Abstract
Knysna estuarine bay in South Africa's Garden Route National Park is that country's most significant estuarine system for biodiversity and conservation value. One outstanding feature is support of 40% of South Africa's—and maybe 20% of the world's—remaining vulnerable and decreasing dwarf-eelgrass, Zostera capensis, whose associated benthic macrofauna has been studied since 2009. For these invertebrates, Knysna comprises several significantly different compartments: sandy mouth; well-flushed marine embayment; poorly flushed central sea-water 'lagoon'; and two disjunct but faunistically similar peripheral regions–marine backwater channels, and low-salinity upper estuary. Although macrofauna ranges from dilute brackish to fully marine, its abundance, local patchiness, and over considerable stretches, species density remains remarkably constant; further, one-third of species occur throughout. Intertidally, all but peripheral compartments are low density and infaunally dominated, while some peripheral areas, and much of the subtidal, are higher density and epifaunally dominated. Overall, seagrass macrobenthos appears maintained below carrying capacity (e.g., by abundant juvenile fish) and of random species composition within a site. Two further characteristics are notable: Unusually, seagrass supports fewer animals than adjacent unvegetated areas, probably because of lack of bioturbatory disturbance in them, and the vegetation cover may ameliorate ambient habitat conditions. Unfortunately, continual heavy and effectively unpreventable exploitation for bait occurs, and chlorophyte blooms have developed because of high nutrient input. Knysna presents a microcosm of problems facing biodiverse and high-value habitats set within areas of high unemployment where subsistence fishing provides the main source of protein and seagrass provides the only source of bait.
Similar content being viewed by others
Introduction
The permanently open Knysna estuarine bay (34º03′S, 23º03′E) is a drowned river valley in South Africa's Western Cape separated from the adjacent Indian Ocean by a narrow gorge (300 m wide, 700 m long, and 4 m deep at low tide) carved through the coastal quartzite ridge by the Knysna River during times of lower sea level. The bay forms part of the open-access Garden Route National Park, and on a basket of criteria, including its size, diversity of habitat, zonal rarity, and biodiversity, is ranked South Africa's most significant estuarine system in terms of conservation importance (Turpie and Clark 2007; van Niekerk et al. 2019). Known locally as the Knysna Lagoon, the system has an area of some 10 km2 at low tide and 16 km2 at high tide and receives the inflow of the Knysna River at its head and a large number of smaller streams along its northern and eastern shores. Nevertheless, it is marine-dominated, consequent on low average rates of freshwater inflow and a very large tidal prism, during spring tides equaling 19 × 106 m3 [the largest of any South African estuary (Grindley 1985)], causing semi-diurnal flushing of its main channel. The estuarine bay can be divided hydrologically into three linear compartments, which vary in areal extent and precise geographical position with the tidal cycle and magnitude of river flow: An outer marine bay tidally flushed with cool water from the Indian Ocean and with salinities usually > 34; a middle, more isolated lagoonal water body also of high salinity (30–34) but with long residence times (c. 4 weeks); and an inner, stratified and well-flushed estuarine region with low and variable salinity (0–30) as a result of freshwater input from the Knysna River (Largier et al. 2000).
This system is also one of the most thoroughly researched of any South African estuary with more than 100 published articles (Russell et al. 2012; Whitfield and Baliwe 2013), work there beginning in 1947 (Day et al. 1951) (see the summaries of Day 1967; Grindley 1985; Russell et al. 2012; and the articles in Hodgson and Allanson 2000). Among other important features, Knysna supports 40% of South Africa's dwarf-eelgrass, Zostera (Zosterella) capensis [or Nanozostera capensis in the recent revision of the Zosteraceae of Coyer et al. (2013)] which may equate to 20% of its world area (Adams 2016; Wasserman et al. 2020). It also forms the only known African locality of the unusual marine valvatoidean gastropod Cornirostra (GBIF 2020), as well as being the main habitat of several other rare seagrass-associated species, including the endangered Knysna seahorse (Hippocampus capensis), and it is one of the only two localities that support the critically endangered seagrass false-limpet (Siphonaria compressa) and a seagrass population of the dwarf cushion-star Parvulastra exigua. Except in the immediate vicinity of the mouth, Z. capensis, together with some mixed Halophila ovalis, occurs virtually throughout the intertidal zone of the system as one continuous bed (Maree 2000), and it also occurs subtidally though more patchily (Wasserman et al. 2020; Barnes and Claassens 2020). Such meadows support well-developed invertebrate macrofaunas that serve the vital functions of consuming epiphytic algal growths and providing the trophic link between microphytobenthic production and that of the larger, more mobile nekton (Murphy et al. 2021). This article synthesizes the main findings of the disparate series of researches conducted on these invertebrate faunal assemblages at Knysna since 2009, re-analyzing the original data where appropriate, with particular emphasis on patterns of macrofaunal assemblage composition, abundance, species richness, and patchiness along the bay's main axial transitional gradient, as well as along the gradient of shelter, located perpendicular to that axis across its eastern section.
General methodology
Patterns described in this review are based mainly on a series of 23 sites positioned to represent the whole area over which Zostera capensis is present intertidally (Fig. 1), with additional comparison between the macrofaunal eelgrass assemblages at some of those sites and the equivalent assemblages in immediately adjacent areas of bare sediment and/or in subtidal seagrass. Sampling was conducted each year between 2009 and 2020 during the austral summer, the research being approved by SANParks and conducted in accordance with their scientific research regulations and requirements. A standard procedure was used, involving series of core samples, each of 0.0027 m2 diameter prior to 2013 and of 0.0054 m2 diameter thereafter and of 100 mm depth, taken from continuous stretches of seagrass while still covered by > 10 cm of water. Cores were gently sieved ('puddled') through 710-µm mesh on site. This sampling procedure collects the smaller and more numerous members of the benthic and epibenthic macrofauna that constitute the large majority of invertebrate biodiversity (Bouchet et al. 2002; Albano et al. 2011), though not the meiofauna nor much scarcer megafauna nor sessile animals attached to the seagrass leaves.
