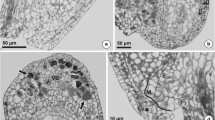
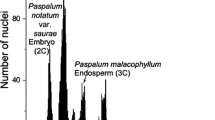
Abstract
About 80% of angiosperms form a monosporic Polygonum-type embryo sac, whereas in the remaining species, eleven other types of embryo sac are found. Evidence as to the type of embryo sac is lacking for many plant species, and the role of higher-ploidy endosperm is unknown. In contrast to the rest of the Apiaceae, where a Polygonum-type embryo sac (3n endosperm) has been reported, the few species of the Azorelloideae studied to form a Drusa-type embryo sac (3n endosperm) or a Penaea-type embryo sac (5n endosperm). This variation within Azorelloideae makes this subfamily, and its genus Azorella in particular, a good candidate for studying the evolutionary importance of the embryo sac and endosperm in diversification. We studied the variation in the type of embryo sac and the ploidy level of the endosperm in Andean-Patagonian Azorella and closely related Pozoa on a sample of 101 individuals from 31 populations of 21 species. We employed flow cytometric seed screening and calibrated the results of ploidy level estimation against embryological observations. In addition, we examined the genome size variation of the species sampled. All species of Azorella formed Penaea-type embryo sacs and a pentaploid endosperm, whereas one species of Pozoa formed triploid and the other tetraploid endosperms. Variations in the type of embryo sac and endosperm ploidy have probably shaped the evolution of the different lineages of Azorelloideae in the southern Andes. A Penaea-type embryo sac, which represents a likely synapomorphy of Azorella, is a feature of underestimated significance in the evolution of angiosperms.




Similar content being viewed by others
References
Andersson L, Kocsis M, Eriksson R (2006) Relationships of the genus Azorella (Apiaceae) and other hydrocotyloids inferred from sequence variation in three plastid markers. Taxon 55:270–280. https://doi.org/10.2307/25065577
Baroux C, Spillane C, Grossniklaus U (2002) Evolutionary origins of the endosperm in flowering plants. Genome Biol 3:1–5
Bell CR, Constance L (1957) Chromosome numbers in Umbelliferae. Amer J Bot 44:565–572. https://doi.org/10.2307/2438928
Benton MJ, Wilf P, Sauquet H (2022) The Angiosperm Terrestrial Revolution and the origins of modern biodiversity. New Phytol 233:2017–2035. https://doi.org/10.1111/nph.17822
Calviño CI, Fernández M, Martínez SG (2016) Las Especies de Azorella (Azorelloideae, Apiaceae) con distribució extra-Argentina. Darwiniana 4:57–82. https://doi.org/10.14522/darwiniana.2016.41.681
Caparelli KF, Peruzzi L, Cesca G (2006) A comparative analysis of embryo-sac development in three closely-related Gagea species (Liliaceae), with some considerations on their reproductive strategies. Pl Biosyst 140:115–122
Carputo D, Monti L, Werner JE, Frusciante L (1999) Uses and usefulness of endosperm balance number. Theor Appl Genet 98:478–484. https://doi.org/10.1007/s001220051095
Constance L, Chuang T, Bell CR (1971) Chromosome numbers in Umbelliferae. IV. Amer J Bot 58:577–587. https://doi.org/10.1002/j.1537-2197.1971.tb10007.x
Constance L, Chuang T, Bell CR (1976) Chromosome numbers in Umbelliferae. V. Amer J Bot 63:608–625. https://doi.org/10.1002/j.1537-2197.1976.tb11849.x
Constance L (1988) Umbelliferae. In: Correa MN (ed) Flora Patagónica. Colección Científica del Instituto Nacional de Tecnología Agropecuaria (INTA), Buenos Aires, pp 310–379
D’Amato F (1984) Role of polyploidy in reproductive organs and tissues. In: Johri BM (eds) Embryology of angiosperms. Springer, Berlin, Heidelberg, New York, Tokyo, pp 519–566
Doležel J, Greilhuber J, Suda J (2007) Flow cytometry with plant cells: analysis of genes, chromosomes and genomes. Wiley, Weinheim
Donoghue MJ, Scheiner SM (1992) The evolution of endosperm: a phylogenetic account. In: Wyatt R (ed) Ecology and evolution of plant reproduction. Chapman & Hall, New York, pp 356–389
Ebert I, Greilhuber J (2005) Developmental switch during embryo sac formation from a bisporic mode to the tetrasporic Fritillaria type in Hyacinthoides vincentina (Hoffmannsegg & Link) Rothm. (Hyacinthaceae). Acta Biol Cracov Bot 47:179–184
Fagerlind F (1938) Wo kommen tetrasporische, durch drei Teilungsschritte vollentwickelte Embryosäcke unter den Angiospermen vor? Bot Not 1938:461–498
Fernández M, Calviño CI (2019) Nueva clasificación infragenérica de Azorella (Apiaceae, Azorelloideae) y sinopsis del subgénero Andinae. Darwiniana Nueva Ser 7:289–304
