Abstract
Pilosocereus is one of the Cactaceae family’s most relevant genera in terms of the number of species and its wide geographical range in the Americas. Within Pilosocereus, five informal taxonomic groups have been recognized, one of which is P. leucocephalus group s.s., whose phylogenetic relationships remain unresolved. Therefore, our objectives are to recognize the circumscriptions of the species in P. leucocephalus group s.s. and to corroborate the monophyly and phylogenetic relationships of this group through a set of morphological and molecular characters. This study is based on representative sampling along the broad distribution of this group in Mexico and Central America using multivariate and phylogenetic analyses. The morphological characters identified to contribute to species recognition and group formation are branch diameter, areole length, the areole length-width ratio, the distance between areoles, the length of the longest radial spine, and branch and spines colors. The chloroplast markers rpl16, trnL-trnF, and petL-psbE and the nuclear marker AT1G18270 support the monophyly of the P. leucocephalus group s.s., and two probable synapomorphies are suggested, including one transversion in rpl16 and another in petL-psbE. Together, our results demonstrate that sampled species of P. leucocephalus group s.s. encompass six species distributed in Mexico and Central America: P. alensis and P. purpusii in the western region, P. chrysacanthus and P. collinsii in the central region, and P. gaumeri and P. leucocephalus in the eastern region. A taxonomic key to recognized species is provided.
Similar content being viewed by others
Introduction
One of the main concerns of biology is to know the species diversity and to understand the limits between them. To know species limits is of great relevance because species represent the fundamental unit of study in multiple biological areas including ecology, population genetics, phylogenetic systematics, and botany among others (Duminil and Di Michele 2009; Su et al. 2015; Valencia-A 2020). In plants, there are many cases that use molecular and morphological evidence to know the boundaries between species complexes, for example, Agave L. (Asparagaceae) (Rivera-Lugo et al. 2018), Crataegus L. (Rosaceae) (Piedra-malagón et al. 2016), Epidendrum L. (Orchidaceae) (Pessoa et al. 2012), Medicago L. (Leguminosae) (Chen et al. 2021), Orinus Hitchc. (Poaceae) (Su et al. 2015), Quercus L. (Fagaceae) (Valencia-A 2020), and Stenocereus (A. Berger) Riccob. (Cactaceae) (Alvarado-Sizzo et al. 2018).
A species complex is recognized as a group of closely related species in which interspecific boundaries are unclear and are often composed of recently diverged lineages (Pinheiro et al. 2018). This occurs in the Pilosocereus aurisetus complex where recognition of divergent lineages using molecular markers don’t correspond precisely to the traditionally recognized species (Moraes et al. 2012). Another case, in the Stenocereus griseus complex, showed some genetic, ecological, and morphological differences among species (Alvarado-Sizzo et al. 2018). It most cases of species complexes, a single method may not be enough to detect the divergence of lineages. This is more difficult among recently formed species (de Queiroz 1998). Therefore, different methods and data from multiple sources increase the power to detect early stages of divergence, improving further attempts to delimitate species complexes (Leaché et al. 2009; Pinheiro et al. 2018).
In Cactaceae, there are many species complexes, probably due to variable morphological traits like the number of ribs or spines, the flower color or the size of the stems and the multiple growth forms such as the globular and globose-depressed, cylindrical, articulated or columnar (Anderson 2001; Hunt et al. 2006; Korotkova et al. 2021; Vázquez-Sánchez et al. 2012). Most of the species with a columnar growth form are members of the tribes Cereeae, Echinocereeae, and Browningieae (Anderson 2001; Hunt et al. 2006). In Cereeae, Pilosocereus Byles & G.D.Rowley is one of the genera with the widest distribution in the Americas compared to the remaining 13 genera of this tribe, which are restricted mostly to eastern South America (Barthlott et al. 2015; Hunt et al. 2006). Furthermore, Pilosocereus stands out in Cactaceae as a genus with a high number of species with 42 to 50 species (Calvente et al. 2016; Franck et al. 2019; Hunt et al. 2006).
Pilosocereus is defined as a tropical genus with a shrubby to treelike habit measuring up to 10 m tall, with species presenting abundant woolly flowering areoles near the apex of the branches. Although its name is derived (pilosus = hairy) from this latter feature, it is absent in several species, and the fruit morphology (a depressed globose, dehiscence by irregular lateral or central slits) remains the most prominent diagnostic feature (Zappi 1994). The flowers have nocturnal anthesis, nearly naked pericarpels and receptacular tubes, and few small scales and fruits with seeds measuring 1.2 to 2.5 mm long (Anderson 2001; Hunt et al. 2006; Zappi 1994). Based on a complete taxonomic revision focused on the native species of Brazil carried out by Zappi (1994), two subgenera have been recognized: Gounellea Zappi (two species), which was recently elevated to genus with the name Xiquexique Lavor, Calvente & Versieux (Lavor et al. 2020), and Pilosocereus (ca. 40 species), both of which are recognized based on different branching patterns (branching candelabriforms/erect or only at the base) and their fruit morphology (floral remnants not deeply embedded in the fruit apex, circular insertion points/floral remnants deeply embedded in the fruit apex, linear insertion points, respectively). The subgenus Pilosocereus includes five informal taxonomic groups based on habit, floral and spine morphology, and geographical distribution patterns (Hunt et al. 2006; Zappi 1994). One of these is the P. leucocephalus group, which broadly in sensu lato (s.l.) includes 13 species; P. fulvilanatus, P. magnificus, P. pachycladus, and P. ulei in Brazil, P. lanuginosus, P. polygonus, and P. royenii in the Caribbean and northern South America, and P. alensis, P. chrysacanthus, P. collinsii, P. leucocephalus, P. purpusii, and P. quadricentralis in Mexico and Central America (Table 1).
Regarding the infrageneric classification of Pilosocereus (Zappi 1994), recent phylogenetic analysis did not support this classification, although a clade including P. leucocephalus and six other species native to Mexico and Central America was strongly supported (Calvente et al. 2016), which we refer to here as the P. leucocephalus group sensu stricto (s.s.) (sensu Calvente et al. 2016). Subsequent phylogenetic studies including all species of the P. leucocephalus group s.s. obtained similar results, but P. lanuginosus and P. polygonus were recovered as members of this group and closely related to P. chrysacanthus and P. quadricentralis (Lavor et al. 2018, 2020). Nevertheless, the phylogenetic relationships within species of this group remain unknown mainly due to the lack of a wider sampling of taxa from Mexico and Central America.
Over time, there have been discrepancies in the number of recognized species inside the P. leucocephalus group s.s., ranging from seven to eleven species (Byles and Rowley 1957; Hunt et al. 2006), with notable controversies on the recognition of P. collinsii, P. cometes, and P. gaumeri, suggesting P. collinsii as a synonym of P. purpusii, P. cometes of P. leucocephalus, and P. gaumeri of P. royenii (Anderson 2001; Hunt et al. 2006; Zappi 1994). As a consequence of these uncertain interspecific boundaries, there is also confusion in recognizing their geographical ranges, but the distributional pattern of this group is well known, extending from the eastern and western coasts of Mexico to Central America (Barthlott et al. 2015; Guzmán et al. 2003; Yetman 2007) (Fig. 1).
Recent studies have expanded the knowledge of Pilosocereus species in the Caribbean and northern South America (Calvente et al. 2016; Franck et al. 2019), however, no solid knowledge of systematics inside the Pilosocereus leucocephalus group s.s. (Calvente et al. 2016) is available. Because we only have the taxonomic contributions made by Byles and Rowley (1957) and the recent phylogenetic study by Calvente et al. (2016), which used a low number of samples within this group, the need of a broader sampling in this particular group is evident, including the use of morphological and molecular characters to better understand their phylogenetic relationships and species delimitation. Therefore, the objectives of this study are (1) to evaluate the circumscriptions of the species in the P. leucocephalus group s.s. (Calvente et al. 2016) and (2) to test monophyly and expand knowledge regarding phylogenetic relationships among this group using a set of morphological (vegetative and reproductive) and molecular characters (three plastid and one nuclear markers) as evidence.
