, Ortiz M, Galván-Villa C, Cupul-Magaña A, Ríos-Jara E (2015) Effect of habitat structure on the most frequent echinoderm species inhabiting coral reef communities at Isla Isabel National Park (Mexico). Community Ecol 16:125–134
Hernández-Morales A, Herrero-Pérezrul M-D, Vázquez-Arce D-I (2021) Variability of size and food type of Acanthaster planci (Echinodermata: Asteroidea) in the southern Gulf of California, Mexico
Herrero-Pérezrul M (2008) Diversity and abundance of reef macro invertebrates (Mollusca; Echinodermata) in the southern Gulf of California, México Proceedings of the 11th international coral reef symposium, pp 7–11
Heydorn A (1972) Tongaland’s coral reefs-an endangered heritage. Afr Wildl 26:20–23
Hill N, Davidson J, Silva I, Mucave S, Muaves L, Guissamulo A, Debney A, Garnier J (2009) Coral and reef fish in the northern Quirimbas Archipelago, Mozambique—a first assessment. West Indian Ocean J Mar Sci. https://doi.org/10.4314/wiojms.v8i1.56680
Høj L, Levy N, Baillie BK, Clode PL, Strohmaier RC, Siboni N, Webster NS, Uthicke S, Bourne DG (2018) Crown-of-thorns sea star, Acanthaster cf. solaris, have tissue-characteristic microbiomes with potential roles in health and reproduction. Appl Environ Microbiol 84:00181
Holguin Quinones O, Wright Lopez H, Solis Marin F (2000) Asteroidea, Echinoidea and Holothuroidea of shallow waters in the Loreto Bay, Baja California Sur, Mexico. Revista De Biologia Tropical [rev Biol Trop] 48(4):749–757
Houk P, Raubani J (2010) Acanthaster planci Outbreaks in Vanuatu coincide with ocean productivity, furthering trends throughout the Pacific Ocean. J Oceanogr 66:435–438. https://doi.org/10.1007/s10872-010-0038-4
James D, Pillai CG, Gopakumar G (1990) A case study of infestation of Acanthaster planci in Andaman waters. Mar Fish Inf Serv Tech Ext Ser 106:1–3
Johansson CL, Francis DS, Uthicke S (2016) Food preferences of juvenile corallivorous crown-of-thorns (Acanthaster planci) sea stars. Mar Biol 163:1–7
Johnson CR, Sutton DC, Olson RR, Giddings R (1991) Settlement of crown-of-thorns starfish: role of bacteria on surfaces of coralline algae and a hypothesis for deepwater recruitment. Mar Ecol Prog Ser 71:143–162
Katz SM, Pollock FJ, Bourne DG, Willis BL (2014) Crown-of-thorns starfish predation and physical injuries promote brown band disease on corals. Coral Reefs 33:705–716. https://doi.org/10.1007/s00338-014-1153-2
Kayal M, Vercelloni J, Lison de Loma T, Bosserelle P, Chancerelle Y, Geoffroy S, Stievenart C, Michonneau F, Penin L, Planes S, Adjeroud M (2012) Predator crown-of-thorns starfish (Acanthaster planci) outbreak, mass mortality of corals, and cascading effects on reef fish and benthic communities. PLoS One 7:e47363. https://doi.org/10.1371/journal.pone.0047363
Keesing JK (1995) Temporal patterns in the feeding and emergence behaviour of the crown-of-thorns starfish Acanthaster planci. Mar Freshw Behav Physiol 25:209–232. https://doi.org/10.1080/10236249509378919
Keesing JK, Lucas JS (1992) Field measurement of feeding and movement rates of the crown-of-thorns starfish Acanthaster planci (L.). J Exp Mar Biol Ecol 156:89–104
Keesing JK, Halford AR, Hall KC, Cartwright CM (1997) Large-scale laboratory culture of the crown-of-thorns starfish Acanthaster planci (L.) (Echinodermata: Asteroidea). Aquaculture 157:215–226
Keesing JK, Thomson DP, Haywood MDE, Babcock RC (2019) Two time losers: selective feeding by crown-of-thorns starfish on corals most affected by successive coral-bleaching episodes on western Australian coral reefs. Mar Biol 166:72. https://doi.org/10.1007/s00227-019-3515-3
Keesing JK, Mortimer N, Hellmrich L, Godoy D, Babcock RC, Heyward A, Paton D, Harvey ES (2023) The short spined crown-of-thorns starfish Acanthaster brevispinus is a corallivore too. Coral Reefs 42:399–404
Keith I, Dawson TP, Collins KJ, Campbell ML (2016) Marine invasive species: establishing pathways, their presence and potential threats in the Galapagos Marine Reserve. Pac Conserv Biol 22:377–385
