Abstract
Biodiversity on coral reefs depends not only on primary reef-builders, but also on associated taxa that create microhabitats for other species. Hydrocorals of the genus Stylaster, commonly known as lace corals, form small branching colonies that enhance three-dimensional complexity on reefs and are known to support a variety of commensal species. Furthermore, the genus is highly speciose, further increasing biodiversity. Despite their important ecological roles, little is known about the evolutionary history and the intraspecific diversity and structure in these broadly distributed hydrocorals. Here, we assessed the phylogenetic relationships among Atlantic species in the genus Stylaster and examined the genetic structure of S. roseus in the Tropical Western Atlantic (Caribbean and Brazil) and of S. blatteus in the Tropical Eastern Atlantic (Africa), using DNA sequences from the 16S ribosomal gene. Time-calibrated phylogenetic analyses showed that S. roseus and S. blatteus diverged at ~ 24.6 Ma. A well-supported Brazilian clade within S. roseus indicates a possible cryptic species that diverged at ~ 11.6 Ma, consistent with the formation of the Amazon River at 9 Ma (Hoorn et al. in Glob Planet Change 153:51–65, 2017). Strong genetic structure was observed even over moderate distances, with ΦST values over all populations being 0.98 for S. roseus and 0.90 for S. blatteus. Nearly, all haplotypes were private (found in a single location) and diverged by many mutational steps from one another. In contrast, genetic diversity was low at the local scale for both species, with most sites showing no variation (a single haplotype). These results are coherent with the reproductive strategy of Stylasteridae, where larvae are brooded and are highly developed at the time of release, often settling near the parental colony. Limited dispersal coupled with possible clonal reproduction have likely contributed to the high levels of genetic differentiation observed here. Lace corals show unusual reproductive and population dynamics compared to other reef inhabiting cnidarians. Future work may reveal additional cryptic diversity in this poorly studied family.
Similar content being viewed by others
Introduction
Tropical marine ecosystems are highly diverse, but the drivers of diversification and speciation are often illusive, particularly in environments without obvious barriers to gene flow (Palumbi 1994; Briggs and Bowen 2012). Intraspecific phylogeography evaluates the spatial genetic structure of species by examining the non-random distribution of the genetic variation among individuals within a population and populations across the species range. By examining the spatial patterns of genetic structure at both local and regional levels, the physical or biological barriers that restrict gene flow between populations can be identified, providing clues to the processes that lead to diversification and speciation in the sea (Avise 1992, 2009).
While much effort has been made toward understanding the processes that shape genetic structure and diversity in reef fish (Rocha and Bowen 2008; Selkoe and Toonen 2011; Gaither et al. 2015; Bowen et al. 2016) and scleractinians (Baums et al. 2006; Vollmer and Palumbi 2007; Nunes et al. 2009; Serrano et al. 2014; Peluso et al. 2018; Riquet et al. 2021), less is known about other benthic invertebrates that also serve important ecological roles on coral reefs. For example, the fire corals of the genus Millepora are the only branching reef-building organisms of the Southwestern Atlantic (Laborel 1970; de Souza et al. 2017), and so they provide a unique habitat type for other reef dwellers. However, only a few studies have evaluated genetic connectivity in hydrozoans (de Souza et al. 2017; Postaire et al. 2017a, b).
Hydrocorals of the family Stylasteridae contribute to the three-dimensional complexity of shallow reefs despite their small sizes (up to 25 cm tall × 30 cm wide) (Cairns 1986). In mesophotic reefs, greater species diversity and colony sizes, such as the fan-like colonies of S. duchassaingi (Sánchez et al. 2019), suggest that hydrocorals can make important contributions to community structure and diversity in a variety of marine ecosystems. Stylasterids serve as host species for a number of small commensals, such as brittle stars, annelids, gastropods, hydroids, and sponges (Pica et al. 2015), thereby favoring higher species diversity on reefs. Furthermore, because stylasterids are the second most diverse group of “hard corals” (Lindner et al. 2008), and have broad depth ranges that go from the intertidal to more than 2000 m, they themselves make important contributions to community diversity in shallow as well as deep habitats. However, some hydrocoral species are particularly sensitive to environmental stressors such as high temperatures, often suffering higher mortality rates than scleractinians on the same reefs (Morri et al. 2017; Duarte et al. 2020). Given their functional importance on reefs coupled with the fragility of some hydrocorals to stressors, it is important to better understand population and evolutionary dynamics in this group of understudied cnidarians.
The alarming decline in coral populations around the world has prompted a rising interest in improving conservation efforts for reef communities (Lundgren 2011). The genetic structure and diversity of populations can help estimate larval dispersal and inform on the location of source populations as well as isolated populations, ultimately improving ecosystem management and marine reserve design (Almany et al. 2009). While genomic resources are increasingly available for non-model organisms, they remain hard to implement in lower income countries, where many coral reefs are found. As such, mitochondrial DNA continues to be informative and widely used in phylogenetic studies of Hydrozoa (Schuchert 2005; López et al. 2015; Arrigoni et al. 2018). Whereas scleractinian corals (Anthozoa) have highly conserved mitochondrial DNA, members of the subphylum Medusozoa (which includes Hydrozoa) have mitochondrial evolution rates more similar to Bilateria (Ruiz-Ramos et al. 2014), thus providing adequate variation for population and species-level genetic analyses.