Materials and methods
Taxon sampling
The focal group of this study is the recognized species of Pilosocereus leucocephalus group s.s. (Calvente et al. 2016) from Mexico and Central America—P. alensis, P. chrysacanthus, P. collinsii, P. cometes, P. gaumeri, P. leucocephalus, P. purpusii, and P. quadricentralis—based on recent taxonomic syntheses (Franck et al. 2019; Guzmán et al. 2003; Hunt et al. 2006) and a phylogenetic study of the genus Pilosocereus (Calvente et al. 2016). The geographical distribution of the species of Pilosocereus leucocephalus group s.s. is shown in Fig. 1. As outgroup for the phylogenetic analysis, we included some species of the Cereeae tribe and Pilosocereus species from South America. The functional outgroup is represented by Browningia hertlingiana and Lasiocereus fulvus, both early-divergent members in expanded Cereeae (BCT clade) (Hernández-Hernández et al. 2011; Lendel 2013).
Data collection from herbaria and field work
For the development of this research, 453 herbarium specimens from Instituto Politécnico Nacional, Durango (CIIDIR), Instituto Politécnico Nacional, Mexico City (ENCB), Universidad Nacional Autónoma de México, Mexico City (FCME), Herbario del Centro de Investigación en Alimentación y Desarrollo, A.C. (HCIAD), Universidad de Guadalajara (IBUG), Universidad Nacional Autónoma de México, Iztacala (IZTA), Herbario Nacional de México, Mexico City (MEXU), Instituto Politécnico Nacional, Oaxaca (OAX), Universidad Autónoma Metropolitana Iztapalapa, Mexico City (UAMIZ), Universidad Autónoma de Tamaulipas (UAT), University of Arizona (ARIZ), Harvard University (GH), The New York Botanical Garden (NY), California Botanic Garden (RSA), Universidad Nacional Autónoma de Honduras (TEFH), and Smithsonian Institution (US) were examined. Subsequently, extensive field work including all the species recognized in the P. leucocephalus group s.s. was carried out; a set of specimens was deposited in the Living Collection of the Botanical Garden, Instituto de Biología UNAM, while the voucher specimens were deposited in MEXU. At each collection site, photographs were taken with reference scales for a set of 21 morphological characters, and observations and complementary measurements were performed on the specimens deposited in the Botanical Garden (Table 2, Fig. 2). We created a database using geographic information from herbaria and our field records to construct a map of the geographical distribution of each species in ArcGIS v.10.5 (Fig. 1).
Morphological characters of Pilosocereus leucocephalus group s.s. analyzed. The abbreviations are defined in Table 2. a, b) P. collinsii (DFE 67), c) P. leucocephalus (DFE 60), d) P. collinsii (DFE 40), and e, f) P. chrysacanthus (DFE 36)
Multivariate analysis
The evaluated morphological characters of mature plants are shown in Table 2, 16 of which were quantitative and five qualitative. The quantitative traits of branches and flowers were measured in the program ImageJ v.1.52a based on our field and greenhouse photographs. For seed measurement, a Celestron Handheld Digital Microscope with the program MicroCapture Pro v.2.4.1 was used. The arithmetic mean of branch characters was obtained by measuring three adult individuals per locality with ten measurement replicates for all samples. A total of 39 localities were sampled to measure branch characters, and for flower and seed characters, we used different sample sizes (Table S1).
Prior to our multivariate analyses, we performed the Royston test in R v.4.0.2 (R Core Team 2013) with the package MVN v.5.8 (Korkmaz et al. 2014) to evaluate whether our data had a multivariate normal distribution. Because our data did not fulfill this assumption, we transformed them into natural logarithm values to improve the distribution and homogenize their variances. Pearson correlation coefficients were calculated for the quantitative characters in R with the package corrplot v.0.84 (Wei and Simko 2017) to identify and exclude strongly correlated characters with a value greater than 0.6 (Akoglu 2018), which could lead to overestimation of the results.
Principal component analyses (PCAs) were carried out to first determine the weights of the characters contributing to the group’s formation and to show the similarities between localities and variables as points on a plane, using the function prcomp in R. The first PCA was performed using only the measurements of seven vegetative branch traits from 39 localities because the reproductive traits of flower and seed could not be obtained either in the field or in cultivation for all the localities. The second PCA was performed with 13 traits (vegetative and reproductive) from branches, flowers, and seeds for 14 localities.
Furthermore, PCAs were carried out with mixed data (numerical and categorical) to include qualitative characters with taxonomic importance, using the function PCAmix in the R package PCAmixdata v.4 allowing to perform a PCA of mixed quantitative and qualitative data (Chavent et al. 2017). The merged data table to be analyzed by PCAmixdata comprises n localities described by p1 numerical variables and p2 categorical variables. A mixed PCA was performed with the quantitative (only vegetative) and qualitative traits combined for 39 localities and another PCA mix based on the quantitative (vegetative and reproductive) and qualitative traits combined for 14 localities. The qualitative traits were habit, areole shape, colors branch at the apex and spine at the branch apex, and fertile part disposition (Table 3).
Finally, linear discriminant analysis (LDA) was performed to evaluate the known group classification a priori. For LDA, the package MASS v.7.3 (Ripley et al. 2013) was used in R. Only quantitative data were used in this analysis based on seven vegetative traits from 39 localities.
DNA extraction, amplification, and sequencing
We amplified the chloroplast markers rpl16, trnL-trnF, and petL-psbE, and the nuclear marker AT1G18270 in 54 individuals of P. leucocephalus group s.s. from Mexico and Central America. For each locality, we obtained the DNA sequences for one to three individuals (Appendix S1).
Tissue samples from approximately 1-cm3 specimens were silica gel dried, frozen and pulverized for DNA extraction following the CTAB method (Doyle and Doyle 1987) with the modifications reported by Bustamante et al. (2016) to avoid mucilage excess. After extraction, the total genomic DNA was stored at −20 °C. PCRs were performed in volumes of 15 µL using the commercial mix “Platinum Taq” (Invitrogen). The reactions included 1.5 µL (1×) of 10× PCR buffer, 0.3 µL of BSA (0.4 %), 0.3 µL of dNTP mix, 0.2 µL of each primer (10 pmol µL−1), 0.5 µL of MgCl2 (1.5 µM), 0.075 µL (0.375 units) of Taq DNA polymerase, 0.5–0.7 µL template DNA and water to reach the final volume. The following primers and thermal cycle profiles were used. For the rpl16 intron, the primers rpl161F and rpl163R (Hernández-Hernández et al. 2011) with a temperature profile of 94 °C for 5 min; 30 cycles of 94 °C for 1 min, 55 °C for 50 s, and 72 °C for 2 min; and a final extension of 72 °C for 4 min. For the trnL-trnF intergenic spacers, we used the primers c, d, e, and f (Taberlet et al. 1991) with a temperature profile of 94 °C for 2 min; 29 cycles of 94 °C for 30 s, 52 °C for 30 s, and 72 °C for 1 min; and a final extension of 72 °C for 7 min. For the petL-psbE intergenic spacer, we used the primers petL and psbE (Shaw et al. 2007) with a temperature profile of 94 °C for 2 min; 30 cycles of 94 °C for 1 min, 52 °C for 30 s, and 72 °C for 1 min; and a final extension of 72 °C for 5 min. For the AT1G18270 intron, we used the c primers (Granados-Aguilar et al. 2021) with a temperature profile of 94 °C for 2 min; 36 cycles of 94 °C for 32 s, 56.5 °C for 30 s, and 72 °C for 1 min and 10 s; and a final extension of 72 °C for 5 min. Finally, the PCR cleaning and Sanger sequencing was performed at the Laboratorio de Biología Molecular de la Biodiversidad y de la Salud, Instituto de Biología, UNAM.
Sequence edition and alignment
Sequences were assembled in Sequencher v.5.4. Then, matrices were generated for each marker, prealigned with Muscle v.3.8 (Edgar 2004) and manually adjusted in PhyDE v.0.9971 (Müller et al. 2005), and a 156-bp highly variable site (nonalignable) only in the nuclear region was excluded. Insertion and deletion events (indels) were identified and coded according to the simple coding method (Simmons and Ochoterena 2000).
Phylogenetic analyses
To determine if there was incongruence between the chloroplast and nuclear data, we performed in PAUP* v.4.0 (Swofford 2003) an incongruence length difference test (ILD) (Farris et al. 1994). Partitions for each marker were designated and we performed a heuristic search with 1,000 homogeneity replicates. We organized our data into three matrices to carry out phylogenetic analyses. The first matrix includes 74 terminals for three chloroplast markers, the second matrix includes the same terminals for the chloroplast markers plus one nuclear marker, and the third includes 64 terminals for the chloroplast and nuclear markers plus morphological data of the P. leucocephalus group s.s. (Appendix S2).