Fernández M, Ezcurra C, Calviño CI (2016) Morphology, fruit anatomy and taxonomy of the South Andean genus Laretia (Azorelloideae, Apiaceae). Syst Bot 41:807–812. https://doi.org/10.1600/036364416X692370
Fernández M, Ezcurra C, Calviño CI (2017a) Molecular phylogenetics and evolution chloroplast and ITS phylogenies to understand the evolutionary history of southern South American Azorella, Laretia and Mulinum (Azorelloideae, Apiaceae). Molec Phylogen Evol 108:1–21. https://doi.org/10.1016/j.ympev.2017.01.016
Fernández M, Ezcurra C, Calviño CI (2017b) Revisión taxonómica del género sudamericano Mulinum (Azorelloideae, Apiaceae). Anales Jard Bot Madrid 74:1–31
Fernández M, Ezcurra C, Calviño CI (2017c) Species limits and morphometric and environmental variation within the South Andean and Patagonian Mulinum spinosum species-group (Apiaceae-Azorelloideae). Syst Biodivers 15:489–505. https://doi.org/10.1080/14772000.2016.1273975
Fernandez M, Martínez SG, Calviño CI (2020) Typifications of 31 names in Azorella (Azorelloideae Apiaceae) from South America. Phytotaxa 433:135–144. https://doi.org/10.11646/phytotaxa.433.2.4
Friedman WE (1998) The evolution of double fertilization and endosperm: an” historical” perspective. Sexual Pl Reprod 11:6–16
Friedman WE, Madrid EN, Williams JH (2008) Origin of the fittest and survival of the fittest: relating female gametophyte development to endosperm genetics. Int J Pl Sci 169:79–92. https://doi.org/10.1086/523354
Haig D (1990) New perspectives on the angiosperm female gametophyte. Bot Rev 56:236–274. https://doi.org/10.1007/BF02858326
Håkansson A (1923) Studienüber die Entwicklungsgeschichite der Umbelliferae. Acta Univ Lund 18:1–120
Håkansson A (1927) Der sechzehnkernige Embryosack von Azorella trifurcata Gaertn. Hook Ber Deutsch Bot Ges 45:654–664
Håkansson A (1952) Seed development in Bowlesia tenera. Bot Not 1:33–45
Härdling R, Nilsson P (2001) A model of triploid endosperm evolution driven by parent-offspring conflict. Oikos 92:417–423. https://doi.org/10.1034/j.1600-0706.2001.920303.x
Henwood MJ, Hart JM (2001) Towards an understanding of the phylogenetic relationships of Australian Hydrocotyloideae (Apiaceae). Edinburgh J Bot 58:269–289
Hojsgaard D, Hörandl E (2015) Apomixis as a facilitator of range expansion and diversification in plants. In: Pontarotti P (ed) Evolutionary biology: biodiversification from genotype to phenotype. Springer, Cham, pp 305–327
Hörandl E (2006) The complex causality of geographical parthenogenesis. New Phytol 171:525–538. https://doi.org/10.1111/j.1469-8137.2006.01769.x
Jurica HS (1922) A morphological study of the Umbelliferae. Bot Gaz 74:292–307
Kaushal P, Dwivedi KK, Radhakrishna A et al (2019) Partitioning apomixis components to understand and utilize gametophytic apomixis. Frontiers Pl Sci 10:256
Kordyum EL, Mosyakin SL (2020) Endosperm of angiosperms and genomic imprinting. Life 10:1–20
Krahulcová A, Rotreklová O (2010) Use of flow cytometry in research on apomictic plants. Preslia 82:23–39
López P, Tremetsberger K, Kohl G, Stuessy T (2011) Progenitor–derivative speciation in Pozoa (Apiaceae, Azorelloideae) of the southern Andes. Ann Bot (Oxford) 109:351–363. https://doi.org/10.1093/aob/mcr291
Maheshwari P (1937) A critical review of the types of embryo sacs in angiosperms. New Phytol 36:359–417
Martinez S (1989) El género Azorella (Apiaceae-Hydrocotyloideae) en la Argentina. Darwiniana 1:139–178
Martínez SG, Calviño CI (2019) Apiaceae. In: Zuloaga FO, Belgrano MJ (eds) Flora Vascular de la República Argentina 20 (2). Talleres Trama S.A., Buenos Aires, pp 1–122
Matzk F, Meister A, Schubert I (2000) An efficient screen for reproductive pathways using mature seeds of monocots and dicots. Pl J 21:97–108. https://doi.org/10.1046/j.1365-313x.2000.00647.x
Moore DM (1981) Chromosome numbers of Fuegian angiosperms. Bol Soc Brot 53:995–1012
Nicolas AN, Plunkett GM (2009) The demise of subfamily Hydrocotyloideae (Apiaceae) and the re-alignment of its genera across the entire order Apiales. Molec Phylogen Evol 53:134–151. https://doi.org/10.1016/j.ympev.2009.06.010
Nicolas AN, Plunkett GM (2012) Untangling generic limits in Azorella, Laretia, and Mulinum (Apiaceae: Azorelloideae): insights from phylogenetics and biogeography. Taxon 61:826–840. https://doi.org/10.1002/tax.614008