Stylaster roseus (Pallas, 1766), commonly known as the rose lace coral, is a hydrocoral that can be found throughout the Caribbean and the tropical Southwestern Atlantic. It ranges from the Gulf of Mexico to Bahia, Brazil (Sanchez and Navas 1994; Leão and Fournier 2007), and it is the only species of its genus to occur in shallow waters in the Western Atlantic (Cairns 1986). Stylaster roseus is most commonly, but not exclusively, present in shaded cryptic habitats, normally inside crevices of complex hard coral structures (living or dead) (Sanchez and Navas 1994) (Fig. 1A). Conversely, it can also be found on rocky walls in the intertidal zone, where the colonies are significantly more exposed to light and wave action. Stylaster blatteus (Boschma, 1961), commonly known as the West African blue coral, was harvested for centuries as “Akori” to manufacture ornamental beads (Boschma 1961; Cairns and Zibrowius 2013); Fig. 1B). The species was redescribed by Zibrowius and Cairns (1992) and is only known to occur on the islands of São Tomé and Príncipe in the Gulf of Guinea, where it was originally described. While most stylasterids tend to have separate sexes, S. roseus and S. blatteus are presumably hermaphroditic, as both species contain male and female gonophores (Goedbloed 1962). The majority of stylasterids brood larvae to an advanced developmental stage before release (Cairns 2011). In some species, such as Stylaster californicus (Verrill, 1866), planulae stay near the bottom after release (Fritchman 1974) often settling near the mother colony (Ostarello 1973, 1976). While the Hydrozoa contains hundreds of species, with more than 90 species in the genus Stylaster alone (Schuchert 2022), few studies have examined speciation and genetic diversity in this diverse class or organisms (Miller et al. 2004; Schuchert 2005; Lindner et al. 2008; Postaire et al. 2017a, 2017b). In this study, a time-calibrated phylogeny was used to estimate divergence times within the genus Stylaster in the Atlantic Ocean, to address the following questions: (i) Are S. roseus and S. blatteus genetically distinct species, and if so, when did they diverge? (ii) Is there differentiation between Caribbean and Brazilian populations of S. roseus? (iii) Is gene flow maintained among Caribbean populations of S. roseus? (iv) Does genetic diversity vary among populations of S. roseus and S. blatteus, and if so, what might be possible explanations for this variation?
Materials and methods
Sample collection
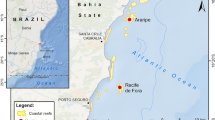
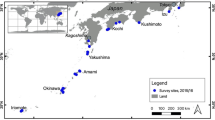
A total of 157 individual colonies of S. roseus and 28 colonies of S. blatteus were collected by snorkeling or scuba diving, in 14 sites in seven countries across the tropical Atlantic (Fig. 1, Table 1). For S. roseus, sampling included one site in Belize, four sites on islands in Colombian territory in the southwestern Caribbean, east of Nicaragua (herein referred to as Colombian Islands), two sites on mainland Colombia, one site in each of Curaçao, Grenada and Trinidad and Tobago and four sites in Brazil (Table 1, Fig. 1). Samples were collected at depths between 0 and 77 m. Below 30 m, for San Andrés and “Parque Nacional Natural Corales de Profundidad,” sample collection was made using Closed Circuit-Rebreather-CCR and Trimix techniques to explore the depth gradient down to 120 m. For S. blatteus, specimens were collected at four sites in the Tropical Eastern Atlantic (Gulf of Guinea, Africa), with three sites being sampled on the Island of São Tomé and one site on the Island of Príncipe, at depths ranging from 10 to 25 m. Small fragments of each colony (1–2 cm) were preserved in tubes with DMSO, ethanol or guanidine thiocyanate (chaos buffer) and stored at − 80 °C for DMSO, or room temperature for ethanol and chaos buffer for later processing. Museum specimen numbers for S. roseus are ANDES-IM5838-5841, ANDES-IM4432-4434, ANDES-IM4600, ANDES-IM5928-5935, UFSCCNI.0357-0371 and USNM1073477. For S. blatteus, specimens are currently stored in the Laboratory of Coastal Benthic Ecology, at the Institut Français de Recherche pour l’Exploitation de la Mer, Plouzané, France. Sample numbers for the island of São Tomé are ST218-221, ST228-229, ST281, ST315-316 and ST324-325 and for Príncipe Island are PSTY1-14 and PSTY16-17.
DNA extraction, amplification and sequencing
Genomic DNA was extracted using the CTAB extraction protocol with modifications according to (Coffroth et al. 1992) for samples from the Caribbean and Brazil, and using the phenol/chloroform/isoamyl alcohol method described in Fukami et al. (2004) for samples from the Tropical Eastern Atlantic. We targeted the large ribosomal subunit gene of the mitochondrial DNA (16S). The 16S gene was amplified using the following primers: SHA: 5′-TCGACTGTTTACCAAAAACATAGC-3′ and SHB: 5′-ACGGAATGAACTCAAATCATGTAAG-3′ (Cunningham and Buss 1993). Polymerase chain reaction (PCR) was performed in a 15-μl reaction containing 1 × PCR buffer, 1.5 mM MgCl2, 1 μl of 10 × bovine serum albumin (BSA), 0.16 mM of each dNTP, 0.52 mM of each primer, 1 U Taq DNA polymerase and 1 μl of DNA (30 ng). PCR conditions were 94 °C for 2 min followed by 35 cycles of 94 °C for 90 s, 50 °C for 90 s and 72 °C for 60 s and a 5 min extension at 72 °C. Resulting PCR products were cleaned with ExoSAP-IT and sequenced using the BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems) and ran on an AB13730xl DNA Analyzer (Applied Biosystems).
Sequences were visually inspected using Geneious software version 4.8.5 (Kearse et al. 2012) or Sequencher 5.4 (Gene Codes Corp). The sequences of the outgroup species and the S. roseus sequence from Trinidad and Tobago (EU645315) were obtained from a previous study (Lindner et al. 2008). The outgroup included 13 species from the genus Stylaster (Gray, 1847), five species from the genus Distichopora (Lamarck, 1816), four species from the genus Conopora (Moseley, 1879), two species of the genus Pliobothrus (Pourtalès, 1868), three species of the genus Crypthelia (Milne Edwards & Haime, 1849) and the species Systemapora ornata (Cairns, 1991). Information on each specimen including species name, isolate number and GenBank accession number are provided in Supplementary Table 1. New sequences have been deposited in GenBank with the accession numbers MT043949–MT044134.