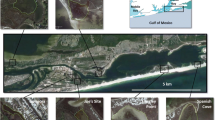
Knysna estuarine bay, showing location of the linear chain of 17 sampling sites along its longitudinal axis and of the six sites a–f set within the backwaters of the Ashmead and Steenbok Channels separating the two large bay islands from the mainland. The approximate geographical extents of the faunally distinct regions suggested in Fig. 2 are also indicated
nMDS plot based on Bray–Curtis similarities between percentage species composition data at the 23 sites, showing the four groups of seagrass macrofaunal assemblage types, i.e., those in: a the sandy mouth region; b the marine bay; c the lagoon and lower estuary; and d the upper estuary and the marine-basin backwater channels. The boundary between backwaters and axial channel conditions in the marine section would appear to lie along the Steenbok Channel in that site D falls within one grouping and E in the other. Envelopes enclose sites with the stated levels of Bray–Curtis similarity, for which the approximate geographical locations are shown in Fig. 1
In the laboratory, retained animals from each core were identified to species level wherever possible, with all organismal nomenclature here being as listed in the World Register of Marine Species (www.marinespecies.org), accessed November 2020, except in respect of the currently genus-less microgastropods 'Assiminea' capensis and 'A'. globulus (see Barnes 2017). It should be noted, however, that the specific identity of several animals, especially among the Polychaeta, is questionable because of lack of recent revision; those of South African taxa of Polycladida, Oligochaeta, and Nemertini, and many members of other groups less than 3 mm–4 mm in largest dimension are virtually unknown. Such animals were treated as morphospecies, an operationally appropriate procedure to detect spatial patterns of numbers of species and their differential abundance (Dethier and Schoch 2006; Gerwing et al. 2020).
All calculations were carried out in Microsoft Excel for Mac 16.37 with the StatPlus:mac Pro 7.1.1 add-on or via PAST 3.24 (Hammer et al. 2019). Numbers of each component zoobenthic species at each site were subjected to similarity analysis, and assemblage metrics were derived and compared. Univariate metrics assessed included: (i) overall faunal numbers per unit area, (ii) observed numbers of species per unit sample, N0 [i.e., 'species density' sensu (Gotelli and Colwell 2001)], and (iii) patchiness in spatial abundance of the macrofaunal assemblages as estimated by the 'index of patchiness' (Ip) of Lloyd (1967), with statistically significant departures from random being determined by Monte Carlo simulation using 9999 iterations. Correlations were assessed using Spearman's rank coefficient Sρ or the Pearson product-moment coefficient Pρ as appropriate; number of species per site unit of 30 cores and per region unit of 180 cores was determined by Mao tau rarefaction; curves were fitted using KaleidaGraph 4.5.4; and, where not known, information on life style of individual species was derived from that of close relatives in compendia such as Macdonald et al. (2010).
Multivariate comparison of macrofaunal assemblage composition used hierarchical clustering analysis of S17 Bray–Curtis similarity, ANOSIM, ANCOVA, SIMPER, and ordination by non-metric multidimensional scaling (nMDS), with 9999 permutations. For such comparison, all data sets were standardized for overall species density (by dividing all ranks by the total number of species in the set) and for sample size (by dividing each species total by the overall number of individuals in the set) to reflect solely differential taxonomic composition and to permit comparison of curve slopes (Passy 2016), a measure of equitability in individual species contribution to the total (Whittaker 1972). Overlaps in quantitative assemblage composition between adjacent regions were measured by the Bray–Curtis similarity index. All multivariate analyses were based on sample sizes of > 250 animals, well above the minimum number recommended by Forcino et al. (2015).
Principal findings
Patterns in assemblage composition
In total, some 67,000 individual macrofauna, representing 160 species, were examined in 2,100 core samples from the Zostera beds during the study. These ranged from typical freshwater/dilute-brackish species such as Afrochiltonia capensis, Corallana africana and Melanoides tuberculata through to fully marine forms such as Gibbula cicer, Limaria tuberculata, Nebalia capensis and Parechinus angulosus. Ordination by nMDS of Bray–Curtis similarity data from the 23 intertidal sites suggested that four significantly different faunal clusters occurred in the system (ANOSIM R = 0.88; P < 0.0001) (Figs. 1and 2), an essentially similar pattern to that derived earlier using non-standardized (but fourth-root transformed) abundance data (Barnes 2013a). These represented: (i) the sandy mouth region immediately adjacent to the true mouth, (ii) the outer marine embayment, and (iii) the lagoon plus lower-estuary divisions of the main axial channel, and (iv) the fringing backwater-creek system of the smaller, saltmarsh-enclosed creeks and channels that separate the bay's two large islands (each c. 82–84 ha) from the mainland, together with sites in the upper estuary. Separation of the backwaters/upper-estuary sites from those along the main axial channel was the most marked, with a Bray–Curtis similarity between the two blocks of sites of only 20%, and the mouth region was an outlier within the axial channel. The three points of change along the longitudinal axis of the bay, however, were not marked by sharp faunal contrasts (Fig. 3). Indeed, SIMPER indicates that most (> 50%) of the differences are brought about by the relative abundances of just eight common and widespread species, the gastropod molluscs Hydrobia knysnaensis and 'Assiminea' capensis (dominant in region iv), Turritella and Alaba (dominant in i), and Nassarius (dominant in iii), and the polychaetes Prionospio (dominant in iii), and Caulleriella and Simplisetia (dominant in ii). Despite statistically significant regionalization, 32% of the species (representing > 75% of the total individuals) occurred in all four regions in more than token quantities (Table 1 lists the more numerous of these shared taxa, and Table 2 displays those characteristic of each region). Patterns of relative species abundance within the four regions did not differ (ANCOVA equality of means F = 0.17, P = 0.92; equality of slopes F = 0.59, P = 0.62) (Fig. 4), further indicating similarity between the different local assemblages. Number of species per sample did not vary across test areas of up to 1.5 ha at a given site, whether in the bay or in the lagoon (Table 3). The observation that the seagrass macrofauna of the brackish upper estuary did not differ from that in the fully saline, saltmarsh-enclosed backwater channels of the marine embayment is noteworthy and reinforces the earlier comments of Day (1959) and Barnes (1989) that so-called estuarine faunas may be as characteristic of sheltered areas of fully marine soft sediment as they are of regions subject to low salinity.