Kenyon JC, Aeby GS (2009) Localized outbreak and feeding preferences of the crown-of-thorns seastar Acanthaster planci (Echinodermata, Asteroidea) on reefs off Oahu, Hawaii. Bull Mar Sci 84:199–209
Kimura T, Chou LM, Huang D, Tun K, Goh E (2022) Status and trends of East Asian Coral Reefs: 1983–2019. ScholarBank@NUS Repository
Kroon FJ, Lefèvre CD, Doyle JR, Patel F, Milton G, Severati A, Kenway M, Johansson CL, Schnebert S, Thomas-Hall P, Bonin MV, Cameron DS, Westcott DA (2020) DNA-based identification of predators of the corallivorous Crown-of-Thorns Starfish (Acanthaster cf. solaris) from fish faeces and gut contents. Sci Rep 10:1–14
Kwong SLT, Villacorta-Rath C, Doyle J, Uthicke S (2021) Quantifying shedding and degradation rates of environmental DNA (eDNA) from Pacific crown-of-thorns seastar (Acanthaster cf. solaris). Mar Biol 168:85. https://doi.org/10.1007/s00227-021-03896-x
Kwong SLT, Villacorta-Rath C, Pratchett M, Uthicke S (2023) Telomere dynamics in the Pacific crown-of-thorns seastar (Acanthaster cf. solaris): effect of age, diet, and tissue type. Coral Reefs. https://doi.org/10.1007/s00338-023-02405-4
Lane DJW (1996) A crown-of-thorns outbreak in the eastern Indonesian Archipelago, February 1996. Coral Reefs 15:209–210. https://doi.org/10.1007/bf01787452
Lane DJW (2012) Acanthaster planci impact on coral communities at permanent transect sites on Bruneian reefs, with a regional overview and a critique on outbreak causes. JMBA J Mar Biol Assoc UK 92:803
Lang BJ, Donelson JM, Caballes CF, Doll PC, Pratchett MS (2021) Metabolic responses of Pacific crown-of-thorns sea stars (Acanthaster sp.) to acute warming. Biol Bull 241:000–000
Lang BJ, Donelson JM, Caballes CF, Uthicke S, Doll PC, Pratchett MS (2022) Effects of elevated temperature on the performance and survival of pacific crown-of-thorns starfish (Acanthaster cf. solaris). Mar Biol 169:1–13
Larkum AWD (1988) High rates of nitrogen fixation on coral skeletons after predation by the crown of thorns starfish Acanthaster planci. Mar Biol 97:503–506
Ling SD, Cowan ZL, Boada J, Flukes EB, Pratchett MS (2020) Homing behaviour by destructive crown-of-thorns starfish is triggered by local availability of coral prey. Proc R Soc B Biol Sci 287:20201341
Loriol P (1895) Catalogue raisonné des Echinodermes recuellis par M. V. de Robillard à l´Ile Maurice. II. Stellérides. Mem Soc Phys Hist Nat Genéve 29:1–84 (pls 87-22)
Lourey MJ, Ryan DAJ, Miller IR (2000) Rates of decline and recovery of coral cover on reefs impacted by, recovering from and unaffected by crown-of-thorns starfish Acanthaster planci: a regional perspective of the Great Barrier Reef. Mar Ecol Prog Ser 196:179–186. https://doi.org/10.3354/meps196179
Lucas JS (1973) Reproductive and larval biology and Acanthaster planci (L.) in Great Barrier Reef. Micronesica 9:197–203
Lucas JS (1982) Quantitative studies on the feeding and nutrition during larval development of the coral reef asteroid Acanthaster planci (L.). J Exp Mar Biol Ecol 65:173–193
Lucas JS, Jones MM (1976) Hybrid crown-of-thorns starfish (Acanthaster planci × A. brevispinus) reared to maturity in the laboratory. Nature 263:409–412. https://doi.org/10.1038/263409a0
MacNeil MA, Chong-Seng K, Pratchett D, Thompson C, Messmer V, Pratchett M (2017) Age and growth of an outbreaking Acanthaster cf. solaris population within the Great Barrier Reef. Diversity 9:18
Martinez-Sarabia P, Reyes-Bonilla H (2021) Damage caused by crown-of-thorns starfish (Acanthaster cf. solaris) outbreak to restored corals in the southern Gulf of California, Mexico. Bull Mar Sci 97:329–336. https://doi.org/10.5343/bms.2020.0034
Maté J (2003) Corals and coral reefs of the Pacific coast of Panamá. In: Cortéz J (ed) Latin American Coral Reefs. Elsevier, New York, pp 387–417
Matthews S, Shoemaker K, Pratchett MS, Mellin C (2020) COTSMod: a spatially explicit metacommunity model of outbreaks of crown-of-thorns starfish and coral recovery. Adv Mar Biol 87:259–290
McCallum H (1987) Predator regulation of Acanthaster planci. J Theor Biol 127:207–220