Phylogenetic analyses and temporal calibration
Unique DNA sequences were aligned using the L-INS-i (localpairs) algorithm in MAFFT v7.313, with a maximum of 1000 iterations (Katoh and Standley 2013). The optimal model of nucleotide substitution for the dataset (GTR + γ) was selected using Topali v2 (Milne et al. 2009), based on the Akaike Information Criterion (AIC). Phylogenetic relationships and divergence times were estimated using BEAST v2.3.6 (Bouckaert et al. 2019) with input files generated in BEAUTi v2.3.6 (Drummond et al. 2012) using the Calibrated Yule Model of speciation with a uniform birth rate prior and a strict clock. Divergence times were estimated based on the first fossil occurrence of Stylaster cretaceous at 66.4 Ma (Jell et al. 2011). Previous phylogenetic work has shown that the genus Stylaster is polyphyletic (Lindner et al. 2008), and as such, the taxon set for the calibration node included all species in the genera Stylaster, Calyptopora, Systemapora and Distichopora. The prior for the calibration node had a lognormal distribution with an offset of 66.4. BEAST was run 5 times to estimate the appropriate number of Monte Carlo Markov chains and sampling frequency to reach convergence. Log files were examined in Tracer v1.6 (Rambaut et al. 2018) to assess convergence and proper mixing of the chains, making sure that ESS values were above 200. The final run was conducted with 2 × 109 chains, sampled every 10,000 chains. The final tree was generated with TreeAnnotator v2.3.6, omitting the first 25% of trees as burn-in. Maximum likelihood analyses were also run on the dataset, using IQtree v2.0.3 (Nguyen et al. 2015; Minh et al. 2020). ModelFinder was used to select the most likely model of sequence evolution (Kalyaanamoorthy et al. 2017). Node support was evaluated with 1000 nonparametric bootstrap replicates as well as with the ultrafast bootstrap approximation (UFBoot) (Minh et al. 2013; Hoang et al. 2018) implemented in IQTree. Removal of selected outgroup taxa (S. papuensis and Stylaster sp. D) was conducted to examine the effect on the support values of certain internal nodes. Trees were inspected and edited with FigTree v1.4.4.
Population genetic analyses
Haplotype networks were obtained separately for S. roseus and S. blatteus using the TCS method implemented in PopART (Population Analysis with Reticulate Trees) (Leigh and Bryant 2015). The number of segregating sites (S), haplotypes (Hap), gene diversity (H), pairwise differences (k), nucleotide diversity (π) and statistics for neutral sequence evolution (Tajima’s D and Fu’s Fs) were calculated for each location using Arlequin v3.5.1.2 (Excoffier and Lischer 2010). Hierarchical analysis of molecular variance (AMOVA) was used to test for population differentiation among all populations, as well as within and among regions for both species. For S. roseus, populations were grouped into a Caribbean group and a Brazil group. Given the limited sampling for S. blatteus, hierarchical structure was not considered (all populations were considered as belonging to one Tropical Eastern Atlantic group). AMOVA and pairwise ϕST values were also calculated in Arlequin v3.5.1.2.
Results
Phylogenetic analyses
Phylogenetic relationships were inferred based on a total of 68 unique sequences (including outgroups) that led to a final alignment of 600 bp. The phylogenetic tree had overall high support, with nodes at the genus level having posterior probabilities of > 0.95, except for a polyphyletic clade composed of species from the genera Calyptopora, Stylaster and Systemapora, also recovered in an analysis based on three genes for the group (Lindner et al. 2008). Stylaster roseus and S. blatteus were reciprocally monophyletic with 95% UFBoot in the ML analysis, but only 68% support when nonparametric bootstrapping was used, and a posterior probability of 0.85 in the Bayesian analysis. When outgroup species S. papuensis and Stylaster sp. D were removed from the analysis, reciprocal monophyly of S. roseus and S. blatteus was recovered with a support of 99% UFBoot, 94% with nonparametric bootstrapping and 0.95 posterior probability in the Bayesian analysis. Samples collected in the Gulf of Guinea aside, all individuals collected for this study clustered within the S. roseus clade, except for two samples (codes SAI064 and SAI107), collected at 100 m depth in San Andrés (Colombian Islands) that were genetically most similar to Stylaster duchassaingi, as shown in Fig. 2. These two individuals were excluded from the population-level genetic analyses for S. roseus; however, they constitute the first record of S. duchassaingi in Colombian waters. The divergence time between S. roseus and S. blatteus was estimated at 24.6 Ma (95% HPD of 30.5–19.1 Ma). The S. roseus clade was further subdivided into two reciprocally phylogenetic clades (100% UFBoot, 100% bootstrap, 1.0 posterior probability): one composed of all individuals collected from Brazil and another composed of all individuals from the Caribbean. Divergence between the Brazilian and Caribbean clades was estimated at 11.3 Ma (95% HPD of 8.0–15.0 Ma) (Fig. 2). Additional structure was observed within the Caribbean clade of S. roseus, with the four Colombian Islands sites forming one clade, the two Colombian Mainland sites forming a second clade and the remaining sites forming a third clade. While this topology was observed in both ML and Bayesian analyses, node support differed considerably (posterior probabilities of 1, 1 and 0.95; UFBoot bootstrap values of 95, 91 and 65; bootstrap values of 78, 58 and 26 for the Colombian Islands, Colombian Mainland and other Caribbean sites, respectively).
Time-calibrated phylogeny for lace corals obtained using Bayesian phylogenetic analysis based on mtDNA 16S. Maximum likelihood analyses produced similar topologies within the Stylaster + Calyptopora + Systemapora clade. A second run was conducted excluding S. papuensis and S. sp. D (highlighted in gray) in order to test internal node support. The support values for the S. roseus + S. blatteus clade for these analyses are also shown and highlighted in gray. Values above branches indicate Bayesian posterior probabilities, UFBoot and nonparametric bootstrap values, respectively. Colored codes represent the 14 sampling locations for Stylaster roseus, and four locations for S. blatteus are shown in the legend. Violet circles indicate specimens of Stylaster duchassaingi collected in San Andrés Island (first occurrence of the species in Colombian waters)
Genetic diversity
A 16S gene fragment of 569 bp length was obtained from 157 individuals of S. roseus collected at 14 sites across the Western Atlantic, and a cropped alignment of 512 bp was kept for population genetic analyses based on the 27 individuals of S. blatteus collected at six sites in the Tropical Eastern Atlantic (Fig. 1C). In total, 24 haplotypes were found for S. roseus and seven haplotypes were found for S. blatteus (Fig. 3). Of these, nearly all were private haplotypes, present in a single site, apart from three haplotypes that were shared between adjacent locations in S. roseus and one shared haplotype in S. blatteus. The shared haplotypes for S. roseus were found between the following populations: one shared between Roncador and Serrana (both Colombian Islands, 90 km apart), one shared between Trinidad and Tobago and Grenada (Eastern Caribbean, 150 km apart) and one shared between Rocas Atoll and Fernando de Noronha (Brazil, Oceanic, 150 km apart). One shared haplotype for S. blatteus was found between the population of Diogo Vaz on São Tomé Island and the population sampled in Príncipe Island (São Tomé & Príncipe, West Africa, 100 km apart). The alignment showed 67 polymorphic sites. Among populations of S. roseus, the two of the Colombian Island populations in Providence Island (h = 0.791 ± 0.067) and Serrana (h = 0.786 ± 0.113) had the highest genetic diversity followed by intermediate levels in two other Colombian Island sites Roncador (h = 0.533 ± 0.172) and San Andrés (h = 0.488 ± 0.095). Low levels of diversity were observed in Rocas Atoll, Brazil (0.303 ± 0.147). There was no genetic diversity (i.e., all haplotypes were identical) in Curaçao (n = 17), in both Colombian Mainland sites “PNN Corales de Profundidad” (n = 5) and Cartagena (n = 7) and in three Brazilian sites Fernando de Noronha Island (n = 11), Pernambuco (n = 12) and Bahia (n = 17), (Table 2). In S. blatteus, genetic diversity was the highest in Príncipe (h = 0.700 ± 0.0506), followed by Diogo Vaz (h = 0.667 ± 0.314) and Lagoa Azul (h = 0.500 ± 0.260), two sites on São Tomé Island. In the southernmost population of São Tomé, Santana, all individuals were identical, but differed by > 12 mutations from haplotypes collected in the nearest neighboring population of Lagoa Azul, only 20 km away. Two populations of S. roseus in the Caribbean showed significant departures from neutrality (p < 0.05), San Andrés (− 1.523) and Providence (− 1.852), while no significant departures from neutrality were detected for S. blatteus.