Molecular phylogenies were reconstructed using probabilistic methods. For Bayesian inference analysis (BI), five partitions were required: four for DNA and one for indels. For DNA partitions, the molecular model of evolution was estimated using the Bayesian Information Criterion (BIC) as implemented in jModelTest v.2.1 (Darriba et al. 2012), and for indels partition, a restriction site model was used according to Ronquist et al. (2011). The BI analysis was performed in MrBayes v.3.2 (Ronquist et al. 2012) and consisted of two independent runs of four chains for 10,000,000 generations, sampling one tree each 1,000 generations, and starting with one random tree. In the Markov Chain Monte Carlo (MCMC) search, 25 % of the initial trees were discarded as burn-in, and with the remaining trees, a majority-rule consensus tree with nodal posterior probabilities (PP) was generated. A maximum likelihood analysis (ML) was performed in the chloroplast and chloroplast plus nuclear marker matrices in RAxML v.8.2 (Stamatakis 2014) using the default model GTR +G model and 10,000 bootstrap support (BS).
A total evidence analysis was performed under maximum parsimony (MP) and BI using molecular and morphological characters. The 16 quantitative morphological characters measured from branches were evaluated by ANOVA followed by the Tukey test to obtain feature intervals for each measured character using the package agricolae v.1.3 (de Mendiburu 2013) in R. Finally, 12 morphological characters were incorporated into the phylogenetic analysis. The MP analysis was performed in PAUP* v.4.0a168 (Swofford 2003) with a heuristic search of 1,000 random addition replicates and tree-bisection-reconnection (TBR) branch swapping and the option MulTrees, and the best score trees among 10 to 40 of the most parsimonious trees were filtered. The MaxTrees option was set at 100,000. Bootstrap analyses were performed using 1,000 replicates with TBR branch swapping and simple addition sequences. The MaxTrees option was set at 1,000 to avoid entrapment in local optima. For BI analysis, the same parameters described above were used with incorporation of the Mk model for morphological characters (Lewis 2001). Trees obtained from phylogenetic analyses were edited in FigTree v.1.4.2 (Rambaut 2014).
Results
Morphological analysis
The measured morphological characters are detailed in Fig. 2. The following characters were excluded due to their strong correlations with others: branch diameter, rib height, rib width, rib distance, areole length, areole width, flower length, style length, and seed width (Fig. S1). Furthermore, for subsequent analyses, we calculated the rib width-distance, areole length-width, and seed length-width ratios.
We performed a PCA whereby population groups were determined using seven branch traits in 39 localities (Fig. 3a). The first two components explained 63.7 % of the variation. The traits with the highest weights in the first component were the length of the longest radial spine and areole length, while those in the second component were branch diameter and the areole length-width ratio (Table S2). In the PCA performed for 14 localities with 13 traits from branches, flowers, and seeds, we obtained 51.6 % for the explained variation, and we excluded P. quadricentralis due to a lack of flowers or seeds by locality (Fig. 3b).
Scatter plots of the principal component analysis (PCA) of quantitative characters in Pilosocereus leucocephalus group s.s. a) PCA based on seven quantitative characters in specimens from 39 localities and b) PCA based on the vegetative and reproductive characters of 13 quantitative variables in specimens from 14 localities. Numbers refer to the ID of Table S1
The combined analysis of quantitative and qualitative traits in 39 localities (mixed PCA, Fig. 4a) revealed that the first two components explain 52.9 % of the variation, where the traits with the highest weights on the first component were the branch diameter, fertile part distribution, and branch color at the apex, while for the second component, they were the spine color at the branch apex, the distance between areoles, and areole length (Table S3). In the mixed PCA performed for 14 localities using 13 quantitative traits from branches, flowers, and seeds plus five qualitative traits, we obtained a similar result to the analysis of the 39 localities which form six species groups (Fig. 4b). The qualitative character states are shown in Table 3.
Scatter plots of the principal component analysis of mixed data (PCA mix) for quantitative and qualitative characters of Pilosocereus leucocephalus group s.s. a) PCA mix based on seven quantitative characters and five qualitative characters in specimens from 39 localities and b) PCA based on the vegetative and reproductive characters of 13 quantitative and five qualitative variables in specimens from 14 localities. Numbers refer to the ID of Table S1
The results derived from the LDA revealed that 100 % of P. alensis, P. chrysacanthus, P. collinsii, P. gaumeri, and P. purpusii individuals were correctly assigned and therefore classified in their own species, while only 91.6 %, 88.8 %, and 33.3 % of P. leucocephalus, P. quadricentralis, and P. cometes individuals were correctly assigned, respectively (Table 4). The characters that best discriminate between the species of P. leucocephalus group s.s. are areole length-width ratio, areole length, branch diameter, and distance between areoles (Table S4).
Phylogenetic analyses
Phylogenetic analyses included 74 terminals with 263 sequences, 237 of which were new sequences, while 26 were sequences from GenBank, mainly from the previous work of Calvente et al. (2016) and Schlumpberger and Renner (2012) (Appendix S1). According to the ILD test, no significant incongruence was found between the plastid and nuclear data (P = 0.49), thus all markers were concatenated and analyzed combined. The concatenate matrix with all markers consisted of 3,265 nucleotides and 19 indels, 83 of which were informative (2.54 %), with a greater number of informative characters in rpl16 (27). Finally, the 19 indels mainly included insertions and deletions with only one inversion. Data of molecular evolution models for each marker and the concatenate matrix as well as the number of indels are shown in Table S5.
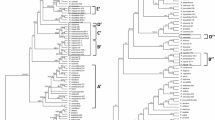
Our results in the analysis performed under BI and ML were highly congruent, the Bayesian majority-rule consensus tree in Fig. 5 has only plastid markers whereas the tree in Fig. 6 also includes a nuclear gene. The genus Pilosocereus is recovered as monophyletic with high to moderate support (Fig. 5; 1 PP/75 % BS). Within Pilosocereus, two main clades can be recognized—one with the species included from Brazil: P. aureispinus, P. pachycladus, and P. vilaboensis (Fig. 5; 0.68 PP/− BS); and the second with all species from Mexico and Central America highlighted as P. leucocephalus group s.s., which are closely related to P. brooksianus, P. millspaughii, and P. robinii from the Caribbean and to P. moritzianus from northern South America (Fig. 5; 0.54 PP/87 % BS). P. leucocephalus group s.s. is monophyletic with high to low support (Fig. 5; 1 PP/66 % BS). This clade includes 54 terminals and within this were recovered at species level three clades: P. alensis (Fig. 5; 0.58 PP/89 % BS), P. gaumeri (Fig. 5; 0.7 PP/67 % BS), and P. purpusii (Fig. 5; 1 PP/73 % BS).
Majority-rule consensus tree of 15,002 trees resulting from the Bayesian analysis based on the concatenation of three chloroplast DNA markers (rpl16, trnL-trnF, petL-psbE) and indels. The value at the node corresponds to PP/BS from the BI/ML analyses. The dotted line delimits Pilosocereus leucocephalus group s.s., and within the group, the names of the species that recover under the criterion of reciprocal monophyly are highlighted
Majority-rule consensus tree of 15,002 trees resulting from the Bayesian analysis based on the concatenation of three chloroplast DNA markers (rpl16, trnL-trnF, petL-psbE), indels, and one nuclear marker (AT1G18270). The value at the node corresponds to PP/BS from the BI/ML analyses. The dotted line delimits Pilosocereus leucocephalus group s.s., and within the group, the name of the species that recovers under the criterion of reciprocal monophyly is highlighted
The majority-rule consensus tree for three plastid markers and one nuclear marker showed similar results to those obtained with only plastid markers. P. leucocephalus group s.s. is monophyletic with high support in BI (Fig. 6; 0.98 PP/− BS), and within this clade, only P. purpusii is recovered as monophyletic with high to moderate support (Fig. 6; 1 PP/77 % BS). In the analyses performed with only plastid markers and plastid plus nuclear markers, within the P. leucocephalus group s.s., P. chrysacanthus, P. collinsii, P. cometes, P. leucocephalus, and P. quadricentralis were not recovered as monophyletic.
