Abstract
Larvae and juveniles of three species in the family Gobiidae are described based on laboratory-reared material: Bathygobius fuscus to post-settlement, Bathygobius cyclopterus to 2 days old, and Bathygobius petrophilus to just after hatching. Newly hatched larvae of these species are distinguishable from each other by the melanophore arrangements on the dorsal region of the trunk, ventrally near the tip of the tail, and along the lateral midline. The early development of Bathygobius fuscus was similar to that described previously for Bathygobius soporator but differed in the appearance of melanophores and the shape of the second dorsal fin and anal fin.
Similar content being viewed by others
Introduction
The genus Bathygobius (Gobiidae) currently comprises 29 species (Tornabene and Pezold 2011), distributed worldwide in tropical to temperate waters (Miller and Stefanni 2001; Tornabene et al. 2010; Tornabene and Pezold 2011). Information on the larval morphology of Bathygobius is limited to four species: Bathygobius soporator, for newly hatched larvae (Tavolga 1950) and postflexion larvae (Tavolga 1950; Baldwin and Smith 2003; Tornabene et al. 2010), as well as a continuous morphological description of laboratory-reared fish from just after hatching to after settlement (Peters 1983); Bathygobius fuscus, for newly hatched larvae and postflexion larvae (Dotsu 1955); Bathygobius curacao, for postflexion larvae (Baldwin and Smith 2003; Tornabene et al. 2010); and Bathygobius lacertus, for postflexion larvae (Tornabene et al. 2010).
It should be noted that species identification for the larvae of B. soporator and B. lacertus are confusing. Baldwin and Smith (2003) reported a variation in pigmentation among the larvae described as B. soporator. Tornabene et al. (2010) compared the larvae identified to B. soporator by DNA barcoding method with the larvae described by Baldwin and Smith (2003). As a result, Tornabene et al. (2010) suggested that some of the B. soporator larvae in Baldwin and Smith (2003) are actually B. lacertus, and only their variant B. soporator larvae is true B. soporator. On the other hand, the B. soporator larvae described by Peters (1983) differed in pigmentation from those described by Baldwin and Smith (2003) and Tornabene et al. (2010), suggesting that the B. soporator larvae described by Peters (1983) may be a different species.
In this study, we describe the early development of three species from laboratory-reared fish: Bathygobius cyclopterus, B. fuscus, and Bathygobius petrophilus. Bathygobius cyclopterus and B. fuscus are widely distributed in the Indo-Pacific Ocean, and B. petrophilus in the Western-Central Pacific Ocean (Akihito et al. 2002). In addition, B. fuscus is known as a very common species that occur in the intertidal habitats (Arakaki and Tokeshi 2005, 2012). Thus, description of the early life history of each species is useful for a variety of studies, such as ecology and phylogenetic relationships, covering a wide range of marine ecosystems.
As mentioned above, the species identification for the larvae of B. soporator and B. lacertus are confusing. In this study, we followed Peters (1983) for the morphology of B. soporator and Tornabene et al. (2010) for the morphology of B. lacertus as comparing the morphology with other species.
Materials and methods
Parent fish were collected along stretches of beach at Tateyama, Chiba Prefecture, Japan, using hand nets (Fig. 1). Bathygobius cyclopterus was captured in December 2019 (one male: 56.8 mm standard length [SL]; four females: 44.9–52.0 mm SL), and B. fuscus on 10 July 2020 (two males: 58.4 and 69.4 mm SL; two females: 50.4 and 59.8 mm SL), both at Banda Coast. Bathygobius petrophilus (one male: 57.2 mm SL; one female: 43.8 mm SL) was collected at Hojo Beach on 14 November 2020. The fish were brought to the laboratory and reared in aquaria: B. cyclopterus in a 182-l tank (90 × 45 × 45 cm) and B. fuscus and B. petrophilus in separate 65-l tanks (60 × 30 × 36 cm). An opaque polyvinyl chloride (PVC) pipe (inside diameter 5 cm, length 6 cm) was placed as shelter for spawning and hiding for B. cyclopterus, and an opaque PVC pipe (inside diameter 6 cm, length 15 cm) cut in half lengthwise was placed for B. fuscus and B. petrophilus. Fish were fed artemia nauplii and an artificial diet (Del Marine Food Ex Co. Ltd., Hyogo, Japan) twice daily to apparent satiation. Throughout the rearing experiment, the water temperature was maintained at ~24 °C and salinity at about 29 ppt, and the photoperiod was 14 h light and 10 h dark.