Nuñez CI, Aizen MA, Ezcurra C (1999) Species associations and nurse plant effects in patches of high-Andean vegetation. J Veg Sci 10:357–364. https://doi.org/10.2307/3237064
Otto F (1990) DAPI staining of fixed cells for high-resolution flow cytometry of nuclear DNA. Methods Cell Biol 33:105–110
Plunkett GM, Nicolas AN (2017) Assessing Azorella (Apiaceae) and its allies: phylogenetics and a new classification. Brittonia 69:31–61. https://doi.org/10.1007/s12228-016-9446-0
Rangan P (2020) Endosperm variability: from endoreduplication within a seed to higher ploidy across species, and its competence. Seed Sci Res 30:173–185
Simpson BB, Todzia CA (1990) Patterns and processes in the development of the high Andean flora. Amer J Bot 77:1419–1432. https://doi.org/10.1002/j.1537-2197.1990.tb12552.x
Sklenář P (2009) Presence of cushion plants increases community diversity in the high equatorial Andes. Flora-Morphology, Distrib, Funct Ecol Pl 204:270–277
Sklenář P, Dušková E, Balslev H (2011) Tropical and temperate: evolutionary history of páramo flora. Bot Rev 77:71–108. https://doi.org/10.1007/s12229-010-9061-9
Soltis DE, Albert VA, Leebens-Mack J et al (2009) Polyploidy and angiosperm diversification. Amer J Bot 96:336–348
Stephens EL (1909) The embryo-sac and embryo of certain Penaeaceae. Ann Bot (Oxford) 23:363–378
Subba Rao AM (1937) A note on the development of the female gametophyte of some Malpighiaceae and polyembryony in Hiptage madablota. Curr Sci 6:280–282
Temsch EM, Koutecký P, Urfus T et al (2021) Reference standards for flow cytometric estimation of absolute nuclear DNA content in plants. Cytometry Part A 9:710–724. https://doi.org/10.1002/cyto.a.24495
Testolin R, Attorre F, Borchardt P et al (2021) Global patterns and drivers of alpine plant species richness. Global Ecol Biogeogr 30:1218–1231
Tseng CC (1967) Anatomical studies of flower and fruit in the Hydrocotyloideae (Umbelliferae). University of California Press, Berkeley
Tucker MR, Araujo A-CG, Paech NA et al (2003) Sexual and apomictic reproduction in Hieracium subgenus Pilosella are closely interrelated developmental pathways. Pl Cell 15:1524–1537. https://doi.org/10.1105/tpc.011742
Virkki N (1962) Meiosis and Development of the Embryo Sac in Gunnera insignis (Oersted) DC (Halorhagaceae). J Agric Univ Puerto Rico 46:254–268
Wilkinson HP, Wanntorp L (2007) Gunneraceae. In: Kubitzki K (ed) Flowering Plants·Eudicots. Springer, Heidelberg, pp 177–183
Williams JH, Friedman WE (2002) Identification of diploid endosperm in an early angiosperm lineage. Nature 415:522–526. https://doi.org/10.1038/415522a
Acknowledgments
We are grateful to Lenka Macková, Veronika Konečná, Katya Romoleroux, Romina Vidal-Russell for their assistance. Fred Rooks kindly improved the English. The necessary research permit was issued by the Ministerio del Ambiente, Ecuador (No. MAE-DNB-CM-2018-0082), the Ministerio de medio ambiente y agua, Bolivia (No. 026/09) and the Administración de Parque Nacionales, Argentina (No. 1621).
Funding
This work was supported by the Grant Agency of the Czech Republic (project No. 17-12420S), the Grant Agency of Charles University (project No. 1554218) and the following institutions in Argentina: Agencia Nacional de Promoción Científica y Tecnológica PICT 2019-007700, CONICET PIP 112-201301-00357 and Universidad Nacional del Comahue PIN B205.
Author information
Authors and Affiliations
Contributions
Carolina I. Calviño, Petr Sklenář and Tomáš Urfus conceived the ideas; Jan Ptáček, Petr Sklenář, Tomáš Urfus and Carolina I. Calviño collected the samples; Jan Ptáček, Tomáš Urfus, Romana Urfusová and Jan Pinc performed the laboratory measurements; Carolina I. Calviño determined the species; and Jan Ptáček analysed the data and led the writing of this article with assistance from Carolina I. Calviño, Petr Sklenář and Tomáš Urfus.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Karol Marhold.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Information on Electronic Supplementary Material
Information on Electronic Supplementary Material
Online Resource 1. Sampling locations of Azorelloideae species.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ptáček, J., Sklenář, P., Pinc, J. et al. A pentaploid endosperm and a Penaea-type embryo sac are likely synapomorphies of Azorella (Apiaceae, Azorelloideae). Plant Syst Evol 308, 40 (2022). https://doi.org/10.1007/s00606-022-01833-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00606-022-01833-z