Levels of Bray–Curtis similarity between the seagrass macrofaunal assemblages of adjacent sites: a along the longitudinal axis of the estuarine bay (arrows indicating points of transition between adjacent faunal assemblage types shown in Fig. 2), and b along the axis of shelter perpendicular to 'A' from the main channel into the fringing backwaters of the marine embayment
Major differences, however, did occur in the relative importance of infauna versus epifauna. Except at the lagoonal site 9, where the small biofilm-feeding cushion star Parvulastra exigua occurs in large numbers, the intertidal zone of the whole axial channel apart from the upper estuary is dominated by infaunal species (Fig. 5a), principally by polychaetes. From sites 1 to 15, the infauna comprised 68% (SE 3.7) of animals with no significant trend in their relative importance along the gradient (Sρ = 0.31; P = 0.26) (Fig. 5B). In contrast, the shores of the upper estuary and the marine backwater channels were dominated by epifaunal truncatelloid microgastropods, especially by 'Assiminea' capensis and Hydrobia knysnaensis, epifauna here comprising 64.4% of individuals. Only a few subtidal Z. capensis sites have so far been examined, but such areas are also overwhelmingly dominated by an epifaunal microgastropod, here by the cerithioid Alaba pinnae, although the importance of this species and hence of the subtidal epifauna in general decreases upstream so that epifauna and infauna contribute equally in the upper estuary (Fig. 5a). Thus in the bay region there is a transition at some LWS between a burrowing polychaete infauna and a seagrass-leaf-associated gastropod epifauna, and although upstream sub- and intertidal faunas are relatively similar, downstream in the bay they are markedly different (Fig. 6a). Few data are available to help explain the great downstream subtidal abundance of the epifaunal Alaba (a mean density of 28,000 m−2), although various studies have suggested that few fish consume significant numbers of shelled gastropods, even relatively small ones (McCormick 1998; Reynolds et al. 2018), not least because of their low nutritive value per unit intake (Vinson and Baker 2008). It is known that in South Africa, mugilids will take microgastropods (Whitfield and Blaber 1978; Whitfield 1988), but at Knysna mugilids do not characterize the dense sublittoral eelgrass beds favored by Alaba (Pollard et al. 2017). Several equivalent subtidal areas of seagrass in other continents are also dominated by species of Alaba, although Knysna is the only known such locality outside the tropics (Barnes and Claassens 2020). These other areas are of relatively high salinity which may help to account for the lesser importance of this gastropod in and near the upper estuary. Why the same suite of truncatelloid microgastropods dominates the otherwise contrasting habitats of the intertidal backwaters and upper estuary is not known for certain, but their common shelter (see paragraph above) is likely to be an important component.
nMDS plots based on Bray–Curtis similarities between percentage species composition data: a at four sites in the Knysna estuarine bay, one in each faunal compartment, showing the similarity between macrofaunal assemblages of seagrass at LWS in the intertidal zone and in the adjacent subtidal area (from data in Barnes and Claassens 2020), and b at three sites in the marine embayment forming a transect from the main channel (site 1) into the backwater Steenbok Channel and its tributaries, showing similarity between macrofaunal assemblages in seagrass and in adjacent areas of bare sediment (from data in Barnes and Barnes 2014a). Envelopes enclose sites at the stated levels of Bray–Curtis similarity, and site codes are those given in Fig. 1, plus in (A) an additional site, 'X', located between sites 1 and 2
With one exception, no evidence of any strong species interactions within any given site was forthcoming. The exception was the positive correlation between numbers of the ectoparasitic pyramidellid snail Sayella sp. and those of its probable host Hydrobia at the backwater site 'A' in Fig. 1 (Rs = 0.78; P < 0.00001). Such a parasite/host association is known from the western Atlantic (e.g., Hershler and Davis 1980), but although the pyramidellid concerned is a widely distributed animal, it is otherwise not recorded from Africa (GBIF 2020). That exception apart, however, in a large sample (325 cores) from the Kingfisher Creek seagrass (site 2 in Fig. 1), for example, Barnes (2013b) recorded 75 macrofaunal species at overall and mean densities of 2581 and 34 m−2, respectively. Considering the 34 relatively common species there that each attained a mean density of at least 10 m−2 (and together comprised 96% of the total individuals), all pairwise correlations of species abundance were very weak to non-existent (sensu Moore et al. 2018), positives averaging only Pρ = 0.069 (± 0.060 SD) and negatives Pρ = 0.047 (± 0.035 SD); and allowing for the familywise errors inherent in such a large correlation matrix (via Bonferroni correction), no negative correlations and only three positive ones were significant at a critical α of < 0.05 (between the polychaetes Simplisetia and Caulleriella, Glycera and Cirriformia, and between the polychaete Prionospio and the gastropod Nassarius). Equivalently, although qualitative co-occurrence patterns across the whole of the marine-influenced embayment at Knysna show deterministic structuring (Barnes and Elwood 2011), as indeed might be expected granted the location of the sampled sites in three distinct faunal regions (sandy mouth, marine bay, and backwater system), syntopic species within a single one of those regions did not differ from random co-occurrences (Barnes and Barnes 2014b).
In the absence of strong bioturbators such as Kraussillichirus kraussi (Callianassa kraussi in the older literature) from the majority of the system, faunal relationships between seagrass and bare sediment at Knysna are not the classic one of seagrass supporting the greater number of species and of individuals per unit area (Hemminga and Duarte 2000; Pillay et al. 2011; Hyman et al. 2019, etc.). To date studies have only concerned the outer marine embayment, but there seagrass macrofauna at a given site is more similar to those occurring in adjacent areas of bare sediment than either habitat is to other areas of the same type in the general region [Bray–Curtis faunal similarity between the two contiguous habitat types being a mean 0.58, whereas within-habitat-type similarity averaged 0.26 for the seagrass and 0.25 for the bare sediment (ANOVA F1,14 = 5.05; P < 0.05)] (see Fig. 6b). In general, seagrass beds supported lower, not higher, levels in half the metric comparisons in which there was a significant difference (Barnes and Barnes 2014a). Overall, faunal abundance was lower in seagrass in the ratio of 0.64: 1, while species density was indeed higher, but only by 1.13 to 1, with in large measure the higher numbers in the unvegetated sediments resulting from a quadrupled abundance of infaunal polychaetes, maybe because of the greater volume of available sedimentary habitat in the absence of eelgrass rootmass, although numbers of epifaunal crustaceans were 15 times less there (from a much smaller base). The same overall effect was not the case, however, in bare areas created by the death of seagrass following blanketing by the chlorophyte blooms described by Allanson et al. (2016) and Human et al. (2016). In these circumstances, the former seagrass sites clustered together, as did the same areas when de-vegetated, although macrofaunal abundance was again significantly lower in the former seagrass than it was in the replacement bare sediment (in a ratio of 0.62: 1) and again largely because of an increased number of polychaetes and decreased number of crustaceans in the unvegetated sediment (Barnes 2019a).
Knysna's marine embayment forms a natural harbor, has been in the past a busy port (Grindley 1985), and today supports several marinas, and hence it is one of the centers of ship-borne alien immigrant species in South Africa (Griffiths et al. 2009). Alien species of Boccardia, Polydora, Dipolydora, Pseudopolydora, Diopatra, Capitella, Desdemona, Ericthonius, Jassa, Monocorophium, Paracerceis, Elysia, Favorinus and Indothais all form part of its seagrass fauna, as do amphipods such as Cymadusa filosa, Melita zeylanica and Americorophium triaeonyx that are regarded by Robinson et al. (2005) and Mead et al. (2011) as being cryptogenic—to which could presumably be added Victoriopisa chilkensis. Relatively recently, these aliens have been joined by more northerly species spreading southward probably as a result of global warming. Smaragdia souverbiana, for example, is now a member of the subtidal seagrass fauna (Barnes and Claassens 2020). In the Knysna intertidal, Melanoides tuberculata has arrived and joined Cerithidea decollata (Hodgson and Dickens 2012) and Austruca occidentalis (formerly Uca annulipes) (Peer et al. 2015), the latter two in the adjacent saltmarsh or at the seagrass/saltmarsh interface.