Spawning of the laboratory-reared B. cyclopterus, B. fuscus, and B. petrophilus occurred on 28 June, 28 July, and 9 December in 2020, respectively. Methods of rearing and observation of the offspring followed Tanaka et al. (2023), as be briefly described here. After the larvae hatched, an appropriate dose of marine microalgae Nannochloropsis sp. (Marine Fresh; Nisshin Marinetech Co., Ltd., Aichi, Japan) was added to the tank. Feeding of the larvae began 1 day post-hatch (DPH). S-type rotifers Brachionus rotundiformis fed with chlorella (Super Fresh Chlorella V12; Chlorella Industry Co., Ltd., Tokyo, Japan) were added to the larval culture tanks once per day; the density of rotifers in the tanks was maintained at 20 individuals/ml. For B. fuscus larvae, artemia nauplii were obtained from 17 DPH.
Larvae and juveniles were sampled and observed under a fluorescence microscope (Olympus BX51; Olympus Co., Ltd., Tokyo, Japan) or a stereomicroscope (Olympus SZ2-LGB) after being euthanized in clove oil. The B. cyclopterus larvae were observed immediately after hatching (n = 5) and at 2 DPH (n = 1); B. fuscus larvae and juveniles were observed after hatching (n = 3) and at 2 (n = 1), 7 (n = 1), 12 (n = 1), 17 (n = 1), 22 (n = 1), 32 (n = 4), and 42 (n = 5) DPH; and B. petrophilus larvae were observed after hatching (n = 5). We selected larger specimens from the tanks each day for various measurements. Body length (BL = notochord length for preflexion and flexion larvae, or standard length [SL] after completion of notochord flexion), total length (TL), and yolk-sac diameter (YD) were measured to the nearest 0.01 mm under a microscope fitted with an ocular micrometer, or to the nearest 0.1 mm with calipers for all specimens. Specimens depicted in illustrations or photographs were also measured for body depth (BD), head length (HL), preanal length (PAL), and eye diameter (ED).
All observed specimens were preserved in 70% ethyl alcohol, after fixation in 10% formalin for adults and 5% formalin diluted with seawater for larvae and juveniles, and then deposited in the ichthyological collection of the Kanagawa Prefectural Museum of Natural History (KPM-NI 63278–63290, 63294–63302) (note: the accession nos. for fishes in the museum are seven digits, with zeros added for convenience in the electronic ledger, but here they are expressed as significant digits). All pigments other than melanin disappeared after fixation. Size and age at settlement were defined by the first individual to settle on the bottom of the tank.
Results
Bathygobius cyclopterus
General morphology. Newly hatched larvae (Fig. 2a) measured 1.95–2.15 mm BL and 2.05–2.24 mm TL (n = 5), with 10–11 + 18 = 28–29 myomeres. The body of all specimens was elongate and compressed, with BD ~14% BL. A slightly ellipsoid yolk sac was visible at hatching, with YD measuring 0.19–0.23 mm (n = 5), and which was completely absorbed at 2.19 mm BL and 2.34 mm TL (2 DPH) (Fig. 2b). The dorsal and ventral finfolds were of similar depth just after hatching. The gas bladder was located on myomeres 2–5 in all specimens. The mouth and anus were open at the time of hatching. The premaxilla, maxilla, and dentary were visible at 2.19 mm BL (2 DPH) (Fig. 2b). HL, PAL, and ED as percentage BL were approximately 20%, 45%, and 7%, respectively.
Fin development. Newly hatched larvae possessed a pair of pectoral fin buds (Fig. 2a).