McCallum H (1992) Completing the circle: stock-recruitment relationships and Acanthaster. Mar Freshw Res 43:653–662
Mellin C, Lugrin C, Okaji K, Francis D, Uthicke S (2017) Selective feeding and microalgal consumption rates by crown-of-thorns Seastar (Acanthaster cf. solaris) larvae. Diversity 9:8
Messmer V, Pratchett M, Chong-Seng K (2017) Variation in incidence and severity of injuries among Crown-of-Thorns Starfish (Acanthaster cf. solaris) on Australia’s Great Barrier Reef. Diversity 9:12
Miller IR, Death G (1996) Effects of training on observer performance in assessing benthic cover by means of the manta tow technique. Mar Freshw Res 47:19–26. https://doi.org/10.1071/mf9960019
Mohammed MS, Muhando C, Machano H (2000) Assessment of coral reef degradation in Tanzania: results of coral reef monitoring–1999. In: Souter D, Obura D and Linden O (eds) Coral Reef Degradation in the Indian Ocean (CORDIO), vol 1 and Institute of Marine Sciences, Zanzibar, Tanzania, pp 35–42
Mohammed S, Muhando C, Machano H, Daniels C, Tyler E, Jiddawi N, Yahya S (2005) Status of coral reefs in Tanzania. In: Obura D, Linden O (eds) Coral Reef degradation in the Indian Ocean. CORDIO, Kalmar, pp 47–53
Moran P (1986) The Acanthaster phenomenon. Oceanogr Mar Biol Ann Rev 24:379–480
Moran PJ, Death G (1992) Suitability of the manta tow technique for estimating relative and absolute abundances of crown-of-thorns starfish (Acanthaster planci) and corals. Aust J Mar Freshw Res 43:357–378
Moran PJ, De’ath G (1992) Estimates of the abundance of the crown-of-thorns starfish Acanthaster planci in outbreaking and non-outbreaking populations on reefs within the Great Barrier Reef. Mar Biol 113:509–515
Moran PJ, Bradbury RH, Reichelf RE (1987) Changes in the distribution and abundance of crown-of-thorns starfish (Acanthaster planci) and corals on John Brewer Reef—diary of an outbreak cycle. Bull Mar Sci 41:638–639
Morri C, Aliani S, Bianchi CN (2010) Reef status in the Rasfari region (North Male Atoll, Maldives) five years before the mass mortality event of 1998. Estuar Coast Shelf Sci 86:258–264. https://doi.org/10.1016/j.ecss.2009.11.021
Moutardier G, Gereva S, Mills SC, Adjeroud M, Beldade R, Ham J, Kaku R, Dumas P (2015) Lime juice and vinegar injections as a cheap and natural alternative to control COTS outbreaks. PLoS One 10:e0137605. https://doi.org/10.1371/journal.pone.0137605
Muhando CA, Lanshammar F (2008) Ecological effects of the crown-of-thorns starfish removal programme on Chumbe Island Coral Park, Zanzibar, Tanzania. In: Reigl BM, Dodge RE (eds) Proceedings of the 11th international Coral Reef Symposium. Ft Lauderdale, Florida, 7–11 July 2008
Nakamura M, Okaji K, Higa Y, Yamakawa E, Mitarai S (2014) Spatial and temporal population dynamics of the crown-of-thorns starfish, Acanthaster planci, over a 24-year period along the central west coast of Okinawa Island, Japan. Mar Biol 161:2521–2530
Nakamura M, Higa Y, Kumagai N, Okaji K (2016) Using long-term removal data to manage a crown-of-thorns starfish population. Diversity 8:24
Narváez K, Zapata FA (2010) First record and impact of the crown-of-thorns starfish, Acanthaster planci (Spinulosida: Acanthasteridae) on corals of Malpelo Island, Colombian Pacific. Rev Biol Trop 58(Suppl 1):139–143
Neil RC, Gomez Cabrera M, Uthicke S (2022) Juvenile age and available coral species modulate transition probability from herbivory to corallivory in Acanthaster cf. solaris (Crown-of-Thorns Seastar). Coral Reefs 41:843–848
Nugues MM, Bak RPM (2009) Brown-band syndrome on feeding scars of the crown-of-thorn starfish Acanthaster planci. Coral Reefs 28:507–510. https://doi.org/10.1007/s00338-009-0468-x
Obura D (2005) Coral status reports: East Africa Summary. In: Obura D, Linden O (eds) Coral Reef Degradation in the Indian Ocean. CORDIO, Kalmar, pp 25–31
Obura D, Church J, Daniels C, Kalombo H, Shleyer M, Suleiman M (2004) Status of Coral Reefs in East Africa 2004: Kenya, Tanzania. In: Wilkinson C (ed) Mozambique and South Africa in 2004. Status of Coral Reefs of the World. Cambridge University Press, Cambridge, pp 171–188