Haplotype networks for A. S. roseus and B. S. blatteus. Circles represent haplotype sequences, and each hatch mark represents one mutational difference. The size of the circle indicates the number of samples that share a given haplotype, and colors correspond to sampling sites and indicated on the legend
Population differentiation
The populations of S. roseus were remarkably differentiated from each other (Table 3), with a global fixation index across all populations of nearly one (ΦST = 0.974), and with high values for the fixation index among regions (ΦSC = 0.901) and within regions (ΦCT = 0.741). Pairwise ϕST values were high and significant (P < 0.05) between all locations, except for two pairwise comparisons: Serrana and Roncador (two Colombian Island sites) and Fernando de Noronha and Rocas Atoll (the two Brazil oceanic islands) (Table 3). Similarly, the global fixation index was high and significant (ΦST = 0.903) for S. blatteus. Pairwise population differentiation was also high and significant for nearly all pairwise comparisons (ϕST ranging from 0.79 to 0.98), even between populations separated by only 10–30 km distance. The only pairwise comparison where differentiation was moderate was between the populations of Diogo Vaz (on São Tomé Island) and Príncipe Island, which shared a haplotype, but were separated by 100 km distance (ϕST = 0.43).
Discussion
Lace corals are important constituents of coral reefs, adding complexity to communities and providing support for commensal species. The two species of lace corals of the genus Stylaster studied here showed remarkable levels of genetic differentiation across their range, and unusual patterns of genetic diversity and connectivity compared to many other marine invertebrates studied so far. Our results confirm species-level distinction between western Atlantic S. roseus and Eastern Atlantic S. blatteus dating as far back as 25 Ma. Although phylogenetic analyses showed somewhat low levels of support for the node demonstrating reciprocal monophyly between S. blatteus and S. roseus when the full dataset was considered (p.p. 85, bootstrap = 68%, UFBoot = 90%), these values improved considerably when the intermediate outgroup taxa Stylaster papuensis and Stylaster sp. D were removed from the dataset (p.p.; bootstrap = 94%; UFboot = 99%). These results indicate that the low support for this node is not necessarily related to whether S. blatteus is monophyletic, but rather its relationship as sister taxa to S. roseus, S. papuensis or another taxon not sampled here. The addition of Western Indian Ocean stylasterids may help refine phylogenetic relationships within the genus and improve support for these nodes in future work. Analyzing additional molecular markers may also help improve node support in the phylogenetic trees. Divergence between S. blatteus and S. roseus is in agreement with a mid-Atlantic Barrier that has led to speciation in many marine taxa, particularly in reef fish, but also sea urchins (Lessios et al. 2003; Floeter et al. 2008; Briggs and Bowen 2012; Araujo et al. 2022).
The phylogenetic analyses further indicated a possible cryptic species within S. roseus. Well-supported reciprocal monophyly and estimated divergence time of 11.3 Ma (Fig. 2) between Caribbean and Brazilian S. roseus indicate a speciation event that would have taken place in the Miocene, at approximately the same time as the establishment of the Amazon River outflow, estimated at 9 Ma (Hoorn et al. 2017). Our results for S. roseus are in agreement with other studies that have shown the Amazon plume as an important barrier to dispersal for a variety of marine species, such as corals (Nunes et al. 2009, 2011), hydrocorals (de Souza et al. 2017), crustaceans (Terossi and Mantelatto 2012), echinoderms (Lessios et al. 2003) and reef fish [e.g., (Mendonça et al. 2013; Volk et al. 2020)]. While there are examples of marine invertebrates and fish capable of maintaining genetic exchange across the Amazon River [e.g., (Lazoski et al. 2001; Nóbrega et al. 2004; Zigler and Lessios 2004; Nunes et al. 2017)], these appear to be exceptions to a more general pattern of distinct populations or sister species across the Amazon. The level of divergence between Caribbean and Brazilian clades of S. roseus is equivalent to the divergence observed between S. laevigatus and S. duchassaingi, two Caribbean stylasterids. Such deep divergence between Caribbean and Brazilian S. roseus incites further work on morphological traits that may allow the description of two separate species.
