Abstract
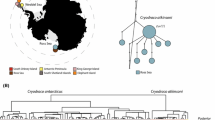
Notothenioids are among the most intensively studied lineages of marine fishes. However, notothenioid research is predominately focused on the approximately 100 species of Antarctic cryonotothenioids. Far less attention is devoted to the non-Antarctic lineages Bovichtidae, Pseudaphritis urvillii, and Eleginops maclovinus, all of which originated prior to the diversification of cryonotothenioid species. Here we utilize DNA sequence data from mitochondrial and nuclear genes, as well as meristic trait morphology to investigate the evolutionary history of Bovichtidae. Bovichtus is the only polytypic lineage of early diverging non-Antarctic notothenioids providing a unique opportunity to contextualize the diversification dynamics of cryonotothenioids with their non-Antarctic relatives. We find strong evidence that species of Bovichtus represent a recent evolutionary radiation with divergence times similar to those estimated among the most closely related species of cryonotothenioids. The divergence in traditional meristic trait morphology among species of Bovichtus is consistent with their phylogenetic relationships. The phylogeny of Bovichtus implies the wide geographic distribution of species in the clade is likely the result of West Wind drift-driven dispersal. The phylogeny and divergence time estimates results reject a hypothesis that species persistence in Bovichtus reflects long periods of evolutionary stasis. Instead, we hypothesize that patterns of extinction and diversification in Bovichtus closely mirror those observed in their Antarctic relatives.





Similar content being viewed by others
References
Andrew TG, Hecht T, Heemstra PC, Lutjeharms JRE (1995) Fishes of the Tristan da Cunha group and Gough Island, South Atlantic Ocean. Ichthyol Bull JLB Smith Inst Ichthol 63:1–43
Balushkin AV (2000) Morphology, classification, and evolution of notothenioid fishes of the Southern Ocean (Notothenioidei, Perciformes). J Ichthyol 40:S74–S109
Balushkin AV (2016) Systematics of the Antarctic thornfishes of the genus Bovichtus (Bovichtidae) of the seamounts of the New Zealand Basin. J Ichthyol 56:631–638. https://doi.org/10.1134/S0032945216050015
Bargelloni L, Marcato S, Zane L, Patarnello T (2000) Mitochondrial phylogeny of notothenioids: a molecular approach to Antarctic fish evolution and biogeography. Syst Biol 49:114–129
Barrera-Oro E (2002) The role of fish in the Antarctic marine food web: differences between inshore and offshore waters in the southern Scotia Arc and west Antarctic Peninsula. Antarctic Sci 14:293–309
Bauret L, Gaudeul M, Sundue MA, Parris BS, Ranker TA, Rakotondrainibe F, Hennequin S, Ranaivo J, Selosse MA, Rouhan G (2017) Madagascar sheds new light on the molecular systematics and biogeography of grammitid ferns: New unexpected lineages and numerous long-distance dispersal events. Mol Phylogenet Evol 111:1–17
Berendzen PB, Olson WM, Barron SM (2009) The utility of molecular hypotheses for uncovering morphological diversity in the Notropis rubellus species complex (Cypriniformes: Cyprinidae). Copeia 2009:661–673
Bouckaert R, Heled J, Kuhnert D, Vaughan T, Wu CH, Xie D, Suchard MA, Rambaut A, Drummond AJ (2014) BEAST 2: a software platform for Bayesian evolutionary analysis. PloS Comput Biol 10
Bravo R, Lloris D, Pequeño G, Rucabado J (1999) Revisión de las distintas especies del género Bovichtus (Perciformes, Bovichtidae) citadas para el cono sur americano y península Antártica. Rev Biol Mar Oceanogr 34:123–137