Obura D, Gudka M, Rabi FA, Gian SB, Bijoux J, Freed S, Maharavo J, Mwaura J, Porter S, Sola E (2017) Coral reef status report for the Western Indian Ocean (2017) Nairobi convention. Global Coral Reef Monitoring Network (GCRMN)/International Coral Reef Initiative
Ormond R, Campbell A (1974) Formation and breakdown of Acanthaster planci aggregations in the Red Sea. In: Proceedings of the second internations symposium on coral reefs. Conducted by the Great Barrier Reef Committee on Board the M.V. Marco Polo Cruising in the Waters of the Great Barrier Reef Province, Australia, 22 June–2 July 1973
Pisapia C, Burn D, Pratchett MS (2019) Changes in the population and community structure of corals during recent disturbances (February 2016–October 2017) on Maldivian coral reefs. Sci Rep. https://doi.org/10.1038/s41598-019-44809-9
Pisapiai C, Hochberg EJ, Carpenter R (2019) Multi-decadal change in Reef-Scale Production and calcification associated with recent disturbances on a Lizard island Reef Flat. Front Mar Sci. https://doi.org/10.3389/fmars.2019.00575
Plaganyi EE, Babcock RC, Rogers J, Bonin M, Morello EB (2020) Ecological analyses to inform management targets for the culling of crown-of-thorns starfish to prevent coral decline. Coral Reefs 39:1483–1499. https://doi.org/10.1007/s00338-020-01981-z
Plass-Johnson JG, Schwieder H, Heiden J, Weiand L, Wild C, Jompa J, Ferse SCA, Teichberg M (2015) A recent outbreak of crown-of-thorns starfish (Acanthaster planci) in the Spermonde Archipelago, Indonesia. Reg Environ Change 15:1157–1162. https://doi.org/10.1007/s10113-015-0821-2
Polunin N (1974) Devastation of a fringing coral reef by Acanthaster. Nature 249:589–590
Pratchett MS (2005) Dynamics of an outbreak population of Acanthaster planci at Lizard Island, northern Great Barrier Reef (1995–1999). Coral Reefs 24:453–462
Pratchett MS (2007) Feeding preferences of Acanthaster planci (Echinodermata: Asteroidea) under controlled conditions of food availability. Pac Sci 61:113–120
Pratchett MS, Cumming GS (2019) Managing cross-scale dynamics in marine conservation: pest irruptions and lessons from culling of crown-of-thorns starfish (Acanthaster spp.). Biol Conserv 238:108211
Pratchett M, Schenk T, Baine M, Syms C, Baird A (2009) Selective coral mortality associated with outbreaks of Acanthaster planci L. in Bootless Bay, Papua New Guinea. Mar Environ Res 67:230–236
Pratchett MS, Trapon M, Berumen ML, Chong-Seng K (2011) Recent disturbances augment community shifts in coral assemblages in Moorea, French Polynesia. Coral Reefs 30:183–193. https://doi.org/10.1007/s00338-010-0678-2
Pratchett M, Caballes CF, Rivera-Posada J, Sweatman H (2014) Limits to understanding and managing outbreaks of crown-of-thorns starfish (Acanthaster spp.). Oceanogr Mar Biol Ann Rev 52:133–200
Pratchett M, Caballes C, Wilmes J, Matthews S, Mellin C, Sweatman H, Nadler L, Brodie J, Thompson C, Hoey J, Bos A, Byrne M, Messmer V, Fortunato S, Chen C, Buck A, Babcock R, Uthicke S (2017a) Thirty years of research on crown-of-thorns starfish (1986–2016): scientific advances and emerging opportunities. Diversity 9:41
Pratchett MS, Cowan Z-L, Nadler LE, Caballes CF, Hoey AS, Messmer V, Fletcher CS, Westcott DA, Ling SD (2017b) Body size and substrate type modulate movement by the western Pacific crown-of-thorns starfish, Acanthaster solaris. PLoS One 12:e0180805. https://doi.org/10.1371/journal.pone.0180805
Pratchett MS, Dworjanyn S, Mos B, Caballes CF, Thompson CA, Blowes S (2017c) Larval survivorship and settlement of crown-of-thorns starfish (Acanthaster cf. solaris) at varying algal cell densities. Diversity 9:2
Pratchett MS, Caballes CF, Cvitanovic C, Raymundo ML, Babcock RC, Bonin MC, Bozec Y-M, Burn D, Byrne M, Castro-Sanguino C, Chen CCM, Condie SA, Cowan Z-L, Deaker DJ, Desbiens A, Devantier LM, Doherty PJ, Doll PC, Doyle JR, Dworjanyn SA, Fabricius KE, Haywood MDE, Hock K, Hoggett AK, Høj L, Keesing J, Kenchington R, Lang B, Ling S, Matthews S, McCallum HI, Mellin C, Mos B, Motti CA, Mumby P, Stump R, Uthicke S, Wolfe K, Vail L, Wilson S (2021a) Knowledge gaps in the biology, ecology, and management of the Pacific crown-of-thorns sea star, Acanthaster sp., on Australia’s Great Barrier Reef. Biol Bull 241:330–346