With regard to intraspecific genetic structure, the phylogenetic analysis showed structure within the Caribbean S. roseus clade, with the four Colombian Islands clustering together, the two Colombian Mainland sites forming a clade, and the remaining Caribbean sites forming a third group. While this topology was consistent between Bayesian and maximum likelihood analyses, support for these nodes differed between the methods (Fig. 2). The haplotype network also showed a similar structure among these three clusters (Fig. 3). These groupings are most likely reflective of intraspecific genetic structure within S. roseus. The four Colombian Island sites (Serrana, Roncador, Providence and San Andrés) are all found in the southwestern Caribbean, adjacent to Nicaragua, Panamá and Colombia (Fig. 1C). The anti-clockwise Panamá–Colombia Gyre likely maintains some level of connectivity among these islands (pairwise ϕST values were more moderate among these populations, ranging from 0.64 to 0.76, with only one non-significant ϕST of 0.05 between Serrana and Roncador) and may partially explain differentiation between the Colombian Islands and the other Caribbean sites as these currents may lead to retention within the gyre (Table 4). This may also explain the higher levels of genetic diversity observed in the Colombian Islands (Table 2), compared to other S. roseus populations in the Caribbean, as the Panamá–Colombian Gyre may favor dispersal and genetic exchange within the region. Genetic similarity among Trinidad and Tobago, Grenada and Curaçao is likely due to their geographical proximity and larval transport which may be mediated by the Caribbean Current. The Caribbean Current may also explain the relative similarity of the Belize haplotype to these Eastern Caribbean sites. The Caribbean Current is thought to favor genetic connectivity across broad distances within the Eastern Caribbean for scleractinian corals, with differentiation often only being observed between Eastern and Western Caribbean populations (Baums et al. 2005; Vollmer and Palumbi 2007; Foster et al. 2012). Isolation of the Colombian Mainland sites may be related to a plume of low salinity from the Magdalena River outflow, which has shown to affect connectivity in other marine organisms (Restrepo et al. 2006; Foster et al. 2012).
Within the Brazilian Biogeographic Province, significant population structure was observed among all sampled locations (ϕST > 0.9 for all pairwise comparisons), except between the oceanic islands of Fernando de Noronha and Rocas Atoll (pairwise ϕST = 0.08), which are approximately 150 km apart. Populations of S. roseus on these islands shared one common haplotype, with one private haplotype being found in Rocas Atoll, but were differentiated with respect to the mainland Brazilian sites. A similar pattern has been observed for scleractinian corals, with oceanic island populations of the endemic reef-builder Mussismilia hispida being genetically differentiated from mainland populations (Peluso et al. 2018), as were oceanic island populations of the coral Favia gravida (Teschima et al. 2021). Strong genetic structure was also observed between mainland populations of S. roseus from Pernambuco and Bahia, which had no shared haplotypes (pairwise ϕST = 1.0). Pernambuco and Bahia are separated by the São Francisco River outflow (Fig. 1), and this river is thought to act as a barrier for two species of fire corals, Millepora braziliensis and Millepora nitida, with the former being restricted to the north and the latter to the south of the river outflow, respectively (de Souza et al. 2017). Overall, our results show high levels of population differentiation, with the global ΦST over all populations being 0.97 for S. roseus and 0.90 for S. blatteus. In the Atlantic Ocean, genetic connectivity between Caribbean, Brazilian and Tropical Eastern Atlantic populations has only been evaluated for one other hydrozoan, the fire coral Millepora alcicornis (Linnaeus, 1758) (de Souza et al. 2017), which showed a global ΦST of 0.69 over all populations in these three biogeographic regions. High levels of genetic differentiation have also been observed for the hydrozoans Lytocarpia brevirostris (Busk, 1852) (Postaire et al. 2017a) and Macrorhynchia phoenicea (Busk, 1852) (Postaire et al. 2017b) among several populations of the Western Indian Ocean and New Caledonia in the Tropical Southwestern Pacific. Mean ϕST values based on microsatellite markers among the most distantly located populations were < 0.6 for both species. Hydrozoans therefore tend to show greater genetic differentiation than scleractinian corals over similar spatial scales (Nunes et al. 2011; Teschima et al. 2021). Limited gene flow is most likely related to the larval traits of Stylaster species, which settle shortly after being released (Cairns 2011). Pelagic larval period has not yet been experimentally determined for S. roseus or S. blatteus, and most reproductive traits are known for only few species of stylasterids. Future work describing reproduction in lace corals and other hydrozoans may help to better understand the mechanisms that lead to such low levels of connectivity among populations of these organisms.
Intraspecific genetic variation was unusual in both S. roseus and S. blatteus. Strikingly, nearly all S. roseus haplotypes were private, in other words, unique to a single site, with only three exceptions where haplotypes were shared among populations between Roncador and Serrana Islands in Colombia, Trinidad and Tobago and Grenada and Rocas Atoll and Fernando de Noronha in Brazil (all pairs of sites < 150 km apart). Likewise, all haplotypes for S. blatteus were private, except for one shared haplotype between Diogo Vaz (São Tomé Island) and Príncipe Island. While genetic diversity was moderate to high in the Colombian Islands (0.401 < h < 0.79) and moderate on Rocas Atoll, Brazil (h = 0.33), genetic diversity was null at most sites; all individuals were genetically identical in Cartagena (n = 14), PNN Corales de Profundidad (n = 5), Curaçao (n = 17), Fernando de Noronha (n = 11), Bahia (n = 17) and Pernambuco (n = 12) for S. roseus and Santana (n = 4) for S. blatteus. Low intrapopulational variation does not appear to be related to slow rates of evolution in the 16S marker, as many mutations have accumulated between sites throughout the distribution of the species. Low genetic diversity in sexually reproducing populations occurs as a result of non-random mating, resulting from inbreeding or genetic drift that occurs in populations that are isolated or that have small population sizes (Frankham 1996; Reed and Frankham 2003). Of these possibilities, inbreeding is likely to occur in stylasterids, given the low levels of gene flow observed here, as well as their short larval period. Although further studies on the reproduction of S. roseus are needed, stylasterid planulae have been observed to stay near the bottom after release (Fritchman 1974) and to settle near the mother colony (Ostarello 1973, 1976), increasing the probability of reproduction between related individuals. Low genetic diversity may also result from asexual reproduction. Clonality can lead to high abundances of one to a few genotypes in a population (Coffroth et al. 1992; Avise 2015), and partial clonality can also have important effects on genetic and genotypic diversity in populations (Arnaud-Haond et al. 2020). Asexual reproduction may occur in Stylaster through fragmentation, selfing or via parthenogenesis (Avise 2015). Future work that studies self-compatibility in S. roseus and S. blatteus is needed, but both species contain male and female gonophores on the same colony (Goedbloed 1962), potentially favoring some level of asexual reproduction. Asexual reproduction via stolons has also been reported for stylasterids, such as in the genus Stylantheca, where stolons have been observed to generate multiple sister colonies in close proximity (Puce et al. 2010). Here, individuals were genotyped with a single mitochondrial marker, which precludes estimation of clonal diversity. Future work employing nuclear markers, such as microsatellites or SNPs, could provide confirmation of clonal reproduction in S. roseus and S. blatteus and would allow for estimates in differences in the clonal diversity among populations of these two species. Further experimental work that tests whether clonal reproduction is possible through fragmentation or that could verify whether parthenogenesis occurs in either or both species may also help determine the causes of such low levels of genetic diversity observed here.