Pigmentation. Melanophore deposition on the eyes was complete by the time of hatching (Fig. 2a). Melanophores were present on the ventral part of the abdomen, and internal melanophores lined the dorsal surface of the abdominal cavity around the gas bladder in all specimens (Fig. 2). There was a single row of melanophores on the dorsal region of the body between the 3rd and 20th myomeres (Fig. 2), and another single row of melanophores on the ventral region of the body between the posterior edge of the gas bladder and the 20th myomere in all specimens (Fig. 2). Separated from this single row of melanophores on the ventral region of the body, small melanophores present ventrally near the tip of the tail (Fig. 2). Lateral midline with 1–4 dendritic melanophores (Fig. 2). A small melanophore at the posterior angle of the lower jaw appeared at 2.19 mm BL (2 DPH) (Fig. 2b).
Some xanthophores were present laterally between the dorsal and ventral series of melanophores (Fig. 2).
Bathygobius fuscus
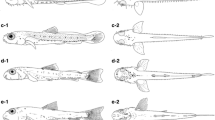
General morphology. Newly hatched larvae (Fig. 3a) measured 1.96–2.13 mm BL and 2.14–2.26 mm TL (n = 3). Larvae in the flexion stage (Fig. 3e) measured 3.69 mm BL and 4.04 mm TL (17 DPH). Juveniles at 42 DPH (Fig. 4b) were 7.6–9.8 mm BL and 9.8–13.2 mm TL (n = 5). The body was elongate and compressed initially. Myomeres were initially V-shaped and then became W-shaped at 5.19 mm BL (27 DPH), with 10–11 + 17–18 = 27–29 myomeres. BD was 12–16% BL initially, and reached ~20% BL after completion of notochord flexion. A slightly ellipsoid yolk sac was visible at hatching, with YD measuring 0.23–0.24 mm (n = 3), and which was completely absorbed by 2.24 mm BL and 2.43 mm TL (2 DPH) (Fig. 3b). Notochord flexion had begun by 3.69 mm BL (17 DPH) (Fig. 3e) and was complete at 5.19 mm BL and 6.17 mm TL (27 DPH) (Fig. 3f). The dorsal and ventral finfolds were of similar depth just after hatching (Fig. 3a); the caudal peduncle part of the finfolds diminished by 3.69 mm BL (17 DPH) (Fig. 3e) and had disappeared at 5.19 mm BL (27 DPH) (Fig. 3f). The anterior edge of the gas bladder was located at myomeres 3–4 (Fig. 3), with the posterior edge extending from myomeres 5–6 (Fig. 3a–d) and then to myomeres 7–8 (Fig. 3e–f). The mouth and anus were open by the time of hatching (Fig. 3a). The premaxilla, maxilla, and dentary were visible at 2.24 mm BL (2 DPH) (Fig. 3b). Scales began to develop on the caudal peduncle at 5.8–6.2 mm BL and 7.6–8.6 mm TL (32 DPH) and covered the whole body and dorsal side of the head at 7.6–9.8 mm BL (42 DPH). Nostrils were divided into anterior and posterior portions at 5.8–6.2 mm BL (32 DPH) (Fig. 4a). HL was 18–22% BL initially, reaching a maximum of ~34% BL after completion of notochord flexion. PAL and ED as percentage BL were 45–55% and 7–9%, respectively, with no trend with increasing BL.