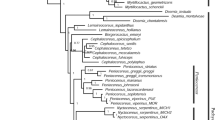
For the total evidence analysis using molecular and morphological characters, P. leucocephalus group s.s. was recovered with high support only in the Bayesian analysis (Fig. 7; 0.91 PP). Within this group, six clades at the species level were recovered with P. cometes nested in P. leucocephalus as well as P. chrysacanthus and P. quadricentralis in the same clade. The first clade was integrated by all terminals for P. leucocephalus and P. cometes (Fig. 7; 0.81 PP) and is sister to the remaining clades. The P. chrysacanthus-P. quadricentralis clade (Fig. 7; 51 % BS/0.63 PP) is sister to the clades P. alensis (Fig. 7; 92 % BS/87 % JK/1 PP), P. collinsii (Fig. 7; 91 % BS/84 % JK/1 PP), P. gaumeri (Fig. 7; 63 % BS/63 % JK/0.9 PP), and P. purpusii (Fig. 7; 77 % BS/67 % JK/1 PP), which were recovered as monophyletic. The relationships between species do not have support. Last, for the P. leucocephalus group s.s., two putative synapomorphies can be recognized, including a transversion in rpl16 (T → A) and another in petL-psbE (C → G). At a more inclusive level, we recognized only autapomorphies in four species: for P. alensis, the long hairs (7–12 cm) and a deletion of 22 bp in trnL-trnF; for P. purpusii, an insertion of 4 bp in trnL-trnF and a transversion in petL-psbE (C → A); for P. gaumeri, an insertion of 7 bp in rpl16; and in P. collinsii, the areole length-width ratio and elliptical areoles.
Strict consensus tree of the 31 most parsimonious trees resulting from the MP analysis based on molecular (rpl16, trnL-trnF, petL-psbE, AT1G18270, and indels) and morphological data (five quantitative and seven qualitative characters). The value at the top branches corresponds to BS/JK from the MP analysis, at the bottom branches corresponds to PP from the IB analysis, and a dash indicates a lack of support. The dotted line delimits Pilosocereus leucocephalus group s.s., and the colors of the terminals denote the taxa according to Guzmán et al. (2003)
Discussion
Recognition of species with morphological attributes
Based on our results, six species are recognized as having the following combinations of characters. 1) P. alensis is characterized by shrubby habit with small areoles (2–3 mm), orange-brown spines, long hairs (7–15 cm), and large seeds (approximately 2.5 mm long). It is distributed in western Mexico, from Sonora to Jalisco. 2) P. chrysacanthus comprises treelike habit with branches measuring 7.5–12 cm in diameter, usually yellow spines, short hairs (2.5–5 cm) and flowers measuring 6 to 10 cm long. It is distributed in southern Mexico in Guerrero, Morelos, Oaxaca, and Puebla. 3) P. collinsii includes shrubby habit with elliptical areoles, a distance between areoles of 1.7–2.2 cm and dark brown spines. It is distributed in southern Mexico in Chiapas, Guerrero, and Oaxaca. 4) P. gaumeri presents treelike habit with branches measuring 4.1–5.2 cm in diameter, light green branches, and noticeably short hairs (1.7–2.3 cm). It is distributed in southeastern Mexico, in Campeche and Yucatán. 5) P. leucocephalus presents shrubby and treelike habit, usually shrubs, with branches measuring 9 to 14 cm in diameter, high ribs (1.8–3.5 cm), and hairs of 4 to 8 cm in length. It is distributed from eastern Mexico (Chiapas, Querétaro, San Luis Potosí, Tamaulipas, and Veracruz) to Central America (El Salvador, Guatemala, Honduras, and Nicaragua). 6) P. purpusii is characterized by shrubby habit with branches measuring 6 to 9 cm in diameter, low ribs (7–16 mm), areole lengths of approximately 3 mm, and yellow spines. It is distributed in western Mexico, from Sinaloa to Guerrero (see the geographical distribution of all taxa in Fig. 8). For further information, see the taxonomic key after the taxonomic treatment.
For species recognition, P. collinsii has been considered synonymous with P. purpusii because they share some characters, including a shrubby habit and similar branch diameters, radial spine lengths and rib heights (Anderson 2001; Yetman 2007; Zappi 1994). Our results confirm that they are different species. Based on the PCA, the characters with the highest weights (loadings) contributing to different groups are those associated with the areole, distance between areoles, and colors of spines (Tables 3, 4), and in the LDA, the 18 sampled P. collinsii individuals were all correctly classified in this species. Similarly, for P. purpusii, the 15 sampled individuals were all correctly classified (Table 4). P. gaumeri had previously been designated as synonym for P. royenii because they share the treelike habit, yellow spines, and flowers of approximately 7 cm long (Anderson 2001; Barthlott et al. 2015; Hunt et al. 2006; Zappi 1994). However, according to Franck et al. (2019), they are different species considering branching (divergent/ascending to strict), stem thickness (slender/thick), and fruit color (purple/red). Our morphological measurements in P. gaumeri which agree with the diagnostic characters presented by Franck et al. (2019), including the hair length of 1.7 to 2.3 cm for P. gaumeri while P. royenii has hairs up to 4 cm long. Therefore, these remarkable morphological differences between P. gaumeri and P. royenii allows us to support them as different species.
On the other hand, previous knowledge of P. cometes and P. leucocephalus shows that the main difference between them is the length of hairs, which is short (2 cm) in P. cometes and long (10 cm) in P. leucocephalus, thus considering them to be independent species (Bravo-Hollis 1978; Britton and Rose 1920; Byles and Rowley 1957; Guzmán et al. 2003). However, in this study, we found hair lengths from 4 to 8 cm for both taxa, and the LDA results show that only 2 of 6 P. cometes individuals sampled were correctly classified in this species, including an P. chrysacanthus individual and three P. leucocephalus individuals. Conversely, 22 of 24 sampled P. leucocephalus individuals were correctly classified in this species, including one P. chrysacanthus individual and one P. cometes individual (Table 4). These results support the previous proposal to consider P. cometes as a synonym of P. leucocephalus (Anderson 2001; Hunt et al. 2006; Zappi 1994). For P. chrysacanthus and P. quadricentralis, the main differences were yellow spines in P. chrysacanthus and orange-brown spines in P. quadricentralis, without recognition of central spines in P. chrysacanthus and up to four spines in P. quadricentralis, and the distances between areoles were approximately 1 cm in P. chrysacanthus and usually 1.5–1.7 cm in P. quadricentralis (Anderson 2001; Bravo-Hollis 1978; Hunt et al. 2006). Nevertheless, based on the results of mixed PCA, no clear distinction was evident between P. chrysacanthus and P. quadricentralis (Fig. 4a), and the LDA showed that 1 of 9 P. quadricentralis individuals was classified as P. chrysacanthus, while all the sampled (18) P. chrysacanthus individuals were correctly classified in this species (Table 4). Therefore, here, these species are considered a unique entity with shades of yellow to orange-brown coloration in spines, 1–4 central spines on the middle part of mature branches, and a distance between areoles of 1.2 to 2.2 cm, in addition to an areole size of 3.5 to 5.5 mm length and 3 to 5 mm width.
This study reveals that the morphological variation among the species of Pilosocereus distributed in Mexico and Central America is very narrow or has overlapping ranges; for example, a diameter of branches between 7.5 and 14.5 cm, a length of longer radial spines between 1 and 2 cm, an areole length from 3.5 to 5.3 mm, and a distance between areoles from 1.3 to 2.3 cm overlap between P. chrysacanthus (= P. quadricentralis) and P. leucocephalus (= P. cometes). Nevertheless, the contributions of five qualitative characters with taxonomic importance allowed us to maximize the differences between groups and showed a clear distinction between P. collinsii and P. purpusii (Fig. 4a). In some groups of Cactaceae, several morphological characters with continuous variation have been recognized between related species, but only some characters offer information to recognize their circumscription at the species level, as in, for example, Cylindropuntia (C. multigeniculata and C. whipplei: Baker 2016), Echinocereus (E. acanthosetus and E. pulchellus: Sánchez et al. 2020), and Escobaria (E. guadalupensis and E. sneedii: Baker and Johnson 2000). Similarly, in the study group, a gradation can be observed for branch diameter and the distance between areoles, but notably, P. leucocephalus group s.s. species have areas of distribution that do not overlap, namely, they are almost completely allopatric (Fig. 8). Regarding reproductive structures, the seed is revealed as potentially informative because the length of the hilum-micropylar region is among the weightiest characters in the PCA for species group formation. Future studies on seed morphology may provide more information on its taxonomic value, as has been recognized for other groups of cacti (e.g., Melocactus: Lemus-Barrios et al. 2021; Stenocereus: Arroyo-Cosultchi et al. 2006).