Pratchett MS, Nadler LE, Burn D, Lang BJ, Messmer V, Caballes CF (2021b) Reproductive investment and fecundity of Pacific crown-of-thorns starfish (Acanthaster cf. solaris) on the Great Barrier Reef. Mar Biol 168:1–8
Quinn NJ, Kojis BL (1987) Distribution and abundance of Acanthaster planci in Papua New Guinea. Bull Mar Sci 41:576–578
Quinn NJ, Kojis BL (2003) The dynamics of coral reef community structure and recruitment patterns around Rota, Saipan, and Tinian, western Pacific. Bull Mar Sci 72:979–996
Ravindran J, Raghukumar C, Raghukumar S (1999) Disease and stress-induced mortality of corals in Indian reefs and observations on bleaching of corals in the Andamans. Curr Sci 76:233–237
Reimer JD, Kise H, Wee HB, Lee CL, Soong K (2019) Crown-of-thorns starfish outbreak at oceanic Dongsha Atoll in the northern South China Sea. Mar Biodivers 49:2495–2497. https://doi.org/10.1007/s12526-019-01021-2
Reverter M, Jackson M, Daraghmeh N, von Mach C, Milton N (2020) 11-yr of coral community dynamics in reefs around Dahab (Gulf of Aqaba, Red Sea): the collapse of urchins and rise of macroalgae and cyanobacterial mats. Coral Reefs 39:1605–1618
Reyes-Bonilla H, Calderon-Aguilera LE (1999) Population density, distribution and consumption rates of three corallivores at Cabo Pulmo reef, Gulf of California, Mexico. Mar Ecol Pubbl Stn Zool Napoli 20:347–357. https://doi.org/10.1046/j.1439-0485.1999.2034080.x
Riegl B, Berumen M, Bruckner A (2013) Coral population trajectories, increased disturbance and management intervention: a sensitivity analysis. Ecol Evol 3:1050–1064. https://doi.org/10.1002/ece3.519
Rivera-Posada J, Owens L, Caballes CF, Pratchett MS (2012) The role of protein extracts in the induction of disease in Acanthaster planci. J Exp Mar Biol Ecol 429:1–6
Rivera-Posada J, Caballes CF, Pratchett MS (2013) Lethal doses of oxbile, peptones and thiosulfate-citrate-bile-sucrose agar (TCBS) for Acanthaster planci, exploring alternative population control options. Mar Pollut Bull 75:133–139. https://doi.org/10.1016/j.marpolbul.2013.07.053
Roads C, Ormond RF (1971) New studies on the Crown-of-Thorns Starfish (Acanthaster planci), from investigations in the Red Sea. Cambridge Coral Starfish Research Group, Cambridge
Roche R, Pratchett M, Carr P, Turner J, Wagner D, Head C, Sheppard C (2015) Localized outbreaks of Acanthaster planci at an isolated and unpopulated reef atoll in the Chagos Archipelago. Mar Biol 162:1695–1704
Rodríguez-Vilalobos J, Ayala-Bocos A (2018) Coral colonies in the eastern tropical Pacific: predation by Acanthaster cf. solaris. Pac Conserv Biol 24:419–420
Rodríguez-Villalobos JC, Work TM, Calderon-Aguilera LE, Reyes-Bonilla H, Hernández L (2015) Explained and unexplained tissue loss in corals from the Tropical Eastern Pacific. Dis Aquat Org 116:121–131
Rodríguez-Villalobos JC, Hernandez-Carreon C, Reyes-Bonilla H, Rojas-Montiel B, Weaver AH (2020) Temporal and spatial abundance of Acanthaster cf. solaris (Echinodermata: Asteroidea) in a National Park in the Gulf of California, Mexico: 2005–2016. Pac Sci 74:269–282. https://doi.org/10.2984/74.3.5
Rodriguez-Villalobos JC, Ayala-Bocos A (2021) Massive predation by Acanthaster planci in El Corralito, gulf of California: a short-term threat. Rev Biol Trop 69:272–286
Rogers JGD, Pláganyi ÉE, Babcock RC (2017) Aggregation, Allee effects and critical thresholds for the management of the crown-of-thorns starfish Acanthaster planci. Mar Ecol Prog Ser 578:99–114
Saponari L, Montano S, Seveso D, Galli P (2015) The occurrence of an Acanthaster planci outbreak in Ari Atoll, Maldives. Mar Biodivers 45:599–600