Laboratory-reared larvae of Bathygobius fuscus: a newly hatched larva, KPM-NI 63294, 2.01 mm BL; b at 2 days post-hatch (DPH), KPM-NI 63295, 2.24 mm BL; c at 7 DPH, KPM-NI 63296, 2.38 mm BL; d at 12 DPH, KPM-NI 63297, 2.66 mm BL; e at 17 DPH (flexion larva), KPM-NI 63298, 3.69 mm BL; f at 27 DPH (postflexion larvae), KPM-NI 63299, 5.19 mm BL
Fin development. Newly hatched larvae possessed a pair of pectoral fin buds (Fig. 3a). Thereafter, pectoral fin rays appeared, and all rays (16) were visible and already segmented at 5.19 mm BL (27 DPH) (Fig. 3f). Pelvic fin buds appeared at 3.69 mm BL (17 DPH) (Fig. 3e). Pelvic fin rays appeared and all elements (I, 5) were visible and segmented at 5.19 mm BL (27 DPH). Emerging caudal fin rays appeared at 3.69 mm BL (17 DPH) (Fig. 3e), and all fins had formed at 5.19 mm BL (27 DPH) (Fig. 3f). Incipient second dorsal and anal fins appeared and were well elongated at 3.69 mm BL (17 DPH) (Fig. 3e). The first dorsal fin appeared at 5.19 mm BL (27 DPH) (Fig. 3f). The rays of the second dorsal fin and anal fin were segmented and reached the counts characteristic of this species by 5.19 mm BL (27DPH): second dorsal fin I, 10 and anal fin I, 8 (Fig. 3f). Likewise, the first dorsal fin had all spines (VI) at 5.19 mm BL (27 DPH) (Fig. 3f).
Pigmentation. Melanophore deposition on the eyes was complete by the time of hatching (Fig. 3a). Newly hatched larvae had a large dendritic melanophore on the yolk sac (Fig. 3a). Some melanophores on the ventral part of the abdomen were present in all specimens (Figs. 3, 4a). Internal melanophores lined the dorsal surface of the abdominal cavity around the gas bladder, but eventually became indistinguishable due to the development of pigment on the body surface (Figs. 3, 4a). Early larvae had a single row of melanophores on the dorsal region of the body between the 13th and 24th myomeres (Fig. 3a–e); the dorsal melanophore series became intermittent at 5.19 mm BL (27 DPH) (Fig. 3f), and faded at 5.8–6.2 mm BL (32 DPH) (Fig. 4a). A single row of melanophores on the ventral region of the body was also present between the posterior edge of the gas bladder and the 22nd myomere (Fig. 3); this ventral melanophore series was restricted to the area around the anal fin base in juveniles (Fig. 4). A small melanophore at the posterior angle of the lower jaw appeared at 2.19 mm BL (2 DPH). Dense melanophores appeared along the anterior edge of membranes of the second dorsal fin and anal fin after the flexion stage and then faded in juveniles (Figs. 3e–f, 4a). The number of melanophores on the head much increased at 5.8–6.2 mm BL (32 DPH) (Fig. 4a). A black transverse band appeared on the first dorsal fin in juveniles (Fig. 4). The pigment distribution pattern was similar to that of adult fish at 7.6–9.8 mm BL (42 DPH) (Fig. 4b).
Some xanthophores appeared laterally between the initial series of dorsal and ventral melanophores; the xanthophores had expanded by 3.69 mm BL (17 DPH) (Fig. 3e), but their extent diminished by 5.19 mm BL (27 DPH) (Fig. 3f).
Some erythrophores appeared ventrally near the tip of the tail after 2.66 mm BL (12 DPH) (Figs. 3d–f, 4).
Bathygobius petrophilus
General morphology. Newly hatched larvae (Fig. 5) measured 1.56–1.60 mm BL and 1.68–1.70 mm TL (n = 5), with 11 + 18 = 29 myomeres. The body was elongate and compressed, with BD ~18% BL. A circular yolk sac was visible at hatching, with YD measuring 0.21–0.25 mm (n = 5). The dorsal and ventral finfolds were of similar depth just after hatching. The gas bladder was located on myomeres 3–6 in all specimens. The anus was open but the mouth was not open at the time of hatching. HL, PAL, and ED as percentage BL were approximately 20%, 51%, and 9%, respectively.
Fin development. Newly hatched larvae possessed a pair of pectoral fin buds (Fig. 5).
Pigmentation. Melanophore deposition on the eyes was complete by the time of hatching (Fig. 5). There were melanophores on the ventral part of the abdomen from below the posterior part of the head to just posterior of the yolk sac. Additionally, internal melanophores lined the dorsal surface of the abdominal cavity around the gas bladder. There was a single row of melanophores on the dorsal region of the body between the 3rd and 21st myomeres, and another single row of melanophores on the ventral region of the body between the posterior edge of the gas bladder and the 24th myomere.