Patterns in assemblage metrics along the axial gradient
As would be expected, the number of species at the 17 sites that were spaced along the system's longitudinal axis decreased with distance upstream (Sρ = −0.82; P < 0.0001; Fig. 7a), but the form of the decrease in species density suggests the occurrence of a step change within the general area of the lower estuary, with the downstream sites showing a considerable degree of uniformity of species density (Fig. 7b; Table 4). The points in Fig. 7 are based on the whole available 12-year dataset, and hence, the location of faunal and regional boundaries will have been blurred by temporal shifts, but an individual survey of macrofaunal animals along the axial channel in 2012 showed an almost identical (and sharper) feature (Barnes and Ellwood 2012) in the same general location. Comparison of data across different spatial scales shows that decline upstream in number of species when assessed per site (Fig. 7) is greater than when assessed per region (Table 4): Clearly, the bay and lagoon + lower-estuary regions are large and, as also would be expected, the total fauna contained in each is considerably in excess of that at any individual site.
Change in number of seagrass macrofaunal species along the longitudinal axis of the estuarine bay a. Note in b the apparent break between sites 10 and 11, corresponding to the one in the same area shown by Barnes and Ellwood (2012)
Assemblage abundance per unit area (Sρ = -0.35; P = 0.16; Fig. 8a) and patchiness in assemblage abundance (Sρ = −0.20; P = 0.45; Fig. 8b), however, showed no significant change with distance upstream; indeed, degree of patchiness along the axial gradient was significantly unchanging (Barnes 2019b). Neither was there any significant relationship between number of species per site and overall assemblage abundance there (Sρ = 0.43; P = 0.08). However, significant relationships have been found between how patchy an individual species is and its occupancy and, to a lesser extent, its abundance: The more abundant and widespread the species, the less its patchiness, both in subtidal and in intertidal seagrass (Barnes 2019c, 2020), and both in interspecific comparisons (Barnes 2020) and intraspecifically (Barnes, in prep.) (Fig. 9). This suggests that the well-known macroecological abundance-occupancy pattern (e.g., He and Gaston 2003) can be extended into a patchiness-abundance-occupancy one, at least in this habitat type. As can be seen in Fig. 9, the slopes of the power laws relating logit occupancy to log patchiness in individual species are much more variable than those interspecifically in the different faunal regions; thus, while the interspecific occupancy-patchiness slopes representing different regions do not differ (ANCOVA F = 1.3, P = 0.3), the equivalent intraspecific slopes are heterogeneous (ANCOVA F = 4.9, P < 0.0001) with a further six of the dominants (including the epifaunal Alaba and Cymadusa, and infaunal Caulleriella and Salmacoma) not showing significant occupancy-patchiness relationships at all. This also indicates that disparate species together form assemblages with similar properties in the various regions. There were no discernable trends in either metric upstream, although the upper estuary did display the largest value of both β and R2.
Relative constancy of intertidal seagrass macrofaunal assemblage metrics along the longitudinal axis of the estuarine bay: a assemblage abundance, with an inset showing abundances at the six backwater-channel sites; and b(i) assemblage patchiness (data from Barnes 2019b). b(ii) illustrates spatial variation in macrofaunal density 0.01 m−2 across an area of site 2 (from data in Barnes 2016)
General discussion and conclusions
The most striking feature of the Knysna intertidal seagrass-associated macrobenthos is its relative spatial uniformity. Macrofaunal abundance does not vary markedly along the longitudinal axis, neither does patchiness of macrofaunal density. Number of species per unit area at a given site is a constant, while species density along whole sections of the gradient can be relatively uniform, and its fauna appears to form a single assemblage with only local variation in relative frequency of its dominant components. Even the subtidal fauna does not differ qualitatively from the intertidal one, although there are marked quantitative differences and overall it is much more abundant especially in the most marine-influenced regions where Alaba dominates (Barnes and Claassens 2020). Admittedly, being a marine-dominated system with under normal circumstances relatively little freshwater input (Day et al. 1951), for an estuarine system salinity is relatively constant; however, occasional episodes of severe freshwater flooding do occur once every 10–12 years or so, rendering most or all of the system temporarily fresh (see, e.g., Korringa 1956; Blake and Chimboza 2010). But, although sea water may penetrate far upstream and dominate most areas, there is much change along the Knysna axis in other features of direct relevance to macrobenthos, as in transitional paralic systems in general (Tagliapietra et al. 2012; Pérez-Ruzafa et al. 2019). Sediment changes from clean sand at the mouth, to soft organic mud in the lagoonal and lower estuarine regions, and to mud with admixed riverine gravel in the upper estuary (Day et al. 1951); shelter changes both as the estuary narrows and on transition from axial channel into backwater creeks (Day 1967); and shore profiles change from extensive tidal flats near the mouth to narrow steep slopes in the estuary supporting only linear strips of seagrass (Day 1967; Maree 2000). Rates of water exchange vary along the channel (Largier et al. 2000); characteristic density and shoot length of the eelgrass change with shore height and exposure (den Hartog 1970; Adams and Talbot 1992); and so on.
Being located at 34°S, Knysna lies within the narrow mid-latitude belt recently identified by Whalen et al. (2020) as that displaying peak intensity of animal food consumption and hence potential top-down control of prey species. Like other South African estuarine areas supporting dwarf-eelgrass (Whitfield et al. 1989; Nel et al. 2018), it is a nursery area for many nektonic species (Whitfield and Kok 1992), schools of juvenile fish being a common sight in its seagrass beds. Indeed, seagrass beds have been regarded as one the most important types of coastal nursery (Whitfield 2017; Lefcheck et al. 2019), both because of the food they provide (Whitfield 2017) and as refuge from larger fish predators (Whitfield 2020a). If, as is generally held to be the case in seagrass (Moksnes et al. 2008; Lewis and Anderson 2012; Duffey et al. 2015, etc.), the effect of this consumption, together with that exerted by adult fish (Pollard et al. 2017) and predatory members of the invertebrate macrobenthos, is top-down control of the seagrass microphytobenthic-biofilm consumers that dominate both epifauna and infauna, then many of features of Knysna's seagrass macrobenthos become more understandable.