Carlton JT, Chapman JW, Geller JB, Miller JA, Carlton DA, McCuller MI, Treneman NC, Steves BP, Ruiz GM (2017) Tsunami-driven rafting: transoceanic species dispersal and implications for marine biogeography. Science 357:1402–1405
Cheng C-HC, Detrich HW (2007) Molecular ecophysiology of Antarctic notothenioid fishes. Philos Trans R Soc B 362:2215–2232
Clarke A, Johnston IA (1996) Evolution and adaptive radiation of Antarctic fishes. Trends Ecol Evol 11:212–218
Collins MA, Brickle P, Brown J, Belchier M (2010) The Patagonian Toothfish: biology, ecology and fishery. Advances in marine biology, vol 58, pp 227–300
Damerau M, Matschiner M, Salzburger W, Hanel R (2012) Comparative population genetics of seven notothenioid fish species reveals high levels of gene flow along ocean currents in the southern Scotia Arc, Antarctica. Polar Biol 35:1073–1086
DeWitt HH (1971) Costal and deep-water benthic fishes of the Antarctic. In: Bushnell VC (ed) Antarctic map folio series. American Geographical Society, New York, pp 1–10
Dornburg A, Moore J, Beaulieu JM, Eytan RI, Near TJ (2015) The impact of shifts in marine biodiversity hotspots on patterns of range evolution: evidence from the Holocentridae (squirrelfishes and soldierfishes). Evolution 69:146–161
Dornburg A, Eytan RI, Federman S, Pennington JN, Stewart AL, Jones CD, Near TJ (2016a) Molecular data support the existence of two species of the Antarctic fish genus Cryodraco (Channichthyidae). Polar Biol 39:1369–1379. https://doi.org/10.1007/s00300-015-1859-9
Dornburg A, Federman S, Eytan RI, Near TJ (2016b) Cryptic species diversity in sub-Antarctic islands: a case study of Lepidonotothen. Mol Phylogenet Evol 104:32–43. https://doi.org/10.1016/j.ympev.2016.07.013
Dornburg A, Federman S, Lamb AD, Jones CD, Near TJ (2017) Cradles and museums of Antarctic teleost biodiversity. Nat Ecol Evol 1:1379–1384. https://doi.org/10.1038/s41559-017-0239-y
Eastman JT (1985a) The evolution of neutrally buoyant notothenioid fishes: their specializations and potential interactions in the Antarctic marine food web. In: Siegfried WR, Condy PR, Laws RM (eds) Antarctic nutrient cycles and food webs. Springer, Berlin, pp 430–436
Eastman JT (1985b) Pleuragramma antarcticum (Pisces, Nototheniidae) as food items for other fishes in McMurdo Sound, Antarctica. Polar Biol 4:155–160
Eastman JT (1993) Antarctic fish biology: evolution in a unique environment. Academic Press, San Diego
Federman S, Dornburg A, Downie A, Richard AF, Daly DC, Donoghue MJ (2015) The biogeographic origin of a radiation of trees in Madagascar: implications for the assembly of a tropical forest biome. BMC Evol Biol 15:216
Fell HB (1962) West-Wind-drift dispersal of echinoderms in the Southern Hemisphere. Nature 193:759. https://doi.org/10.1038/193759a0
Fraser CI, Nikula R, Waters JM (2011) Oceanic rafting by a coastal community. Proc R Soc B 278:649–655
Gillespie RG, Baldwin BG, Waters JM, Fraser CI, Nikula R, Roderick GK (2012) Long-distance dispersal: a framework for hypothesis testing. Trends Ecol Evol 27:47–56
Hardy GS (1988) A revision of Bovichtus Cuvier, 1831 (Pisces: Bovichthyidae) from Australasia, with description of a new deepwater species from the New Zealand Subantarctic. J Nat Hist 22:1639–1655
Hou ZG, Li SQ (2018) Tethyan changes shaped aquatic diversification. Biol Rev 93:874–896
Hüne M, González-Wevar C, Poulin E, Mansilla A, Fernández DA, Barrera-Oro E (2015) Low level of genetic divergence between Harpagifer fish species (Perciformes: Notothenioidei) suggests a Quaternary colonization of Patagonia from the Antarctic Peninsula. Polar Biol 38:607–617. https://doi.org/10.1007/s00300-014-1623-6