Monophyly in the P. leucocephalus group s.s.
Sampled members of P. leucocephalus group s.s. were recovered in a monophyletic clade with high to low support (Figs. 5–7), with 54 terminals incorporated into the analysis and six clades at the species level distributed in Mexico and Central America (Fig. 8). According to this result, the monophyly of P. leucocephalus group s.s. reported by Calvente et al. (2016) is corroborated. For this group, two base pairs were recognized as putative synapomorphies, including one in rpl16 (an A in site 729 from the alignment) and one in petL-psbE (a G in site 277 from the alignment), and a combination of morphological characters indicated the taxonomic treatment section.
Based on our results and following the regionalization of the neotropical region (Morrone 2014), the members of P. leucocephalus group s.s. included in this study are distributed in the Mesoamerican dominion and are suggested to belong to a Mesoamerican clade that is sister to the species from the Caribbean and northern South America included in our analyses, including P. brooksianus, P. millspaughii, P. moritzianus, and P. robinii sensu Franck et al. (2019). Previous phylogenetic analyses focused on Pilosocereus mainly from South America (Calvente et al. 2016), reported that P. leucocephalus group s.s. was integrated by P. alensis, P. purpusii, P. leucocephalus, P. collinsii, P. chrysacanthus, P. quadricentralis, and P. gaumeri (as P. royenii: mistaken name; for more details, see Franck et al. (2019)). In subsequent analyses (Lavor et al. 2020) including more species, a clade integrated by P. alensis, P. chrysacanthus, P. collinsii, P. gaumeri, P. lanuginosus, P. leucocephalus, P. polygonus, P. purpusii, and P. quadricentralis was recovered, which was named a non-Brazilian species clade, and the former name—P. leucocephalus group s.s.—was omitted. Thus, although our results are in agreement with those of Calvente et al. (2016), the inclusion of a higher number of Caribbean and northern South American species can help to define whether P. leucocephalus group s.s. recovers as a natural group or is only an artifact of an artificial classification, as observed for the rest of the informal Pilosocereus groups (Calvente et al. 2016). Furthermore, wider sampling of non-Brazilian Pilosocereus species may help to clarify whether the Mesoamerican clade and the species from the Caribbean and northern South America are sister groups or whether the Mesoamerican clade is nested within the clade AII or non-Brazilian clade (Lavor et al. 2018, 2020).
Interestingly, similar results to those obtained for P. leucocephalus group s.s. have been recorded in other groups of organisms with wide geographical distribution in the Americas, recognizing monophyletic groups that are distributed in the Mesoamerican dominion. For instance, among plants, Granados et al. (2017) found that in Poales, the genus Tillandsia L. subg. Tillandsia (which belongs to the clade K in the study with 82 spp.) shows a similar pattern in North and Central America. The same pattern is observed in the genus Zamia L. (Cycadales) (Calonje et al. 2019) with a group of 18 species restricted to the Mesoamerican dominion. While in animals, inside the genus Sturnira Gray (Chiroptera) the S. parvidens lineage from tropical areas of Mexico to northern Costa Rica is closely related to their South American sisters (Hernández-Canchola and León-Paniagua 2017). Similarly, in the genus Alouatta Lacépède (Primates) a Mesoamerican clade is strongly supported, comprising A. paliatta and A. pigra (Doyle et al. 2021).
Internal relationships in P. leucocephalus group s.s.
The monophyly of P. leucocephalus group s.s. is a corroborated hypothesis, but the interrelationships of its species remain quite ambiguous. For example, in the work of Calvente et al. (2016), the authors report P. gaumeri as a sister to the remaining species, whereas in Lavor et al. (2018), P. gaumeri and P. collinsii are sisters of two clades, one supporting the relationships of P. leucocephalus with P. purpusii and P. alensis and other with P. chrysacanthus (= P. quadricentralis). In both works, P. gaumeri was a basal terminal, and a close relationship was identified between P. leucocephalus, P. purpusii, and P. alensis. A more recent analysis showed basal polytomy in P. leucocephalus group s.s. (non-Brazilian species) and maintenance of the aforementioned relationships, although an additional terminal of P. leucocephalus is a sister to P. gaumeri (Lavor et al. 2020). In our study, we were not able to recover the relationships within the group using only molecular markers, but notably, the molecular data are consistent with the morphological recognition of P. alensis, P. gaumeri, and P. purpusii. The problem of the poor resolution of phylogenetic relationships between species is probably associated with the recent divergence of P. leucocephalus group s.s. (a mean divergence of 0.90 million years with an interval of 1.77–0.31; Lavor et al. 2018), incomplete lineage sorting and/or long generational times. Similar situations occur in other plant groups, such as Myosotis (Meudt et al. 2015), Astragalus (Bagheri et al. 2017), and Agave (Jiménez-Barron et al. 2020), which also show recent diversification, which complicates determination of the phylogenetic relationships between their species.
On the other hand, when jointly analyzing morphological and molecular characters, we found clear recognition of six species and their probable internal relationships within P. leucocephalus group s.s. This analysis allows us to infer the lineages P. alensis, P. chrysacanthus (including P. quadricentralis), P. collinsii, P. gaumeri, P. leucocephalus (including P. cometes), and P. purpusii at the species level. Within P. leucocephalus group s.s., the relationships lack support and differ from the results obtained in previous works (Calvente et al. 2016; Lavor et al. 2018, 2020), mainly because we identified P. leucocephalus as the sister species to the remaining species constituting the group, and P. collinsii appears to be the sister species of P. gaumeri.
In conclusion, the group Pilosocereus leucocephalus s.s. distributed in Mexico and Central America is supported as a monophyletic group, in which we recognize six species based on morphological and molecular characters. The most important morphological characters that contribute to the formation of groups and in being able to correctly discriminate between certain species are areole length, branch diameter, distance between areoles, and spines colors, for which its potential use in other Pilosocereus species is suggested. Regarding molecular characters, only P. alensis, P. gaumeri, and P. purpusii were recovered with reciprocal monophyly using chloroplast markers, although by including a nuclear marker only P. purpusii was recovered. Given the uncertainty in the taxonomic circumscription of the closely related species in this group with the previous suggestion of a likely recent divergence, the combination of morphological and molecular characters offers good results in the delimitation of its species and reveals as one same species P. chrysacanthus and P. quadricentralis as well as P. cometes and P. leucocephalus, while P. collinsii and P. purpusii turned out to be distinct species, and P. gaumeri closely related to the Mesoamerican species, differing from the Caribbean. For future research, we suggest that other unexplored characters should be evaluated, such as chromosome numbers or anatomical information, and conduct genomic or microsatellite analyses to broaden our knowledge of this rather complex group.
Taxonomic treatment
The P. leucocephalus group s.s. occurring in Mexico and Central America is characterized here with amendment of the proposal made by Hunt et al. (2006) as follows: shrubby cacti, very rarely treelike, between 3.5 and 10 m high; thick branches, not very woody except at the base, often glaucous, usually 7 to 15 ribs; few to many spines, mostly thick, differentiated into central and radial; weakly differentiated floriferous areoles with long dense tufts of hairs; medium to large flowers with straight tubes, medium to large seeds (rarely small), and smooth and shiny cuticles.
Based on the results of this study, we recognize the following six species in the P. leucocephalus group s.s.:
-
1.
Pilosocereus alensis (F.A.C.Weber) Byles & G.D.Rowley, Cact. Succ. J. Gr. Brit. 19: 66. 1957. ≡ Pilocereus alensis F.A.C.Weber ex Rol.-Goss., Bull. Mus. Hist. Nat. (Paris) 11(6): 508 (−509). 1905. ≡ Cephalocereus alensis (F.A.C.Weber) Britton & Rose, Contr. U.S. Natl. Herb. 12: 415. 1909. ≡ Cereus alensis Vaupel, Monatsschr. Kakteenk. 23: 23, 24, 83. 1913. TYPE: Mexico, Jalisco, Sierra del Alo, near Manzanillo, L.Diguet s.n. (holotype: P?; isotypes: US?, RB 00537920!).