Three lines of evidence suggest that across the whole system seagrass macrofaunal abundance is below carrying capacity and not structured by density-dependent factors. First, the prevailing intertidal density along the longitudinal axis of some 4,000 m−2 is very low compared to the > 40,000 m2 animals occurring in similar intertidal dwarf-eelgrass beds in cool-temperate Europe (Blanchet et al. 2004; Barnes and Ellwood 2011; etc.) where predator rates are almost certainly lower on a fauna of similarly sized animals that are often members of the same families as represented at Knysna (Barnes and Hendy 2015b). Secondly, constancy of number of species per unit area, as demonstrated at the Kingfisher Creek site at Knysna (Barnes 2013b), is exactly what would be expected were the various species to be distributed independently of each other (granted their overall frequencies of occurrence) (Barnes and Barnes 2014b). Such independence of distribution is likely only if the whole assemblage is being maintained below the level at which species would otherwise interact. Thirdly, the large quantitative dataset of Barnes (2013b) from the same Kingfisher Creek site also showed that there were very few significant correlations (0.5%) between the abundances of pairs of species and all those were very weak. Moreover, as seen elsewhere, for example within Pacific Canadian Zostera marina meadows (Stark et al. 2020), weak and very weak positive relationships greatly out-numbered negative ones, further suggesting the lack of competitive interspecific interactions. In such overall circumstances of low and unpredictable density of potential invertebrate prey species, predators could thus be expected to have to forage optimally (Beseres and Feller 2007) and to graze down local prey stocks to threshold levels before moving and repeating the process elsewhere, and having reduced their food stocks to low levels over wide areas could themselves then experience food limitation (Saulnier et al. 2000). The actions of such predators roaming widely over the surface might also help explain the uniform levels of macrofaunal patchiness characterizing large areas (Barnes and Hamylton 2019).
A second process that could help account for the observed features of the Knysna macrobenthic assemblages, and particularly their relative uniformity along the longitudinal axis, is if the presence of the seagrass ameliorates variation in the local ambient microclimatic conditions, as a macro-algal cover has been shown to do (Scrosati 2017; Monteiro et al. 2017). Within the relatively uniform and stable conditions provided by the Z. capensis bed, species can penetrate upstream further than they might otherwise be able to do (Barnes and Ellwood 2012). Seagrass beds and other structurally complex systems (Hyman et al. 2019) generally appear to support macrofaunal assemblages that display spatial and temporal stability of abundance and composition, and high levels of resilience (Whanpetch et al. 2010; Blake et al. 2014; Gartner et al. 2015), and this may have important knock-on effects on the whole local coastal food web (Jankowska et al. 2019).
Granted the current high loss rates of seagrass throughout the world (Waycott et al. 2009; Short et al. 2011) and of Zostera capensis in southern Africa (Adams 2016), an important question is the implication of the operation of such potential structuring factors at Knysna for the future of its important seagrass system. Threats to the health of Knysna estuarine bay have recently been reviewed by Claassens et al. (2020), and the two that might particularly affect the seagrass and its inhabitants are uncontrolled bait harvesting by destructive means and chlorophyte blooms. Bait harvesting by 'pushing' for mudprawns (Upogebia capensis) and trenching for worms (Marphysa, Polybrachiorhynchus and Arenicola spp.) is rife in Knysna (Simon et al. 2019), even (and arguably especially) in the formally protected bait-reserve area (Fig. 10) that also supports 79% of the estuarine bay's seagrass-associated species. Unfortunately, there are immense logistic and social problems associated with preventing illegal and restricting legal bait harvesting in southern Africa, especially in areas of high unemployment where subsistence fishing provides the main or only source of protein (Bandeira and Gell 2003; Napier et al. 2009) and the local intertidal provides the only source of bait (Barnes and Claassens 2020). Subsistence harvesting at Knysna is worth some ZAR 1 × 106 (Turpie 2007). Its precise effect on the seagrass macrofauna is unknown; however, basically because the extent of harvesting means that a like-with-like, harvested versus unharvested, comparison is not possible: No area of intertidal seagrass remains un-pushed or un-pumped. At other localities, however, it and the associated trampling are known to have severe consequences (e.g., Pillay et al. 2010; Garmendia et al. 2017; Short et al. 2011; Adams 2016). Nevertheless, the inherent resilience and spatial uniformity of the Knysna seagrass beds referred to above, together with the fact that they form one large interconnected system with dispersal possible between all sections, does offer hope.
Destructive effects of bait-collecting activities on intertidal seagrass habitat in the 'no-take bait sanctuary' section of the Knysna estuarine bay: a The hole and jettisoned plug created by pushing for mudprawn; b, a substratum pock-marked by such mudprawn pushing; and c, the resultant plugs scattered over the seagrass surface; d, an area of seagrass destroyed by trenching for bait worms (from Barnes and Claassens 2020)
In respect of the second major threat, at the moment the problems of eutrophication, to which Z. capensis is known to be sensitive (Mvungi and Pillay 2019), and consequent algal blooms are only local, affecting mainly the backwater channels into which the municipal sewage treatment plant discharges and in which there is a legacy of organic matter retention (Human et al. 2020). Knysna's large tidal prism proves invaluable insofar as minimizing blooms in the main axial channel is concerned. But the local effect of the chlorophyte blanket is dramatic and destructive. Animal numbers may increase on its seasonal dieback, except in the very local areas of anoxia, although this increase is almost entirely confined to densities of the dominant polychaete groups (except, for some reason, cirratulids) (Barnes 2019a). Crustaceans do not bounce back so readily, and as they provide the food for most fish in the nearby Swartvlei Estuary (Whitfield 1988, 2020b) and presumably therefore do so at Knysna, the fish populations may suffer the consequences: There is some evidence that this is indeed the case (Pollard et al. 2018). The chlorophyte problem is soluble (no pun intended) but at considerable cost (see Human et al. 2020). The financial reward, however, might also be large, not least because such blooms impact on tourism (Boesch et al. 1996) and tourists contribute some ZAR 1 × 109 per annum to the Knysna economy (Turpie 2007).
Knysna is perhaps not a typical South African estuary in being permanently open (see van Niekerk et al. 2020), without a significant presence of Kraussillichirus, and with a subtidal dominated by the little-known Alaba pinnae, but nevertheless its fauna generally appears to be the classic Cape estuarine one (Day 1981; de Villiers et al. 1999); indeed, it supports > 40% of South African estuarine biodiversity. It also seems a microcosm of the country's estuarine problems, both environmental and socio-economic, including loss of area to reclamation (> 30% of the saltmarsh has already gone, and 25% of that remaining is under threat). Thus far, at least, it has managed to fare better than many other South African estuarine systems (van Niekerk et al. 2013), and it may yet continue to do so.
Data availability and material
Datasets on which these analyses are based have been lodged in electronic format in the Rondevlei Office of SANParks Scientific Services (http://dataknp.sanparks.org/sanparks/metacat/Nerinak.23.11/sanparks) and are available on request.