Lace corals show distinct patterns of genetic diversity and connectivity compared to scleractinian corals, indicating that evolutionary histories among reef inhabitants can vary greatly. To better understand the various evolutionary processes at work in reef communities, it is important to examine a variety of organisms. Additional studies on hydrozoans may help elucidate the diversity of evolutionary processes at work on coral reefs at local and basin-wide scales and mechanisms that lead to diversification in the tropical seas.
References
Almany GR, Connolly SR, Heath DD, Hogan JD, Jones GP, McCook LJ, Mills M, Pressey RL, Williamson DH (2009) Connectivity, biodiversity conservation and the design of marine reserve networks for coral reefs. Coral Reefs 28:339–351
Araujo GS, Rocha LA, Lastrucci NS, Luiz OJ, Di F, Sergio D (2022) The Amazon–Orinoco Barrier as a driver of reef–fish speciation in the Western Atlantic through time. J Biogeogr 1–13
Arnaud-Haond S, Stoeckel S, Bailleul D (2020) New insights into the population genetics of partially clonal organisms: When seagrass data meet theoretical expectations. Mol Ecol 29:3248–3260
Arrigoni R, Maggioni D, Montano S, Hoeksema BW, Seveso D, Shlesinger T, Terraneo TI, Tietbohl MD, Berumen ML (2018) An integrated morpho-molecular approach to delineate species boundaries of Millepora from the Red Sea. Coral Reefs 37:967–984
Avise JC (1992) Molecular population structure and the biogeographic history of a regional fauna - a case history with lessons for conservation biology. Oikos 63:62–76
Avise JC (2009) Phylogeography: retrospect and prospect. J Biogeogr 36:3–15
Avise JC (2015) Evolutionary perspectives on clonal reproduction in vertebrate animals. Proc Natl Acad Sci U S A 112:8867–8873
Baums IB, Miller MW, Hellberg ME (2005) Regionally isolated populations of an imperiled Caribbean coral, Acropora palmata. Mol Ecol 14:1377–1390
Baums IB, Miller MW, Hellberg ME (2006) Geographic variation in clonal structure in a reef-building Caribbean coral, Acropora palmata. Ecol Monogr 76:503–519
Boschma H (1961) Stylasterina. Camp La Calypso Golf Guinée 5:193–225
Bouckaert R, Vaughan TG, Barido-Sottani J, Duchêne S, Fourment M, Gavryushkina A, Heled J, Jones G, Kühnert D, De Maio N, Matschiner M, Mendes FK, Müller NF, Ogilvie HA, Du Plessis L, Popinga A, Rambaut A, Rasmussen D, Siveroni I, Suchard MA, Wu CH, Xie D, Zhang C, Stadler T, Drummond AJ (2019) BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLoS Comput Biol 15:1–28
Bowen BW, Gaither MR, DiBattista JD, Iacchei M, Andrews KR, Grant WS, Toonen RJ, Briggs JC (2016) Comparative phylogeography of the ocean planet. Proc Natl Acad Sci U S A 113:7962–7969
Briggs JC, Bowen BW (2012) A realignment of marine biogeographic provinces with particular reference to fish distributions. J Biogeogr 39:12–30
Cairns SD (1986) A revision of the northwest Atlantic Stylasteridae. Smithson Contrib to Zool 131
Cairns SD (2011) Global diversity of the stylasteridae (Cnidaria: Hydrozoa: Athecatae). PLoS One 6:e21670
Cairns SD, Zibrowius H (2013) Stylasteridae (Cnidaria, Hydrozoa, Filifera) from South Africa. Zootaxa 3691:1–57
Coffroth MA, Lasker HR, Diamond ME, Bruenn JA, Bermingham E (1992) DNA fingerprints of a gorgonian coral: a method for detecting clonal structure in a vegetative species. Mar Biol 114:317–325
Cunningham CW, Buss LW (1993) Molecular evidence for multiple episodes of paedomorphosis in the Family Hydractiniidae. Biochem Syst Ecol 21:57–69
Drummond AJ, Suchard MA, Xie D, Rambaut A (2012) Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol 29:1969–1973
Duarte GAS, Villela HDM, Deocleciano M, Silva D, Barno A, Cardoso PM, Vilela CLS, Rosado P, Messias CSMA, Chacon MA, Santoro EP, Olmedo DB, Szpilman M, Rocha LA, Sweet M, Peixoto RS (2020) Heat waves are a major threat to turbid coral reefs in Brazil. Front Mar Sci 7:179
Excoffier L, Lischer HEL (2010) Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Resour 10:564–567
Floeter SR, Rocha LA, Robertson DR, Joyeux JC, Smith-Vaniz WF, Wirtz P, Edwards AJ, Barreiros JP, Ferreira CEL, Gasparini JL, Brito A, Falcón JM, Bowen BW, Bernardi G (2008) Atlantic reef fish biogeography and evolution. J Biogeogr 35:22–47
Foster NL, Paris CB, Kool JT, Baums IB, Stevens JR, Sanchez JA, Bastidas C, Agudelo C, Bush P, Day O, Ferrari R, Gonzalez P, Gore S, Guppy R, McCartney MA, McCoy C, Mendes J, Srinivasan A, Steiner S, Vermeij MJA, Weil E, Mumby PJ (2012) Connectivity of Caribbean coral populations: complementary insights from empirical and modelled gene flow. Mol Ecol 21:1143–1157
Frankham R (1996) Relationship of genetic variation to population size in wildlife. Conserv Biol 10:1500–1508
Fritchman H (1974) The planula of the stylasterine hydrocoral Allopora petrograpta Fisher: its structure, metamorphosis and development of the primary cyclosystem. Proc Second Int Coral Reef Symp 245–258
Fukami H, Budd AF, Paulay G, Sole A, Chen CA, Iwao K, Knowlton N (2004) Conventional taxonomy obscures deep divergence between Pacific and Atlantic corals. 427:2–5
Gaither MR, Bernal MA, Coleman RR, Bowen BW, Jones SA, Simison WB, Rocha LA (2015) Genomic signatures of geographic isolation and natural selection in coral reef fishes. Mol Ecol 24:1543–1557
Goedbloed AF (1962) On the structure and the development of the gonophores of Allopora blattea and Stylaster roseus. Proc K Ned Akad Van Wet C 65:522–531