Hureau JC, Tomo AP (1977) Bovichtus elongatus n.sp., poisson Bovichthyidae, famille nouvelle pour l'Antarctique. Cybium 3:67–74
Kingsford MJ, Schiel DR, Battershill CN (1989) Distribution and abundance of fish in a rocky reef environment at the subantarctic Auckland Islands, New Zealand. Polar Biol 9:179–186. https://doi.org/10.1007/BF00297173
Kocher TD, Conroy JA, McKaye KR, Stauffer JR, Lockwood SF (1995) Evolution of NADH dehydrogenase subunit 2 in east African cichlid fish. Mol Phylogenet Evol 4:420–432
Kock K-H (1985) Krill consumption by Antarctic notothenioid fishes. In: Siegfried WR, Condy PR, Laws RW (eds) Antarctic nutrient cycles and food webs. Springer, Berlin, pp 437–444
Kock KH (1992) Antarctic fish and fisheries. Cambridge University Press, Cambridge
Kozal LC, Simmons JW, Mollish JM, MacGuigan DJ, Benavides E, Keck BP, Near TJ (2017) Phylogenetic and morphological diversity of the Etheostoma zonistium species complex with the description of a new species endemic to the Cumberland Plateau of Alabama. Bull Peabody Mus Nat Hist 58:263–286. https://doi.org/10.3374/014.058.0202
La Mesa M, Eastman JT, Vacchi M (2004) The role of notothenioid fish in the food web of the Ross Sea shelf waters: a review. Polar Biol 27:321–338
La Mesa M, Caputo V, Eastman JT (2010) Some reproductive traits of the Tristan klipfish, Bovichtus diacanthus (Carmichael 1819) (Notothenioidei: Bovichtidae) from Tristan da Cunha (South Atlantic). Polar Biol 33:337–346
Last PR, Balushkin AV, Hutchins JB (2002) Halaphritis platycephala (Notothenioidei: Bovichtidae): a new genus and species of temperate icefish from Southeastern Australia. Copeia 2002:433–440
Li CH, Ortí G, Zhang G, Lu GQ (2007) A practical approach to phylogenomics: the phylogeny of ray-finned fish (Actinopterygii) as a case study. BMC Evol Biol 7:44
Lopez JA, Chen WJ, Ortí G (2004) Esociform phylogeny. Copeia 2004:449–464
Luiz OJ, Allen AP, Robertson DR, Floeter SR, Madin JS (2015) Seafarers or castaways: ecological traits associated with rafting dispersal in tropical reef fishes. J Biogeogr 42:2323–2333
Matschiner M, Hanel R, Salzburger W (2009) Gene flow by larval dispersal in the Antarctic notothenioid fish Gobionotothen gibberifrons. Mol Ecol 18:2574–2587
McDowall RM (1978) Generalized tracks and dispersal in biogeography. Syst Zool 27:88–104
Miller RG (1987) Origins and pathways possible for the fishes of the Antarctic Ocean. In: Kullander SO, Fernholm B (eds) Proceedings of the Fifth Congress of European Ichthyologists. Swedish National Museum, Stockholm, pp 373–380
Nagalingum NS, Marshall CR, Quental TB, Rai HS, Little DP, Mathews S (2011) Recent synchronous radiation of a living fossil. Science 334:796–799
Near TJ (2004) Estimating divergence times of notothenioid fishes using a fossil-calibrated molecular clock. Antarctic Sci 16:37–44
Near TJ (2009) Notothenioid fishes (Notothenioidei). In: Hedges SB, Kumar S (eds) The timetree of life. Oxford University Press, New York, pp 339–343
Near TJ, Dornburg A, Kuhn KL, Eastman JT, Pennington JN, Patarnello T, Zane L, Fernandez DA, Jones CD (2012) Ancient climate change, antifreeze, and the evolutionary diversification of Antarctic fishes. Proc Nat Acad Sci USA 109:3434–3439. https://doi.org/10.1073/pnas.1115169109
Near TJ, Dornburg A, Tokita M, Suzuki D, Brandley MC, Friedman M (2014) Boom and bust: ancient and recent diversification in bichirs (Polypteridae: Actinopterygii), a relictual lineage of ray-finned fishes. Evolution 68:1014–1026. https://doi.org/10.1111/evo.12323