References
JB Adams 2016 Distribution and status of Zostera capensis in South African estuaries—a review S Afr J Bot 107 63 73 https://doi.org/10.1016/j.sajb.2016.07.007
Adams JB, Talbot MMB (1992) The influence of river impoundment on the estuarine seagrass Zostera capensis Setchell. Bot Mar 35: 69–75
PG Albano B Sabelli P Bouchet 2011 The challenge of small and rare species in marine biodiversity surveys: microgastropod diversity in a complex tropical coastal environment Biodivers Conserv 20 3223 3237 https://doi.org/10.1007/s10531-011-0117-x
BR Allanson LRD Human L Claassens 2016 Observations on the distribution and abundance of a green tide along an intertidal shore, Knysna Estuary S Afr J Bot 107 49 54 https://doi.org/10.1016/j.sajb.2016.02.197
AI Azovsky MV Chertoprood NV Kucheruk PV Rybnikov FV Sapozhnikov 2000 Fractal properties of spatial distribution of intertidal benthic communities Mar Biol 136 581 590 https://doi.org/10.1007/s002270050718
SO Bandeira F Gell 2003 The seagrasses of Mozambique and southeastern Africa EP Green FT Short Eds World Atlas of Seagrasses University of California Press Berkeley 93 100
RSK Barnes 1989 What, if anything, is a brackish-water fauna? Earth Environ Sci Trans Roy Soc Edinb 80 235 240 https://doi.org/10.1017/S0263593300028674
RSK Barnes 2013a Distribution patterns of macrobenthic biodiversity in the intertidal seagrass beds of an estuarine system, and their conservation significance Biodivers Conserv 22 357 372 https://doi.org/10.1007/s10531-012-0414-z
RSK Barnes 2013b Spatial stability of macrobenthic seagrass biodiversity Mar Ecol Progr Ser 493 127 139 https://doi.org/10.3354/meps10546
RSK Barnes 2016 Spatial homogeneity of benthic macrofaunal biodiversity across small spatial scales Mar Environ Res 122 148 157 https://doi.org/10.1016/j.marenvres.2016.10.006
RSK Barnes 2017 Little-known and phylogenetically obscure South African estuarine microgastropods (Mollusca: Truncatelloidea) as living animals J Nat Hist 57 87 113 https://doi.org/10.1080/00222933.2017.1408867
RSK Barnes 2019a Context dependency in the effect of Ulva-induced loss of seagrass cover on estuarine macrobenthic abundance and biodiversity Aquat Conserv 29 163 174 https://doi.org/10.1002/AQC.2977
RSK Barnes 2019b Local patchiness of macrobenthic faunal abundance displays homogeneity across the disparate seagrass systems of an estuarine bay Mar Environ Res 148 99 107 https://doi.org/10.1016/j.marenvres.2019.05.001
RSK Barnes 2019c Abundance/occupancy/patchiness relations in estuarine seagrass macrobenthos Estuar Coast Shelf Sci 228 106360 https://doi.org/10.1016/j.ecss.2019.106360
RSK Barnes 2020 Interspecific relationship of patchiness to occupancy and abundance, as exemplified by seagrass macrobenthos Ecol Indic 121 107083 https://doi.org/10.1016/jecolind.2020.107083
RSK Barnes MKS Barnes 2014a Biodiversity differentials between the numerically-dominant macrobenthos of seagrass and adjacent unvegetated sand in the absence of sandflat bioturbation Mar Environ Res 99 34 43 https://doi.org/10.1016/j.marenvres.2014.05.013
RSK Barnes MKS Barnes 2014b Spatial uniformity of biodiversity is inevitable if the available species are distributed independently of each other Mar Ecol Progr Ser 516 263 266 https://doi.org/10.3354/meps11067
RSK Barnes L Claassens 2020 Do beds of subtidal estuarine seagrass constitute a refuge for macrobenthic biodiversity threatened intertidally? Biodivers Conserv 29 3227 3244 https://doi.org/10.1007/s10531-020-02019-0
RSK Barnes MDF Ellwood 2011 Macrobenthic assemblage structure in a cool-temperate intertidal dwarf-eelgrass bed in comparison to those in lower latitudes Biol J Linn Soc 104 527 540 https://doi.org/10.1111/j.1095-8312-2011.01738.x
RSK Barnes MDF Ellwood 2012 Spatial variation in the macrobenthic assemblages of intertidal seagrass along the long axis of an estuary Estuar Coast Shelf Sci 112 173 182 https://doi.org/10.1016/j.ecss.2012.07.013
RSK Barnes SM Hamylton 2019 Isometric scaling of faunal patchiness: seagrass macrobenthic abundance across small spatial scales Mar Environ Res 146 89 100 https://doi.org/10.1016/j.marenvres.2019.03.011
RSK Barnes IW Hendy 2015a Seagrass-associated macrobenthic functional diversity and functional structure along an estuarine gradient Estuar Coast Shelf Sci 164 233 243 https://doi.org/10.1016/j.ecss.2015.07.050
RSK Barnes IW Hendy 2015b Functional uniformity underlies the common spatial structure of macrofaunal assemblages in intertidal seagrass beds Biol J Linn Soc 115 114 126 https://doi.org/10.1111/bij.12483
JJ Beseres RJ Feller 2007 Changes in the spatial distribution of subtidal macrobenthos due to predation by the white shrimp (Litopenaeus setiferus) Estuar Coast 30 591 600 https://doi.org/10.1007/BF02841956
RE Blake JE Duffy JP Richardson 2014 Patterns of seagrass community response to local shoreline development Estuar Coast 37 1549 1561 https://doi.org/10.1007/s12237-014-9784-7
Blake D, Chimboza N (2010) Sea Level Rise and Flood Risk Assessment for a Select Disaster Prone Area Along the Western Cape Coast. Phase 2 Report: Eden District Municipality Sea Level Rise and Flood Risk Modelling. Umvoto Africa (Pty) Ltd / Western Cape Department of Environmental Affairs and Development Planning. (https://www.westerncape.gov.za/eadp/files/atoms/files/Eden%20DM%20SLR%20Phase%202%20Modelling%20FInal_0.pdf)
H Blanchet X Montaudouin de A Lucas P Chardy 2004 Heterogeneity of macrozoobenthic assemblages within a Zostera noltii seagrass bed: diversity, abundance, biomass and structuring factors Estuar Coast Shelf Sci 61 111 123 https://doi.org/10.1016/j.ecss.2004.04.008
Boesch DF, Anderson DM, Horner RA, Shumway SE, Tester PA, Whitledge TE (1996) Harmful algal blooms in coastal waters: Options for prevention, control and mitigation. NOAA Coastal Ocean Program Decision Analysis Series No. 10. NOAA Coastal Ocean Office, Silver Spring MD