Saponari L, Montalbetti E, Galli P, Strona G, Seveso D, Dehnert I, Montano S (2018) Monitoring and assessing a 2-year outbreak of the corallivorous seastar Acanthaster planci in Ari Atoll, Republic of Maldives. Environ Monit Assess 190:12. https://doi.org/10.1007/s10661-018-6661-z
Schleyer M (1998) Crown of thorns starfish in the western Indian Ocean. Reef Encount 23:25–27
Schleyer M, Celliers L (2005) Coral reefs Bazaruto. West Indian Ocean J Mar Sci 4:227–236
Scott CM, Mehrotra R, Hein MY, Moerland MS, Hoeksema BW (2017) Population dynamics of corallivores (Drupella and Acanthaster) on coral reefs of Koh Tao, a diving destination in the Gulf of Thailand. Raffles Bull Zool 65:68–79
Seymour RM (1990) Very long period cycles in a near-optimal model of the population-dynamics of Acanthaster planci. IMA J Math Appl Med Biol 7:157–174
Smith D, McMellor S (2006) Conservation value index assessment of Ras Mohammed National Park, July–August 2006. Coral Reef Research Unit, University of Essex. https://cdn.yello.link/opwall/files/2017/11/Opwall-Egypt-Ras-Mohammed-Monitoring-Report-2006.pdf. Accessed 09 Dec 2023
Solís-Marín F, Alvarado J, Abreu-Pérez M, Aguilera O, Alió J, Bacallado-Aránega J, Barraza E, Benavides-Serrato M, Benítez-Villalobos F, Betancourt-Fernández L, Borges M, Brandt M, Brogger M, Borrero-Pérez G, Buitrón-Sánchez B, Campos L, Cantera J, Clemente S, Cohen-Renjifo M, Coppard S, Costa-Lotufo L, del Valle-García R, Díaz Y, Díaz de Vivar M, Díaz-Martínez J, Durán-González A, Epherra L, Escolar M, Francisco V, Freire C, García-Arrarás J, Gil D, Guarderas P, Hadel V, Hearn A, Hernández J, Hernández-Delgado E, Herrera-Moreno A, Herrero-Pérezrul M, Hooker Y, Honey-Escandón M, Lodeiros C, Luzuriaga M, Manso C, Martín A, Martinez M, Martínez S, Moro-Abad L, Mutschke E, Navarro J, Neira R, Noriega N, Palleiro-Nayar J, Pérez A, Pérez-Ruzafa A, Prieto-Rios E, Reyes J, Rodríguez R, Rubilar T, Sancho-Mejía T, Sangil C, Silva J, Sonnenholzner J, Ventura C, Tablado A, Tavares Y, Tiago C, Tuya F, Williams S (2012) Appendix. In: Solís-Marín FA, Alvarado JJ (eds) Echinoderm research and diversity in Latin America. Springer, Berlin
Solís-Marín F, Laguarda-Figueras A, Durán-González A, CONABIO (2016) Estrellas, erizos y pepinos de mar (Echinodermata). Apéndice 1. Lista de equinodermos. La Biodiversidad en Colima Estudio de Estado. pp 295–304
Sonnenholzner J, Brandt N, Hearn A, Luzuriaga M, Guarderas P, Navarro J (2013) Chapter 6. Echinoderms of Ecuador. In: Alvarado JJ, Solís-Marín FA (eds) Echinoderm research and diversity in Latin America. Springer, Berlin, pp 183–233
Souter DW, Cameron AM, Endean R (1997) Implications of sublethal predation, autotomy and regeneration: Pigment bands on their spines can not be used to determine the ages of adult specimens of the corallivore Acanthaster planci. Mar Freshw Res 48:321–328
Stevens GM, Froman N (2019) The Maldives Archipelago. In: Sheppard C (ed) World seas: an environmental evaluation. Elsevier, New York, pp 211–236
Stump RJW, Lucas JS (1990) Linear growth in spines from Acanthaster planci (L.) involving growth lines and periodic pigment bands. Coral Reefs 9:149–154. https://doi.org/10.1007/bf00258227
Timmers MA, Andrews KR, Bird CE, deMaintenton MJ, Brainard RE, Toonen RJ (2011) Widespread dispersal of the crown-of-thorns sea star, Acanthaster planci, across the Hawaiian Archipelago and Johnston Atoll. J Mar Biol 2011:11
Tkachenko KS, Britayev TA, Huan NH, Pereladov MV, Latypov YY (2016) Influence of anthropogenic pressure and seasonal upwelling on coral reefs in Nha Trang Bay (Central Vietnam). Mar Ecol Evol Perspect 37:1131–1146. https://doi.org/10.1111/maec.12382
Tkachenko KS, Huan NH, Thanh NH, Britayev TA (2020) Extensive coral reef decline in Nha Trang Bay, Vietnam: Acanthaster planci outbreak: the final event in a sequence of chronic disturbances. Mar Freshw Res 72:186–199
Tokeshi M, Daud J (2011) Assessing feeding electivity in Acanthaster planci: a null model analysis. Coral Reefs 30:227–235
Tusso S, Morcinek K, Vogler C, Schupp PJ, Caballes CF, Vargas S, Worheide G (2016) Genetic structure of the crown-of-thorns seastar in the Pacific Ocean, with focus on Guam. PeerJ 4:22. https://doi.org/10.7717/peerj.1970