Xanthophores were present laterally on the dorsal and ventral surfaces and were more widely spread than the melanophores; additionally, a large dendritic xanthophore was present just posterior to the eyes.
Ecological note. The larvae of all three Bathygobius species hatched 4 days after fertilization. The newly hatched larvae showed positive phototaxis. Bathygobius fuscus began to settle at 5.8–6.2 mm BL (32 DPH).
Discussion
Newly hatched larvae of Bathygobius cyclopterus (this study), B. fuscus (Dotsu 1955; this study), B. petrophilus (this study), and B. soporator (Tavolga 1950; Peters 1983) share the following traits: pigment on the dorsal surface of the abdominal cavity around the gas bladder, single rows of melanophores on the dorsal and ventral regions of the body, melanophores on the ventral part of the abdomen, and xanthophores on various parts of the body. These traits may be useful to identify members of the genus.
Newly hatched larvae of the present three species and other known species of Bathygobius can be distinguished from each other by the melanophore arrangements on the dorsal region of the trunk, ventrally near the tip of the tail (separated from the single row of melanophores on the ventral region of the body), and along the lateral midline (Table 1). Bathygobius fuscus and B. soporator are difficult to differentiate but can be distinguished by their distribution (Pacific vs. Atlantic).
Comparing the early development of B. fuscus to B. soporator (Peters 1983), the timing of fin formation, changes in proportions in relation to BL, the beginning of flexion, and the timing of settlement were similar. The appearance of numerous melanophores on the head during the juvenile stage was also consistent between the two species. However, melanophores appeared along the lateral midline in the flexion stage in B. soporator but not in B. fuscus. In addition, the second dorsal fin and anal fin of B. fuscus are elongate in the flexion stage, with dense melanophores along the anterior edge of the fin membranes, unlike in B. soporator in which the fins are not elongate and the melanophores do not appear (Peters 1983).
The postflexion larvae of Bathygobius curacao and B. lacertus has also been described (Baldwin and Smith 2003; Tornabene et al. 2010). Although based on limited descriptions of its early developmental stages, B. curacao and B. lacertus share some common features of the genus: a dorsal and ventral series of melanophores, the presence of xanthophores on the body, and the presence of erythrophores ventrally near the tip of the tail.
The pelagic larval duration is variable among members of the genus Bathygobius. Aquarium experiments have reported the pelagic duration of B. fuscus to 32 days (this study) and that of B. soporator to 26–31 days (Peters 1983); estimated from otolith readings, the pelagic durations are 32–50 days for Bathygobius coalitus (Shafer 2000) and ~20 days for Bathygobius cocosensis (Thia et al. 2018; da Silva et al. 2019). Differences in larval traits including pelagic duration are caused by several factors, not least of all the environment encountered by the given species (Borges et al. 2011). Further investigation into the early development of multiple species is required to understand the early developmental mechanisms and early life ecology of Bathygobius.
In this study, we described the early development of three laboratory-reared Bathygobius species. Since laboratory-reared individuals are known to differ from wild individuals in morphology (Houde 1971; Ellis et al. 1997; Morioka et al. 2012), comparison with wild individuals is important for species identification. Dotsu (1955) described the post-settlement B. fuscus larva collected in the field. The body length and pigmentation of this post-settlement B. fuscus larva are similar to those of the postflexion larva in this study (Fig. 3f). Thus, the morphology of the laboratory-reared B. fuscus postflexion larvae described in this study is similar to that of wild specimens. However, similarities with wild specimens of other developmental stages and species have not been verified, and future comparisons are needed.