P Bouchet P Lozouet P Maestrati V Heros 2002 Assessing the magnitude of species richness in tropical marine environments: exceptionally high numbers of molluscs at a New Caledonia site Biol J Linn Soc 75 421 436 https://doi.org/10.1046/j.1095-8312.2002.00052.x
L Claassens RSK Barnes J Wasserman SJ Lamberth NAF Miranda L Niekerk van JB Adams 2020 Knysna estuary health: ecological status, threats and options for the future Afr J Aquat Sci 45 65 82 https://doi.org/10.2989/16085914.2019.1672518
JA Coyer G Hoarau J Kuo A Tronholm J Veldsink JL Olsen 2013 Phylogeny and temporal divergence of the seagrass family Zosteraceae using one nuclear and three chloroplast loci Syst Biodivers 11 271 284 https://doi.org/10.1080/14772000.2013.821187
DS Moore WI Notz M Fligner 2018 The basic practice of statistics 8E Macmillan Gordonsville
JH Day 1959 The biology of Langebaan Lagoon: a study of the effect of shelter from wave action Trans R Soc S Afr 35 475 547 https://doi.org/10.1080/00359195909519025
JH Day 1967 Biology of the Knysna Estuary, South Africa GF Lauff Eds Estuaries American Association for the Advancement of Science Washington 397 407
JH Day NAH Millard AD Harrison 1951 The ecology of South African estuaries. Part III. Knysna: a clear open estuary Trans R Soc S Afr 33 367 413 https://doi.org/10.1080/00359195109519891
MN Dethier GC Schoch 2006 Taxonomic sufficiency in distinguishing natural spatial patterns on an estuarine shoreline Mar Ecol Progr Ser 306 41 49 https://doi.org/10.3354/meps306041
JE Duffy PL Reynolds C Boström JJ Stachowicz 2015 Biodiversity mediates top-down control in eelgrass ecosystems: a global comparative experimental approach Ecol Lett 18 696 705 https://doi.org/10.1111/ele.12448
FL Forcino LR Leighton P Twerdy JF Cahill 2015 Reexamining sample size requirements for multivariate, abundance-based research: When resources are limited, the research does not have to be PLoS ONE 10 6 e0128379 https://doi.org/10.1371/journal.pone.0128379
GBIF (2020) Global Species Diversity Information Facility: Cornirostra Ponder, 1990. https://www.gbif.org/occurrences/1101139915, and Sayella Dall, 1885. https://www.gbif.org/occurrences/2298827. Accessed 1 Nov 2020
Garmendia JM, Valle M, Borja Á, Chust G, Lee DJ, Rodríguez JG, Franco J (2017) Effect of trampling and digging from shellfishing on Zostera noltei (Zosteraceae) intertidal seagrass beds. Sci Mar 81: 121–128 https://doi.org/10.3989/scimar.04482.17A
A Gartner PS Lavery H Lonzano-Montes 2015 Trophic implications and faunal resilience following one-off and successive disturbances to an Amphibolis griffithii seagrass system Mar Poll Bull 94 131 143 https://doi.org/10.1016/j.marpolbul.2015.03.001
TG Gerwing K Cox AM Allen Gerwing L Campbell T Macdonald SE Dudas F Juanes 2020 Varying intertidal invertebrate taxonomic resolution does not influence ecological findings Estuar Coast Shelf Sci 232 106516 https://doi.org/10.1016/j.ecss.2019.106516
NJ Gotelli RK Colwell 2001 Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness Ecol Lett 4 379 391 https://doi.org/10.1046/j.1461-0248.2001.00230.x
CL Griffiths TB Robinson A Mead 2009 The status and distribution of marine alien species in South Africa G Rilov JA Crooks Eds Biological invasions in marine ecosystems Springer Berlin
Grindley JR (1985) Estuaries of the Cape, Part II Synopses of Available Information on individual Systems No. 30 Knysna. CSIR, Stellenbosch.
Ø Hammer DAT Harper PD Ryan 2019 PAST: Paleontological statistics software package for education and data analysis Version 3 24
C Hartog den 1970 The sea-grasses of the world North Holland Amsterdam
F He KJ Gaston 2003 Occupancy, spatial variance and the abundance of species Am Nat 162 366 375 https://doi.org/10.1086/377190
R Hershler GM Davis 1980 The morphology of Hydrobia truncata (Gastropoda: Hydrobiidae): relevance to systematics of Hydrobia Biol Bull 158 195 219 https://doi.org/10.2307/1540931
AN Hodgson J Dickens 2012 Activity of the mangrove snail Cerithidea decollata (Gastropoda: Potamididae) in a warm temperate South African estuary Estuar Coast Shelf Sci 109 98 106 https://doi.org/10.1016/j.ecss.2012.05.033
Hodgson AN, Allanson BR (eds) (2000) The Knysna Basin Project, 1995–1998. A scientific report on the Knysna Estuary. Trans Roy Soc S Afr 55(2): 97–240.
LRD Human JB Adams BR Allanson 2016 Insights into the cause of an Ulva lactuca Linnaeus bloom in the Knysna Estuary S Afr J Bot 17 55 62 https://doi.org/10.1016/j.sajb.2016.05.016
LRD Human R Weitz BR Allanson JB Adams 2020 Nutrient fluxes from sediments pose management challenges for the Knysna Estuary, South Africa Afr J Aquat Sci 45 1 9 https://doi.org/10.2989/16085914.2019.1671787
AC Hyman TK Frazer CA Jacoby JR Frost M Kowalewski 2019 Long-term persistence of structured habitats: seagrass meadow as enduring hotspots of biodiversity and faunal stability Proc Roy Soc B 286 201901861 https://doi.org/10.1098/rspb.2019.1861
JH Day Eds 1981 Estuarine Ecology with particular reference to southern Africa Balkema Rotterdam
E Jankowska LN Michel G Lepoint M Wlodarska-Kowalczuk 2019 Stabilizing effects of seagrass meadows on coastal water benthic food webs J Exp Mar Biol Ecol 510 54 63 https://doi.org/10.1016/j.jembe.2018.10.004
P Korringa 1956 Oyster culture in South Africa Hydrological, biological and ostreological observations in the Knysna Lagoon, with notes on conditions in other South African waters Invest Rep Div Sea Fish S Afr 20 85
JL Largier C Attwood J-L Harcourt-Baldwin 2000 The hydrographic character of the Knysna Estuary Trans R Soc S Afr 55 107 122 https://doi.org/10.1080/00359190009520437
JS Lefcheck BB Hughes AJ Johnson BW Pfirrman DB Rasher AR Smyth BL Williams MW Beck RJ Orth 2019 Are coastal habitats important nurseries? A Meta-Anal Conserv Lett 12 e12645 https://doi.org/10.1111/conl.12645
LS Lewis TW Anderson 2012 Top-down control of epifauna by fishes enhances seagrass production Ecology 93 2746 2757 https://doi.org/10.1890/12-0038.1
M Lloyd 1967 Mean crowding J Anim Ecol 36 1 30 https://doi.org/10.2307/3012
MA Hemminga CM Duarte 2000 Seagrass ecology Cambridge University Press Cambridge