UNEP (1989) Coastal and marine environmental problems of the United Republic of Tanzania. UNEP Regional Seas Reports and Studies No 106 pp 33 + annex
Ussi A (2009) Population dynamics and impact of Crown-of-Thorns Starfish, Acanthaster planci (L.), on Coral Reefs of Zanzibar. MSc Theses, University of Dar es Salaam
Uthicke S, Schaffelke B, Byrne M (2009) A boom-bust phylum? Ecological and evolutionary consequences of density variations in echinoderms. Ecol Mon 79:3–24
Uthicke S, Doyle J, Duggan S, Yasuda N, McKinnon AD (2015) Outbreak of coral-eating crown-of-thorns creates continuous cloud of larvae over 320 km of the Great Barrier Reef. Sci Rep 5:16885. https://doi.org/10.1038/srep16885
Uthicke S, Lamare M, Doyle JR (2018a) eDNA detection of corallivorous seastar (Acanthaster cf. solaris) outbreaks on the Great Barrier Reef using digital droplet PCR. Coral Reefs 37:1229–1239. https://doi.org/10.1007/s00338-018-1734-6
Uthicke S, Liddy M, Patel F, Logan M, Johansson C, Lamare M (2018b) Effects of larvae density and food concentration on Crown-of-Thorns seastar (Acanthaster cf. solaris) development in an automated flow-through system. Sci Rep 8:642
Uthicke S, Fisher EE, Patel F, Diaz-Guijarro B, Doyle JR, Messmer V, Pratchett MS (2019) Spawning time of Acanthaster cf. solaris on the Great Barrier Reef inferred using qPCR quantification of embryos and larvae: do they know it’s Christmas? Mar Biol 166:133. https://doi.org/10.1007/s00227-019-3582-5
Uthicke S, Robson B, Doyle JR, Logan M, Pratchett MS, Lamare M (2022) Developing an effective marine eDNA monitoring: eDNA detection at pre-outbreak densities of corallivorous seastar (Acanthaster cf. solaris). Sci Total Environ 851:158143
van Woesik R, Cacciapaglia CW (2019) Carbonate production of Micronesian reefs suppressed by thermal anomalies and Acanthaster as sea-level rises. PLoS One 14:17. https://doi.org/10.1371/journal.pone.0224887
Verrill AE (1869) On new and imperfectly known echinoderms and corals. Proc Boston Soc Nat Hist 12:381–396
Vogler C, Benzie J, Lessios H, Barber P, Wörheide G (2008) A threat to coral reefs multiplied? Four species of crown-of-thorns starfish. Biol Let 4:696–699
Vogler C, Benzie J, Barber PH, Erdmann MV, Sheppard C, Tenggardjaja K, Gérard K, Wörheide G (2012) Phylogeography of the Crown-of-Thorns Starfish in the Indian Ocean. PLoS One 7:e43499
Vogler C, Benzie JAH, Tenggardjaja K, Ambariyanto BPH, Wörheide G (2013) Phylogeography of the crown-of-thorns starfish: genetic structure within the Pacific species. Coral Reefs 32:515–525. https://doi.org/10.1007/s00338-012-1003-z
Wada N, Yuasa H, Kajitani R, Gotoh Y, Ogura Y, Yoshimura D, Toyoda A, Tang SL, Higashimura Y, Sweatman H, Forsman Z, Bronstein O, Eyal G, Thongtham N, Itoh T, Hayashi T, Yasuda N (2020) A ubiquitous subcuticular bacterial symbiont of a coral predator, the crown-of-updates thorns starfish, in the Indo-Pacific. Microbiome 8:14. https://doi.org/10.1186/s40168-020-00880-3
Wagner G (2008) The Dar es Salaam seascape: a case study of an environmental management ‘hotspot.’ West Indian Ocean J Mar Sci. https://doi.org/10.4314/wiojms.v6i1.48229
Wakeford M, Done TJ, Johnson CR (2008) Decadal trends in a coral community and evidence of changed disturbance regime. Coral Reefs 27:1–13. https://doi.org/10.1007/s00338-007-0284-0
Walbran PD, Henderson RA, Jull AJT, Head MJ (1989) Evidence from sediments of long-term Acanthaster planci predation on corals of the Great Barrier Reef. Science 245:847–850
Wilkinson CC (2004) Status of coral reefs of the world: 2004. Volume 1. Australian Institute of Marine Science (AIMS)
Williams ST, Benzie JAH (1998) Evidence of a biogeographic break between populations of a high dispersal starfish: congruent regions within the Indo-west Pacific defined by color morphs, mt DNA, and allozyme data. Evolution 52:87–99
Wilmes JC, Hoey AS, Messmer V, Pratchett MS (2019) Incidence and severity of injuries among juvenile crown-of-thorns starfish on Australia’s Great Barrier Reef. Coral Reefs 38:1187–1195