Hoang DT, Chernomor O, Von Haeseler A, Minh BQ, Vinh LS (2018) UFBoot2: Improving the ultrafast bootstrap approximation. Mol Biol Evol 35:518–522
Hoorn C, Bogotá-A GR, Romero-Baez M, Lammertsma EI, Flantua SGA, Dantas EL, Dino R, do Carmo DA, Chemale F (2017) The Amazon at sea: Onset and stages of the Amazon River from a marine record, with special reference to Neogene plant turnover in the drainage basin. Glob Planet Change 153:51–65
Jell JS, Cook AG, Jell PA (2011) Australian Cretaceous cnidaria and porifera. Alcheringa 35:241–284
Kalyaanamoorthy S, Minh BQ, Wong TKF, Von A, Jermiin LS (2017) ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods 14:587–589
Katoh K, Standley DM (2013) MAFFT Multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A (2012) Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649
Laborel J (1970) Les peuplements de Madréporaires des cotês tropicales du Brésil. Ann l’Université D’Abidjan Série E II:1–261
Lazoski C, Solé-Cava AM, Boury-Esnault N, M K, Russo CAM (2001) Cryptic speciation in a high gene flow scenario in the oviparous marine sponge Chondrosia reniformis. Mar Biol 139:421–429
Leão ZMAN, Fournier J (2007) Contribution à l’étude biogéomorphologique d’un archipel d’origine volcanique: une synthèse sur le complexe récifal d’Abrolhos (Bahia, Brésil). Les littoraux volcaniques - une approche environnementale. pp 193–219
Leigh JW, Bryant D (2015) POPART: Full-feature software for haplotype network construction. Methods Ecol Evol 6:1110–1116
Lessios HA, Kane J, Robertson DR (2003) Phylogeography of the pantropical sea urchin Tripneustes: contrasting patterns of population structure between oceans. Evolution (N Y) 57:2026–2036
Lindner A, Cairns SD, Cunningham CW (2008) From offshore to onshore: multiple origins of shallow-water corals from deep-sea ancestors. PLoS One 3:e2429
López C, Clemente S, Almeida C, Brito A, Hernández M (2015) A genetic approach to the origin of Millepora sp. in the eastern Atlantic. Coral Reefs 34:631–638
Lundgren P (2011) Genetics and genetic tools in coral reef management: a synthesis of current research and its application in the management of coral reefs.
Mendonça FF, Oliveira C, Gadig OBF, Foresti F (2013) Diversity and genetic population structure of the Brazilian sharpnose shark Rhizoprionodon lalandii. Aquat Conserv Mar Freshw Ecosyst 23:850–857
Miller KJ, Mundy CN, Chadderton WL (2004) Ecological and genetic evidence of the vulnerability of shallow-water populations of the stylasterid hydro coral Errina novaezelandiae in New Zealand’s fiords. Aquat Conserv Mar Freshw Ecosyst 14:75–94
Milne I, Lindner D, Bayer M, Husmeier D, Mcguire G, Marshall DF, Wright F (2009) TOPALi v2: A rich graphical interface for evolutionary analyses of multiple alignments on HPC clusters and multi-core desktops. Bioinformatics 25:126–127
Minh BQ, Nguyen MAT, Von Haeseler A (2013) Ultrafast approximation for phylogenetic bootstrap. Mol Biol Evol 30:1188–1195
Minh BQ, Schmidt HA, Chernomor O, Schrempf D, Woodhams MD, Von Haeseler A, Lanfear R, Teeling E (2020) IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol Biol Evol 37:1530–1534
Morri C, Bianchi CN, Di Camillo CG, Ducarme F, Allison WR, Bavestrello G (2017) Global climate change and regional biotic responses: two hydrozoan tales. Mar Biol Res 13:573–586
Nguyen LT, Schmidt HA, Von Haeseler A, Minh BQ (2015) IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol 32:268–274
Nóbrega R, Solé-Cava AM, Russo CAM (2004) High genetic homogeneity of an intertidal marine invertebrate along 8000 km of the Atlantic coast of the Americas. J Exp Mar Bio Ecol 303:173–181
Nunes F, Norris RD, Knowlton N (2009) Implications of isolation and low genetic diversity in peripheral populations of an amphi-Atlantic coral. Mol Ecol 18:4283–4297
Nunes FLD, Norris RD, Knowlton N (2011) Long distance dispersal and connectivity in amphi-Atlantic corals at regional and basin scales. PLoS ONE 6:e22298
Nunes FLD, Van Wormhoudt A, Faroni-Perez L, Fournier J (2017) Phylogeography of the reef-building polychaetes of the genus Phragmatopoma in the western Atlantic Region. J Biogeogr 44:1612–1625
Ostarello GL (1973) Natural history of the hydrocoral Allopora californica Verrill (1866). Biol Bull 145:548–564
Ostarello GL (1976) Larval dispersal in the subtidal hydrocoral Allopora californica Verrill (1866). In: Mackie G. (eds) Coelenterate Ecology and Behavior. Plenum Press, New York, pp 331–337
Palumbi SR (1994) Genetic divergence, reproductive isolation, and marine speciation. Annu Rev Ecol Syst 25:547–572
Peluso L, Tascheri V, Nunes FLD, Castro CB, Pires DO, Zilberberg C (2018) Contemporary and historical oceanographic processes explain genetic connectivity in a Southwestern Atlantic coral. Sci Rep 8:2684
Pica D, Cairns SD, Puce S, Newman WA (2015) Southern hemisphere deep-water stylasterid corals including a new species, Errina labrosa sp. n. (Cnidaria, Hydrozoa, Stylasteridae), with notes on some symbiotic scalpellids (Cirripedia, Thoracica, Scalpellidae). Zookeys 472:1–25
Postaire B, Gélin P, Bruggemann JH, Magalon H (2017a) One species for one island? Unexpected diversity and weak connectivity in a widely distributed tropical hydrozoan. Heredity (edinb) 118:385–394
Postaire B, Gélin P, Bruggemann JH, Pratlong M, Magalon H (2017b) Population differentiation or species formation across the Indian and the Pacific Oceans? An example from the brooding marine hydrozoan Macrorhynchia phoenicea. Ecol Evol 7:8170–8186