-
2.
Pilosocereus chrysacanthus (F.A.C.Weber ex K.Schum.) Byles & G.D.Rowley, Cact. Succ. J. Gr. Brit. 19: 66. 1957. ≡ Pilocereus chrysacanthus F.A.C.Weber ex K.Schum., Gesamtbeschr. Kakt. 178. 1897. ≡ Cereus chrysacanthus Orcutt, W. Amer. Sci. 13: 63. 1902. ≡ Cephalocereus chrysacanthus Britton & Rose, Contr. U.S. Natl. Herb. 12: 416. 1909. ≡ Cephalophorus chrysacanthus (F.A.C.Weber) Boom, Succulenta (Netherlands) 46: 107. 1967. TYPE: Mexico, near Tehuacán, Weber s.n. (not preserved). NEOTYPE (designed by Zappi, Succ. Pl. Res. 3: 144. 1994): Mexico, Puebla, Tehuacán, 30 Aug to 08 Sep 1905, J.N.Rose, J.H.Painter & J.S.Rose 9993 (neotype: US 00170926!; isoneotype: NY 02256593!).
= Pilocereus tehuacanus Weing., Z. Sukkulentenk. 3: 58. 1927. ≡ Cephalocereus tehuacanus (Weing.) Borg, Cacti (Borg), ed. 2. 150. 1951. ≡ Pilosocereus tehuacanus (Weing.) Byles & G.D.Rowley, Cact. Succ. J. Gr. Brit. 19: 69. 1957. TYPE: Mexico, Puebla, Tehuacán area, C.A.Purpus s.n., 1907 (not preserved).
= Cephalocereus quadricentralis E.Y.Dawson, Allan Hancock Found. Publ. Occas. Pap. 1: 14, tab. 3, fig. 5. 1948. ≡ Pilosocereus quadricentralis (E.Y.Dawson) Backeb., Cactaceae (Backeberg) 4: 2437. 1960. TYPE: Mexico, Oaxaca, east of Oaxaca-Chiapas, Pan-Pacific Highway, 1,000 m, 25 Jan 1947, E.Y.Dawson 3004 (holotype: AHH 8259). Note: holotype transferred to RSA 0008868!. Synon. nov.
-
3.
Pilosocereus collinsii (Britton & Rose) Byles & G.D.Rowley, Cact. Succ. J. Gr. Brit. 19: 66. 1957. ≡ Cephalocereus collinsii Britton & Rose, Cactaceae (Britton & Rose) 4: 269, fig. 242. 1923. ≡ Pilocereus collinsii (Britton & Rose) F.M.Knuth, Kaktus-ABC [Backeb. & Knuth] 330. 1936. LECTOTYPE (designed by Zappi, Succ. Pl. Res. 3: 150. 1994): Mexico, Oaxaca, Tehuantepec [O.F.Cook & G.N.Collins s.n., 1902] (lectotype: US 00115537!; isolectotypes: NY 00118700!, 00120552!).
-
4.
Pilosocereus gaumeri (Britton & Rose) Backeb., Cactaceae (Backeberg) 4: 2462. 1960. ≡ Cephalocereus gaumeri Britton & Rose, Cactaceae (Britton & Rose) 2: 47. 1920. ≡ Cereus gaumeri Standl., Publ. Field Mus. Nat. Hist., Bot. Ser. 3: 366. 1930. ≡ Pilocereus gaumeri (Britton & Rose) F.M.Knuth, Kaktus-ABC [Backeb. & Knuth] 330. 1936. LECTOTYPE (designed by Zappi, Succ. Pl. Res. 3: 151. 1994): Mexico, Yucatán, Progreso, 1918, G.F.Gaumer 23934 (lectotype: US 00115539!; isolectotypes: NY 00120553!, 00120554!, 00120555!).
-
5.
Pilosocereus leucocephalus (Poselg.) Byles & G.D.Rowley, Cact. Succ. J. Gr. Brit. 19: 67. 1957. ≡ Pilocereus leucocephalus Poselg., Allg. Gartenzeitung (Otto & Dietrich) 21: 126. 1853. ≡ Cephalocereus leucocephalus Britton & Rose, Contr. U.S. Natl. Herb. 12: 417. 1909. TYPE: Mexico, Sonora, near Horcasitas, Poselger (not preserved). NEOTYPE (designed by Zappi, Succ. Pl. Res. 3: 147. 1994): E.Palmer 362 (neotype: US 00115543!; isoneotypes: NY 00120557!, CM 1478!, K 000062714!). Note: this neotype designated by Zappi is the type of Cephalocereus palmeri (see below).
= Cereus cometes Scheidw., Allg. Gartenzeitung (Otto & Dietrich) 8: 339. 1840. ≡ Pilocereus cometes Mittl. ex C.F.Först., Handb. Cacteenk. [Förster] 357. 1846. ≡ Pilocereus jubatus Salm-Dyck, Cact. Hort. Dyck. ed. I. 24; Lem. in Rev. Hortic. 427. 1862. ≡ Cephalocereus cometes Britton & Rose, Contr. U.S. Natl. Herb. 12: 416. 1909. ≡ Pilosocereus cometes (Scheidw.) Byles & G.D.Rowley, Cact. Succ. J. Gr. Brit. 19: 66. 1957. TYPE: Mexico, [San Luis] Potosí, [Galeotti?] (not preserved).
= Cephalocereus maxonii Rose, Contr. U.S. Natl. Herb. 12: 417. 1909. ≡ Cereus maxonii Vaupel, Monatsschr. Kakteenk. 23: 23, 26. 1913. ≡ Pilocereus maxonii A.Berger, Kakteen (Berger) 345. 1929. ≡ Pilosocereus maxonii (Rose) Byles & G.D.Rowley, Cact. Succ. J. Gr. Brit. 19: 67. 1957. TYPE: Guatemala, Jalapa, near El Rancho, 4 Apr 1905, W.R.Maxon & R.H.Hay 3769 (holotype: US 00115542!).
= Cephalocereus palmeri Rose, Contr. U.S. Natl. Herb. 12: 418. 1909. ≡ Cereus victoriensis Vaupel, Monatsschr. Kakteenk. 23: 24, 37. 1913. ≡ Pilocereus palmeri (Rose) F.M.Knuth, Kaktus-ABC [Backeb. & Knuth] 333. 1936. ≡ Pilosocereus palmeri (Rose) Byles & G.D.Rowley, Cact. Succ. J. Gr. Brit. 19: 67. 1957. ≡ Pilosocereus palmeri var. victoriensis (Vaupel) Backeb., Kakteenlexikon 367. 1966. ≡ Cephalophorus palmeri (Rose) Boom, Succulenta (Netherlands) 46: 107. 1967. ≡ Pilosocereus leucocephalus subsp. palmeri (Rose) Scheinvar, Fl. Cactológ. Est. Querétaro 192. 2004. TYPE: Mexico, Tamaulipas, near Victoria, 320 m, 01 May–13 June 1907, E.Palmer 362 (holotype: US 00115543!; isotypes: NY 00120557!, CM 1478!, K 000062714!).
= Cephalocereus sartorianus Rose, Contr. U.S. Natl. Herb. 12: 419. 1909. ≡ Cereus sartorianus (Britton & Rose) Kupper ex A.Berger, Kakteen (Berger) 157. 1929. ≡ Pilocereus sartorianus (Britton & Rose) A.Berger, Kakteen (Berger) 345. 1929. ≡ Pilosocereus sartorianus (Rose) Byles & G.D.Rowley, Cact. Succ. J. Gr. Brit. 19: 69. 1957. ≡ Pilosocereus palmeri var. sartorianus (Rose) Lodé, Fichier Encycl. Cact. Autres Succ. 19: 1776. 1997. TYPE: Mexico, Veracruz, 1908, C.A.Purpus s.n. (holotype: US 00115545!).
-
6.