Wilmes JC, Hoey AS, Pratchett MS (2020) Contrasting size and fate of juvenile crown-of-thorns starfish linked to ontogenetic diet shifts. Proc R Soc B 287:20201052
Wolfe K, Graba-Landry A, Dworjanyn SA, Byrne M (2015) Larval starvation to satiation: Influence of nutrient regime on the success of Acanthaster planci. PLoS One 10:e0122010
Wolff NH, Mumby PJ, Devlin M, Anthony K (2018) Vulnerability of the Great Barrier Reef to climate change and local pressures. Glob Change Biol 24:1978–1991
Wooldridge SA, Brodie JE (2015) Environmental triggers for primary outbreaks of crown-of-thorns starfish on the Great Barrier Reef, Australia. Mar Pollut Bull 101:805–815. https://doi.org/10.1016/j.marpolbul.2015.08.049
Wörheide G, Kaltenbacher E, Cowan Z-L, Haszprunar G (2022) A new species of crown-of-thorns sea star, Acanthaster benziei sp. nov. (Valvatida: Acanthasteridae), from the Red Sea. Zootaxa 5209:379–393
Yasuda N (2018) Distribution expansion and historical population outbreak patterns of crown-of-thorns starfish, Acanthaster planci sensu lato, in Japan from 1912 to 2015. In: Iguchi A, Hongo C (eds) Coral Reef studies of Japan. Springer, Berlin, pp 125–148
Yasuda N, Nagai S, Hamaguchi M, Okaji K, Gerard K, Nadaoka K (2009) Gene flow of Acanthaster planci (L.) in relation to ocean currents revealed by microsatellite analysis. Mol Ecol 18:1574–1590
Yasuda N, Ogasawara K, Kajiwara K, Ueno M, Oki K, Taniguchi H, Kakuma S, Okaji K, Nadaoka K (2010) Latitudinal differentiation in the reproduction patterns of the crown-of-thorns starfish Acanthaster planci through the Ryukyu Island Archipelago. Plankon Benthos Res 5:156–164
Yasuda N, Inoue J, Hall MR, Nair MR, Adjeroud M, Fortes MD, Nishida M, Tuivavalagi N, Ravago-Gotanco R, Forsman ZH (2022) Two hidden mtDNA-clades of crown-of-thorns starfish in the Pacific Ocean. Front Mar Sci. https://doi.org/10.3389/fmars.2022.831240
Yuasa H, Higashimura Y, Nomura K, Yasuda N (2017) Diet of Acanthaster brevispinus, sibling species of the coral-eating crown-of-thorns startfish, Acanthaster planci sensu lato. Bull Mar Sci 93:1009–1010. https://doi.org/10.5343/bms.2017.1032
Yuasa H, Kajitani R, Nakamura Y, Takahashi K, Okuno M, Kobayashi F, Shinoda T, Toyoda A, Suzuki Y, Thongtham N, Forsman Z, Bronstein O, Seveso D, Montalbetti E, Taquet C, Eyal G, Yasuda N, Itoh T (2021) Elucidation of the speciation history of three sister species of crown-of-thorns starfish (Acanthaster spp.) based on genomic analysis. DNA Res 28:dsab012. https://doi.org/10.1093/dnares/dsab012
Zann L, Brodie J, Berryman C, Naqasima M (1987) Recruitment, ecology, growth and behavior of juvenile Acanthaster planci (L.) (Echinodermata: Asteroidea). Bull Mar Sci 41:561–575
Zapata F, Palacios M, Zambrano V, Rodríguez-Moreno M (2017) Filling the gaps: first record of the crown-of-thorns starfish, Acanthaster planci (Linnaeus, 1758)(Spinulosida: Acanthasteridae), at Gorgona Island, Colombia, Tropical Eastern Pacific. Check List 13:1–4
Ziesenhenne FC (1937) Echinoderms from the West Coast of Lower California, the Gulf of California and Clarion Island. Zoologica 12:210–239
Acknowledgements
The manuscript greatly benefitted from the comments of two anonymous reviewers.
Funding
No specific funding was received, and salaries for our time were covered by respective institutes.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The lead author (SU) declares that he is acting as an Associate Editor for Marine Biology.
Ethics approval
Review only, no ethical approval required.
Additional information
Responsible Regional Editor: Ciemon Frank Caballes.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Uthicke, S., Pratchett, M.S., Bronstein, O. et al. The crown-of-thorns seastar species complex: knowledge on the biology and ecology of five corallivorous Acanthaster species. Mar Biol 171, 32 (2024). https://doi.org/10.1007/s00227-023-04355-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-023-04355-5