Puce S, Bo M, Gioia C, Camillo D, Paoli L, Pica D, Bavestrello G (2010) Morphology and development of the early growth stages of an Indonesian Stylaster (Cnidaria: Hydrozoa). J Mar Biol Assoc United Kingdom 90:1145–1151
Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA (2018) Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst Biol 67:901–904
Reed DH, Frankham R (2003) Correlation between fitness and genetic diversity. Conserv Biol 17:230–237
Restrepo JD, Zapata P, Dı JM, Garzo J, Garcı CB (2006) Fluvial fluxes into the Caribbean Sea and their impact on coastal ecosystems : The Magdalena River, Colombia. Glob Planet Change 50:33–49
Riquet F, Japaud A, Nunes FLD, Serrano XM, Baker AC, Bezault E, Bouchon C, Fauvelot C (2021) Complex spatial patterns of genetic differentiation in the Caribbean mustard hill coral Porites astreoides. Coral Reefs
Rocha LA, Bowen BW (2008) Speciation in coral-reef fishes. J Fish Biol 72:1101–1121
Ruiz-Ramos DV, Weil E, Schizas NV (2014) Morphological and genetic evaluation of the hydrocoral Millepora species complex in the Caribbean. Zool Stud 53:4
Sánchez JA, González-Zapata FL, Dueñas LF, Andrade J, Pico-Vargas AL, Vergara DC, Sarmiento A, Bolaños N (2019) Corals in the mesophotic zone (40–115 m) at the barrier reef complex from San Andrés Island (Southwestern Caribbean). Front Mar Sci 6:536
Sanchez JA, Navas GR (1994) Notes on the distribution, habitat and morphology of Stylaster roseus (Pallas 1766) (Hydrozoa:Stylasterina) in the Colombian Caribbean. Boletín Investig Mar y Costeras 193–197
Schuchert P (2005) Species boundaries in the hydrozoan genus Coryne. Mol Phylogenet Evol 36:194–199
Schuchert P (2022) Stylaster Gray, 1831. World Register of Marine Species.
Selkoe KA, Toonen RJ (2011) Marine connectivity: A new look at pelagic larval duration and genetic metrics of dispersal. Mar Ecol Prog Ser 436:291–305
Serrano XM, Baums IBB, O’Reilly K, Smith TBB, Jones RJJ, Shearer TLL, Nunes FLDFLD, Baker ACAC (2014) Geographic differences in vertical connectivity in the Caribbean coral Montastraea cavernosa despite high levels of horizontal connectivity at shallow depths. Mol Ecol 23:4226–4240
de Souza JN, Nunes FLD, Zilberberg C, Sanchez JA, Migotto AE, Hoeksema BW, Serrano XM, Baker AC, Lindner A (2017) Contrasting patterns of connectivity among endemic and widespread fire coral species (Millepora spp.) in the tropical Southwestern Atlantic. Coral Reefs 36:701–716
Terossi M, Mantelatto FLA (2012) Morphological and genetic variability in Hippolyte obliquimanus Dana, 1852 (Decapoda, Caridae, Hippolytidae) from Brazil and the Caribbean Sea. Crustaceana 85:685–712
Teschima MM, Zilberberg C, Nunes FLD (2021) Strong genetic differentiation demarks populations of Favia across biogeographic regions of the Atlantic Ocean. Coral Reefs
Volk DR, Konvalina JD, Floeter SR, Ferreira CEL, Hoffman EA (2020) Going against the flow : Barriers to gene flow impact patterns of connectivity in cryptic coral reef gobies throughout the western Atlantic. J Biogeogr 1–13
Vollmer SV, Palumbi SR (2007) Restricted gene flow in the Caribbean staghorn coral Acropora cervicornis: implications for the recovery of endangered reefs. J Hered 98:40–50
Zibrowius H, Cairns SD (1992) Revision of the northeast Atlantic and Mediterranean Stylasteridae (Cnidaria: Hydrozoa). Mémoirs Du Muséum Natl D’histoire Nat 153:135
Zigler KS, Lessios HA (2004) Speciation on the coasts of the new world: phylogeography and the evolution of binding in the sea urchin genus Lytechinus. Evolution (N Y) 58:1225–1241
Acknowledgements
We would like to thank CORALINA (Convenios No. 13, 2014 and No. 21, 2015), particularly to N. Bolaños and E. Castro, and PNN “Corales de Profundidad” for their support in field logistics and aid with collection permits; to several colleagues that collected or supported sample collection activities: J. Andrade, F. García, O. Ruiz, D. Seguro, M. Forero, L. Gutierrez, M. Gómez, M. Marrugo, A. Henao, L. Buss, R. Arantes, C. Cunha, L. Simone, A. Rojo Prado, F. Negrão, K. Lima, M. Maida, E. Macedo, M. Silva, R. Cordeiro, F. Amaral, L. Vieira, J. Gasparini, C. Ferreira, S. Floeter, L. Rocha, H. Maia, R. Morais, L. Fontoura, K. Nickols and N. Knowlton; the HPC group of Universidad de los Andes for their support with the bioinformatic tools; A. Lindner thanks L. Branco (FAPESP TT-3), SISBIOTA-Mar Network (CNPq 563276/2010-0/FAPESC 6308/2011-8 grant to S. Floeter) and São Paulo Research Foundation (FAPESP 2006/02960-8, 2006/05821-9, 2006/60327-0 grants to A. Lindner and A. Migotto) for funding. Sampling in Príncipe was possible thanks to the support of California Academy of Sciences, The Rufford Foundation (Grant #18424-1), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—CAPES (Brazil) and Rombout Swanborn, Africa's Eden, Roça Belo Monte Resort. Sampling and DNA sequencing for S. blatteus in São Tomé was funded by the John Dove Isaacs Chair in Natural Philosophy to N. Knowlton (CITES permit 139/DP/ME/05).
Funding
Open Access funding provided by Colombia Consortium.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gnecco, M., Nunes, F.L.D., González-Zapata, F.L. et al. Remarkable population structure in the tropical Atlantic lace corals Stylaster roseus (Pallas, 1766) and Stylaster blatteus (Boschma, 1961) . Coral Reefs 42, 181–194 (2023). https://doi.org/10.1007/s00338-022-02329-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-022-02329-5