Pilosocereus purpusii (Britton & Rose) Byles & G.D.Rowley, Cact. Succ. J. Gr. Brit. 19: 67. 1957. ≡ Cephalocereus purpusii Britton & Rose, Cactaceae (Britton & Rose) 2: 56. 1920. ≡ Pilocereus purpusii (Britton & Rose) F.M.Knuth, Kaktus-ABC [Backeb. & Knuth] 333. 1936. LECTOTYPE (designed by Zappi, Succ. Pl. Res. 3: 150. 1994): Mexico, Sinaloa, Mazatlán, near the town overlooking the sea, 31 Mar 1910, J.N.Rose, P.C.Standley & P.G.Russell 13749 (lectotype: US 00115544!; isolectotype: NY 00120558!).
= Pilocereus guerreronis Backeb., Beitr. Sukkulentenk. Sukkulentenpflege 1: 3. 1941. ≡ Pilosocereus guerreronis (Backeb.) Byles & G.D.Rowley, Cact. Succ. J. Gr. Brit. 19: 67. 1957. ≡ Cephalocereus guerreronis (Backeb.) Buxb., Bot. Stud. 12: 101. 1961. TYPE: Mexico, Guerrero, Cañón del Zopilote, 800 m (not preserved). LECTOTYPE (designed by Zappi, Succ. Pl. Res. 3: 144. 1994): Backeberg, in ibid.: 4, photo. 1941. Synon. nov.
In previous works, P. guerreronis had been assumed to be a local form of the widespread P. alensis (Anderson 2001; Hunt et al. 2006), and in some cases had been included in P. alensis with a question mark (Korotkova et al. 2021; Zappi 1994). Moreover, its morphological traits of branches and fertile region show differences with respect to P. alensis. Herein the name P. guerreronis is recognized as a synonym for P. purpusii as by examining the protologue of P. guerreronis (Backeberg 1941) its description and distribution is more consistent with our recognition of P. purpusii by presenting branches of 7 cm diameter, ribs of 14 mm height, distance between areoles of 15 mm, discontinuous fertile region, and whitish flowers.
Species key to Pilosocereus leucocephalus group s.s.

References
Akoglu H (2018) User’s guide to correlation coefficients. Turk J Emerg Med 18:91–93. https://doi.org/10.1016/j.tjem.2018.08.001
Alvarado-Sizzo H, Casas A, Parra F, Arreola-Nava HJ, Terrazas T, Sánchez C (2018) Species delimitation in the Stenocereus griseus (Cactaceae) species complex reveals a new species S. huastecorum. PLoS ONE 13:e0190385
Anderson EF (2001) The cactus family. Timber Press, Portland
Arroyo-Cosultchi G, Terrazas T, Arias S, Arreola-Nava HJ (2006) The systematic significance of seed morphology in Stenocereus (Cactaceae). Taxon 55:983–992. https://doi.org/10.2307/25065693
Backeberg C (1941) Die Kakteen des Zopilote-Cañons (Geierschlucht) in Guerrero (Mexico). Beitr Sukkulentenk Sukkulentenpflege 1:1–7
Bagheri A, Maassoumi AA, Rahiminejad MR et al (2017) Molecular phylogeny and divergence times of Astragalus section Hymenostegis: an analysis of a rapidly diversifying species group in Fabaceae. Sci Rep 7:14033. https://doi.org/10.1038/s41598-017-14614-3
Baker MA (2016) Morphological and cytological analyses in Cylindropuntia (Cactaceae): the taxonomic circumscription of C. echinocarpa, C. multigeniculata, and C. whipplei. J Bot Res Inst Tex 10:325–343
Baker MA, Johnson RA (2000) Morphometric analysis of Escobaria sneedii var. sneedii, E. sneedii var. leei, and E. guadalupensis (Cactaceae). Syst Bot 25:577–587. https://doi.org/10.2307/2666722
Barthlott W, Burstedde K, Geffert J et al (2015) Biogeography and biodiversity of cacti. Schumannia 7:1–205
Bravo-Hollis H (1978) Las cactáceas de México. Universidad Nacional Autónoma de México, México
Britton NL, Rose JN (1920) The Cactaceae: descriptions and illustrations of plants of the cactus family. The Carnegie Institution of Washington, Washington
Bustamante E, Búrquez A, Scheinvar E, Eguiarte LE (2016) Population genetic structure of a widespread bat-pollinated columnar cactus. PLoS ONE 11:e0152329. https://doi.org/10.1371/journal.pone.0152329
Byles RS, Rowley GD (1957) Pilosocereus Byl. & Rowl. nom. gen. nov. (Cactaceae). Cact Succ J Gr Brit 19:66–67
Calonje M, Meerow AW, Griffith MP et al (2019) A time-calibrated species tree phylogeny of the New World cycad genus Zamia L. (Zamiaceae, Cycadales). Int J Plant Sci 180:286–314. https://doi.org/10.1086/702642
Calvente A, Moraes EM, Lavor P et al (2016) Phylogenetic analyses of Pilosocereus (Cactaceae) inferred from plastid and nuclear sequences. Bot J Linn Soc 183:25–38. https://doi.org/10.1111/boj.12491
Chavent M, Kuentz-Simonet V, Labenne A, Saracco J (2017) Multivariate analysis of mixed data: the R package PCAmixdata. http://arxiv.org/abs/14114911
Chen J, Wu G, Shrestha N et al (2021) Phylogeny and species delimitation of Chinese Medicago (Leguminosae) and its relatives based on molecular and morphological evidence. Front Plant Sci 11:619799. https://doi.org/10.3389/fpls.2020.619799
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9:772–772. https://doi.org/10.1038/nmeth.2109
de Mendiburu F (2013) Statistical procedures for agricultural research. Package “agricolae”. CRAN-R. The R Foundation, Vienna
de Queiroz K (1998) The general lineage concept of species, species criteria, and the process of speciation: a conceptual unification and terminological recommendations. In: Howard D, Berlocher S (eds) Endless forms: species and speciation. Oxford University Press, Oxford, pp 57–75
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
Doyle ED, Prates I, Sampaio I et al (2021) Molecular phylogenetic inference of the howler monkey radiation (Primates: Alouatta). Primates 62:177–188. https://doi.org/10.1007/s10329-020-00854-x
Duminil J, Di Michele M (2009) Plant species delimitation: a comparison of morphological and molecular markers. Plant Biosyst 143:528–542. https://doi.org/10.1080/11263500902722964
Edgar RC (2004) Muscle: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. https://doi.org/10.1093/nar/gkh340
Farris JS, Källersjö M, Kluge AG, Bult C (1994) Testing significance of incongruence. Cladistics 10:315–319
Franck AR, Barrios D, Campbell KCSE et al (2019) Revision of Pilosocereus (Cactaceae) in the Caribbean and northern Andean region. Phytotaxa 411:129–182. https://doi.org/10.11646/phytotaxa.411.3.1
Granados MC, Granados-Aguilar X, Donadío S et al (2017) Geographic structure in two highly diverse lineages of Tillandsia (Bromeliaceae). Botany 95:641–651. https://doi.org/10.1139/cjb-2016-0250
Granados-Aguilar X, Granados MC, Cervantes CR et al (2021) Unraveling reticulate evolution in Opuntia (Cactaceae) from southern Mexico. Front Plant Sci 11:2168. https://doi.org/10.3389/fpls.2020.606809
Guzmán U, Arias S, Dávila P (2003) Catálogo de cactáceas mexicanas. Universidad Nacional Autónoma de México, México
Hernández-Canchola G, León-Paniagua L (2017) Genetic and ecological processes promoting early diversification in the lowland Mesoamerican bat Sturnira parvidens (Chiroptera: Phyllostomidae). Mol Phylogenet Evol 114:334–345. https://doi.org/10.1016/j.ympev.2017.06.015
Hernández-Hernández T, Hernández HM, De-Nova JA et al (2011) Phylogenetic relationships and evolution of growth form in Cactaceae (Caryophyllales, Eudicotyledoneae). Am J Bot 98:44–61. https://doi.org/10.3732/ajb.1000129
Hunt D, Taylor N, Charles G (2006) The new cactus lexicon. DH Books, Milborne Port
Jiménez-Barron O, García-Sandoval R, Magallón S et al (2020) Phylogeny, diversification rate, and divergence time of Agave sensu lato (Asparagaceae), a group of recent origin in the process of diversification. Front Plant Sci 11:1651. https://doi.org/10.3389/fpls.2020.536135
Korkmaz S, Goksuluk D, Zararsiz G (2014) MVN: an R package for assessing multivariate normality. R J 6:151–162. https://doi.org/10.32614/RJ-2014-031