Near TJ, Dornburg A, Harrington RC, Oliveira C, Pietsch TW, Thacker CE, Satoh TP, Katayama E, Wainwright PC, Eastman JT, Beaulieu JM (2015) Identification of the notothenioid sister lineage illuminates the biogeographic history of an Antarctic adaptive radiation. BMC Evol Biol 15:109. https://doi.org/10.1186/s12862-015-0362-9
Near TJ, Simmons JW, Mollish J-M, Correa MA, Benavides E, Harrington RC, Keck BP (2017) A new species of logperch endemic to Tennessee (Percidae: Etheostomatinae: Percina). Bull Peabody Mus Nat Hist 58:287–309. https://doi.org/10.3374/014.058.0203
Near TJ, MacGuigan DJ, Parker E, Struthers CD, Jones CD, Dornburg A (2018) Phylogenetic analysis of Antarctic notothenioids illuminates the utility of RADseq for resolving Cenozoic adaptive radiations. Mol Phylogenet Evol 129:268–279. https://doi.org/10.1016/j.ympev.2018.09.001
Papetti C, Windisch HS, La Mesa M, Lucassen M, Marshall C, Lamare MD (2016) Non-Antarctic notothenioids: Past phylogenetic history and contemporary phylogeographic implications in the face of environmental changes. Mar Genomics 25:1–9
Pequeño G, Farías D, Thiel M, Hinojosa I (2004) Peces asociados con la deriva de macroalgas en Aysén, Chile. Rev Biol Mar Oceanogr 39:93–99
Pequeño G, Inzunza AJ (1987) Variabilidad intraespecifica y status sistematico del "torito" Bovichthys chilensis Regan 1913 (Osteichthyes, Bovichthyidae) en Valdivia, Chile. Bol Soc Biol Concepción 58:127–139
Posada D, Crandall KA (1998) Modeltest: testing the model of DNA substitution. Bioinformatics 14:817–818
Regan CT (1913) The Antarctic fishes of the Scottish National Antarctic expedition. Trans R Soc Edinburgh 49:229–292
Reynolds RG, Niemiller ML, Hedges SB, Dornburg A, Puente-Rolon AR, Revell LJ (2013) Molecular phylogeny and historical biogeography of West Indian boid snakes (Chilabothrus). Mol Phylogenet Evol 68:461–470
Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Hohna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542
Sauvage H-E (1879) Mémoire sur la faune ichthyologique d l'île St Paul. Arch Zool Exper Gen 8:1–46
Stadler T (2011) Inferring speciation and extinction processes from extant species data. Proc Nat Acad Sci USA 108:16145–16146
Stankovic A, Spalik K, Kamler E, Borsuk P, Weglenski P (2002) Recent origin of sub-Antarctic notothenioids. Polar Biol 25:203–205
Sutton CA, Bruce BD (1988) Bovichtidae: thornfishes. In: Neira FJ, Miskiewicz AG, Trnski T (eds) Lavae of temperate Australian fishes Laboratory guide for larval fish identification. University of Western Australia Press, Nedlands, pp 338–343
Waters JM (2008) Driven by the West Wind Drift? A synthesis of southern temperate marine biogeography, with new directions for dispersalism. J Biogeogr 35:417–427
Acknowledgements
G. Watkins-Colwell provided outstanding assistance with all aspects arising from the use of specimens housed in museum collections. A. Lamb provided assistance with various aspects in the preparation of this manuscript. All images were obtained through creative commons licenses. Alastair Graham (CSIRO) provided valuable tissue specimens. Financial support was provided by the Bingham Oceanographic fund maintained and managed at the Peabody Museum of Natural History, Yale University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors of this paper have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Near, T.J., Ghezelayagh, A., Ojeda, F.P. et al. Recent diversification in an ancient lineage of Notothenioid fishes (Bovichtus: Notothenioidei). Polar Biol 42, 943–952 (2019). https://doi.org/10.1007/s00300-019-02489-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-019-02489-1