References
Akihito, Sakamoto K, Ikeda Y, Sugiyama K (2002) Gobioidei. In: Nakabo T (ed), Fishes of Japan with pictorial keys to the species, English edn. Tokai University Press, Tokyo, pp 1139–1310, 1596–1619
Arakaki S, Tokeshi M (2005) Microhabitat selection in intertidal gobiid fishes: species- and size-associated variation. Mar Biol Res 1:39–47
Arakaki S, Tokeshi M (2012) Species and size matter: an experimental study of microhabitat use under the influence of competitive interactions in intertidal gobiids. J Exp Mar Biol Ecol 418–419:59–68
Baldwin CC, Smith DG (2003) Larval Gobiidae (Teleostei: Perciformes) of Carrie Bow Cay, Belize, Central America. Bull Mar Sci 72:639–674
Borges R, Faria C, Gil F, Gonçalves EJ (2011) Early development of gobies. In: Patzner R, Van Tassell JL, Kovacic M, Kapoor BG (eds) The biology of gobies. CRC Press, Boca Raton, pp 403–464
da Silva, Wilson RS, Riginos C (2019) Rapid larval growth is costly for post-metamorphic thermal performance in a Great Barrier Reef fish. Coral Reefs 38:895–907
Dotsu Y (1955) Life history of a goby, Gobius poecilichthys Jordan et Snyder. Sci Bull Fac Agric Kyushu Univ 15:77–86
Ellis T, Howell BR, Hayes J (1997) Morphological differences between wild and hatchery-reared turbot. J Fish Biol 50:1124–1128
Houde ED (1971) Developmental abnormalities of the flatfish Achirus lineatus reared in the laboratory. Fish Bull 69:537–544
Miller PJ, Stefanni S (2001) The Eastern Pacific species of Bathygobius (Perciformes: Gobiidae). Rev Biol Trop 49:141–156
Morioka S, Chanthasone P, Phommachan P, Vongvichith B (2012) Growth and morphological development of laboratory-reared larval and juvenile three-spot gourami Trichogaster trichopterus. Ichthyol Res 59:53–62
Peters KM (1983) Larval and early juvenile development of the frillfin goby, Bathygobius soporator (Perciformes: Gobiidae). Northeast Gulf Sci 6:137–153
Shafer DJ (2000) Evaluation of periodic and aperiodic otolith structure and somatic-otolith scaling for use in retrospective life history analysis of a tropical marine goby, Bathygobius coalitus. Mar Ecol Prog Ser 199:217–229
Tanaka S, Takeda Y, Sunobe T (2023) Larvae and juveniles of three gobiid species of the genus Priolepis. Ichthyol Res 70:177–184
Tavolga W (1950) Development of the gobiid fish, Bathygobius soporator. J Morphol 87:467–492
Thia JA, Riginos C, Liggins L, Figueira WF, McGuigan K (2018) Larval traits show temporally consistent constraints, but are decoupled from postsettlement juvenile growth, in an intertidal fish. J Anim Ecol 87:1353–1363
Tornabene L, Pezold F (2011) Phylogenetic analysis of Western Atlantic Bathygobius (Teleostei: Gobiidae). Zootaxa 3042:27–36
Tornabene L, Baldwin CC, Weigt LA, Pezold F (2010) Exploring the diversity of western Atlantic Bathygobius (Teleostei: Gobiidae) with cytochrome c oxidase-I, with descriptions of two new species. Aqua Int J Ichthyol 16:141–170
Acknowledgements
We are grateful to S. Shimizu, S. Ishibashi, and R. Yazawa (Tateyama Science Center, Tokyo University of Marine Science and Technology) for their assistance throughout this study. We thank H. Senou (Kanagawa Prefectural Museum of Natural History) for receiving the deposited specimens. This work was supported in part by JSPS KAKENHI grant no. 16K07507 to T. Sunobe from the Japan Society for the Promotion of Science. We thank Cynthia Kulongowski from Edanz (https://jp.edanz.com/ac) for editing the language of a draft of this manuscript.
Funding
Open access funding provided by The University of Tokyo.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Tanaka, S., Sunobe, T. Laboratory-reared larvae and juveniles of three species of the genus Bathygobius (Gobiidae). Ichthyol Res 71, 180–186 (2024). https://doi.org/10.1007/s10228-023-00916-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10228-023-00916-2