Korotkova N, Aquino D, Arias S et al (2021) Cactaceae at Caryophyllales.org—a dynamic online species-level taxonomic backbone for the family. Willdenowia 51:251–270. https://doi.org/10.3372/wi.51.51208
Lavor P, Calvente A, Versieux LM, Sanmartin I (2018) Bayesian spatio-temporal reconstruction reveals rapid diversification and Pleistocene range expansion in the widespread columnar cactus Pilosocereus. J Biogeogr 46:238–250. https://doi.org/10.1111/jbi.13481
Lavor P, Versieux LM, Calvente A (2020) Phylogenetic relationships of Pilosocereus (Cactaceae) and taxonomic implications. PlantNow 1:52–70. https://doi.org/10.6084/m9.figshare.12895124
Leaché AD, Koo MS, Spencer CL, Papenfuss TJ, Fisher RN, McGuire JA (2009) Quantifying ecological, morphological, and genetic variation to delimit species in the coast horned lizard species complex (Phrynosoma). Proc Natl Acad Sci 106:12418–12423
Lemus-Barrios H, Barrios D, García-Beltrán JA et al (2021) Taxonomic implications of seed morphology in Melocactus (Cactaceae) from Cuba. Willdenowia 51:91–113. https://doi.org/10.3372/wi.51.51108
Lendel A (2013) South American cacti in time and space: studies on the diversification of the tribe Cereeae, with particular focus on subtribe Trichocereinae (Cactaceae). Dissertation, Mathematisch-naturwissenschaftlichen Fakultät der Universität Zürich
Lewis PO (2001) A likelihood approach to estimating phylogeny from discrete morphological character data. Syst Biol 50:913–925. https://doi.org/10.1080/106351501753462876
Meudt HM, Prebble JM, Lehnebach CA (2015) Native New Zealand forget-me-nots (Myosotis, Boraginaceae) comprise a Pleistocene species radiation with very low genetic divergence. Plant Syst Evol 301:1455–1471. https://doi.org/10.1007/s00606-014-1166-x
Moraes E, Perez M, Téo M, Zappi D, Taylor N, Machado M (2012) Cross-species amplification of microsatellites reveals incongruence in the molecular variation and taxonomic limits of the Pilosocereus aurisetus group (Cactaceae). Genetica 140:277–285. https://doi.org/10.1007/s10709-012-9678-1
Morrone JJ (2014) Biogeographical regionalisation of the Neotropical region. Zootaxa 3782:1–110. https://doi.org/10.11646/zootaxa.3782.1.1
Müller J, Müller K, Quandt D, Neinhuis C (2005) PhyDE, phylogenetic data editor v.0.995. http://www.phyde.de/. Accessed 28 Jul 2020
Pessoa EM, Alves M, Alves-Araújo A, Palma-Silva C, Pinheiro F (2012) Integrating different tools to disentangle species complexes: a case study in Epidendrum (Orchidaceae). Taxon 61:721–734. https://doi.org/10.1002/tax.614002
Piedra-malagón EM, Albarrán-lara AL, Rull J, Piñero D, Sosa V (2016) Using multiple sources of characters to delimit species in the genus Crataegus (Rosaceae): the case of the Crataegus rosei complex. Syst Biodivers 14:244–260. https://doi.org/10.1080/14772000.2015.1117027
Pinheiro F, Dantas-Queiroz MV, Palma-Silva C (2018) Plant species complexes as models to understand speciation and evolution: a review of South American studies. Crit Rev Plant Sci 37:54–80
R Core Team (2013) R: a language and environment for statistical computing. http://www.r-project.org/. Accessed 29 Nov 2019
Rambaut A (2014) FigTree. http://tree.bio.ed.ac.uk/software/figtree/. Accessed 28 Jul 2020
Ripley B, Venables B, Bates DM et al (2013) Package “MASS”. CRAN-R. The R Foundation, Vienna
Rivera-Lugo M, García-Mendoza A, Simpson J, Solano E, Gil-Vega K (2018) Taxonomic implications of the morphological and genetic variation of cultivated and domesticated populations of the Agave angustifolia complex (Agavoideae, Asparagaceae) in Oaxaca, Mexico. Plant Syst Evol 304:969–979. https://doi.org/10.1007/s00606-018-1525-0
Ronquist F, Teslenko M, van der Mark P et al (2012) MrBayes 3.2: efficient bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542. https://doi.org/10.1093/sysbio/sys029
Ronquist F, Huelsenbeck J, Teslenko M (2011) MrBayes version 3.2 manual: tutorials and model summaries. http://nbisweden.github.io/MrBayes/manual.html. Accessed 28 Jul 2020
Sánchez D, Gómez-Quintero D, Vargas-Ponce O et al (2020) Species delimitation in the Echinocereus pulchellus complex (Cactaceae). Brittonia 72:433–452. https://doi.org/10.1007/s12228-020-09632-x
Schlumpberger BO, Renner SS (2012) Molecular phylogenetics of Echinopsis (Cactaceae): polyphyly at all levels and convergent evolution of pollination modes and growth forms. Am J Bot 99:1335–1349. https://doi.org/10.3732/ajb.1100288
Shaw J, Lickey EB, Schilling EE, Small RL (2007) Comparison of whole chloroplast genome sequences to choose noncoding regions for phylogenetic studies in angiosperms: the tortoise and the hare III. Am J Bot 94:275–288. https://doi.org/10.3732/ajb.94.3.275
Simmons MP, Ochoterena H (2000) Gaps as characters in sequence-based phylogenetic analyses. Syst Biol 49:369–381. https://doi.org/10.1093/sysbio/49.2.369
Stamatakis A (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313. https://doi.org/10.1093/bioinformatics/btu033
Su X, Wu G, Li L, Liu J (2015) Species delimitation in plants using the Qinghai-Tibet Plateau endemic Orinus (Poaceae: Tridentinae) as an example. Ann Bot 116:35–48. https://doi.org/10.1093/aob/mcv062
Swofford DL (2003) PAUP*: phylogenetic analysis using parsimony (* and other methods). Version 4. Sinauer Associates, Sunderland
Taberlet P, Gielly L, Pautou G, Bouvet J (1991) Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Mol Biol 17:1105–1109. https://doi.org/10.1007/BF00037152
Valencia-A S (2020) Species delimitation in the genus Quercus (Fagaceae). Bot Sci 99:1–12. https://doi.org/10.17129/botsci.2658
Vázquez-Sánchez M, Terrazas T, Arias S (2012) El hábito y la forma de crecimiento en la tribu Cacteae (Cactaceae, Cactoideae). Bot Sci 90:97–108
Wei T, Simko V (2017) Package “corrplot”: visualization of a correlation matrix. CRAN-R. The R Foundation, Vienna
Yetman D (2007) The great cacti: ethnobotany and biogeography. University of Arizona Press, Tucson
Zappi DC (1994) Pilosocereus (Cactaceae): the genus in Brazil. Suc Plant Res 3:1–160
Acknowledgments
This paper constitutes part of the doctoral research of Daniel Franco Estrada, who thanks the graduate program Doctorado en Ciencias Biológicas, UNAM and acknowledges the scholarships provided by CONACyT. We especially thank Abraham Torres, Arturo Mora Olivo, Daniel Sánchez Carbajal, Germán Carnevali Fernández-Concha, Isaías Escalante, Juan Ismael Calzada, and Martha González Elizondo for supporting the field work during this research and Laura M. Márquez Valdelamar and Nelly María López Ortiz for their support in sequencing (LANABIO, Instituto de Biología, UNAM). We thank Jenny Elizabeth Menjívar Cruz, José Gabriel Cerén López, and Lilian Ferrufino for providing samples and Yolanda Morales for her support in the greenhouse. Alice Calvente, Tania Escalante, and José Luis Villaseñor provided valuable comments on the preliminary version; we also thank the anonymous reviewers and Associate Editors for their relevant suggestions that allowed us to improve this contribution. This work was financially supported by UNAM-DGAPA-PAPIIT <IN208619> to S. A.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Franco-Estrada, D., Barrios, D., Cervantes, C.R. et al. Phylogenetic and morphological analyses of Pilosocereus leucocephalus group s.s. (Cactaceae) reveal new taxonomical implications. J Plant Res 135, 423–442 (2022). https://doi.org/10.1007/s10265-022-01384-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-022-01384-x