TA Macdonald BJ Burd VI Macdonald A Roodeselaar van 2010 Taxonomic and feeding guild classification for the marine benthic macroinvertebrates of the Strait of Georgia, British Columbia Can Tech Rep Fish Aquat Sci 2874 63
B Maree 2000 Structure and status of the intertidal wetlands of the Knysna Estuary Trans Roy Soc S Afr 55 163 176 https://doi.org/10.1080/00359190009520441
MI McCormick 1998 Ontogeny of diet shifts by a microcarnivorous fish, Cheilodactylus spectabilis: relationship between feeding mechanics, microhabitat selection and growth Mar Biol 132 9 20 https://doi.org/10.1007/s002270050367
A Mead JT Carlton CL Griffiths M Rius 2011 Revealing the scale of marine bioinvasions in developing regions: a South African re-assessment Biol Invasions 13 1991 2008 https://doi.org/10.1007/s10530-011-0016-9
P-O Moksnes M Gullström K Tryman S Baden 2008 Trophic cascades in a temperate seagrass community Oikos 117 763 777 https://doi.org/10.1111/j.0030-1299.2008.16521.x
C Monteiro GI Zardi CD McQuaid EA Serrão GA Pearson KR Nicastro 2017 Canopy microclimate modification in central and marginal populations of a marine macroalga Mar Biodivers 49 415 424 https://doi.org/10.1007/s12526-017-0824-y
CE Murphy RJ Orth JS Lefcheck 2021 Habitat primarily structures seagrass epifaunal communities: a regional-scale assessment in the Chesapeake Bay Estuar Coast 44 442 452 https://doi.org/10.1007/s12237-020-00864-4
EF Mvungi D Pillay 2019 Eutrophication overrides warming as a stressor for a temperate African seagrass (Zostera capensis) PLoS ONE 14 4 e0215129 https://doi.org/10.1371/journal.pone.0215129
VR Napier JK Turpie BM Clark 2009 Value and management of the subsistence fishery at Knysna Estuary, South Africa Afr J Mar Sci 31 297 310 https://doi.org/10.2989/AJMS.2009.31.3.3.991
L Nel NA Strydom JB Adams 2018 Habitat partitioning in juvenile fishes associated with three vegetation types in selected warm temperate estuaries, South Africa Environ Biol Fish 101 1137 1148 https://doi.org/10.1007/s10641-018-0762-y
L Niekerk van JB Adams GC Bate TH Wooldridge 2013 Country-wide assessment of estuary health: An approach for integrating pressures and ecosystem response in a data limited environment Estuar Coast Shelf Sci 130 239 251 https://doi.org/10.1016/j.ecss.2013.05.006
L Niekerk van JB Adams NC James SJ Lamberth CF MacKay JK Turpie A Rajkaran SP Weerts AK Whitfield 2020 An estuary ecosystem classification that encompasses biogeography and a high diversity of types in support of protection and management S Afr J Aquat Sci 45 199 216 https://doi.org/10.2989/16085914.2019.1685934
SI Passy 2016 Abundance inequality in freshwater communities has an ecological origin Amer Nat 187 505 516 https://doi.org/10.1086/685424
N Peer NAF Miranda R Perissinotto 2015 A review of fiddler crabs (genus Uca Leach, 1814) in South Africa Afr Zool 50 187 204 https://doi.org/10.1080/15627020.2015.1055700
D Pillay GM Branch J Dawson D Henry 2011 Contrasting effects of ecosystem engineering by the cordgrass Spartina maritima and the sandprawn Callianassa kraussi in a marine-dominated lagoon Estuar Coast Shelf Sci 91 169 176 https://doi.org/10.1016/j.ecss.2010.10.010
D Pillay GM Branch CL Griffiths C Williams 2010 Ecosystem change in a South African marine reserve (1960–2009): role of seagrass loss and anthropogenic disturbance Mar Ecol Progr Ser 415 35 48 https://doi.org/10.3354/meps08733
M Pollard AN Hodgson HM Kok AK Whitfield 2017 Eelgrass beds and bare substrata - sparid and mugilid composition in contrasting littoral estuarine habitats Afr J Mar Sci 39 211 224 https://doi.org/10.2989/1814232X.2017.1333043
M Pollard AK Whitfield AN Hodgson 2018 Possible influences of a macroalgal bloom in eelgrass beds on fish assemblages in the lower Knysna Estuary, South Africa Afr J Aquat Sci 43 319 323 https://doi.org/10.2989/16085914.2018.1515063
A Pérez-Ruzafa F Pascalis De M Ghezzo JI Quispe-Becerra MI Hernández-García C Vergara IM Pérez-Ruzafa G Umgiesser C Marcos 2019 Connectivity between coastal lagoons and sea: Asymmetrical effects on assemblages' and populations' structure Estuar Coast Shelf Sci 216 171 186 https://doi.org/10.1016/j.ecss.2018.02.031
PL Reynolds JJ Stachowicz K Hovel JE Duffy 2018 Latitude, temperature, and habitat complexity predict predation pressure in eelgrass beds across the Northern Hemisphere Ecology 99 29 35 https://doi.org/10.1002/ecy.2064
TB Robinson CL Griffiths CD McQuaid M Rius 2005 Marine alien species of South Africa — status and impacts Afr J Mar Sci 27 297 306 https://doi.org/10.2989/18142320509504088
Russell IA, Randall RM, Kruger N (2012) Garden Route National Park, Knysna Coastal Section, State of Knowledge. SANParks Scientific Services, Rondevlei (available at http://www.sanparks.org/docs/conservation/scientific/coastal/knla-may2012.pdf)
E Saulnier H Bris Le A Tableau JC Dauvin A Brind'Amour 2020 Food limitation of juvenile marine fish in a coastal and estuarine nursery Est Coast Shelf Sci 241 106670 https://doi.org/10.1016/j.ecss.2020.106670
RA Scrosati 2017 Community-level facilitation by macroalgal foundation species peaks at an intermediate level of environmental stress Algae 32 41 46 https://doi.org/10.4490/algae.2017.32.2.20
FT Short B Polidoro SR Livingstone JC Zieman 2011 Extinction risk assessment of the world's seagrass species Biol Conserv 144 1961 1971 https://doi.org/10.1016/j.biocon.2011.04.010
C Simon AN Toit du MKS Smith L Claassens F Smith P Smith 2019 Bait collecting by subsistence and recreational fishers in Knysna Estuary may impact management and conservation Afr Zool 54 91 103 https://doi.org/10.1080/15627020.2019.1608862
KA Stark PL Thompson J Yakimishyn L Lee EM Adamczyk M Hessing-Lewis MI OConnor 2020 Beyond a single patch: local and regional processes explain diversity patterns in a seagrass epifaunal community Mar Ecol Progr Ser 655 91 106 https://doi.org/10.3354/meps13527
D Tagliapietra M Sigovini P Magni 2012 Saprobity: a unified view of benthic succession models for coastal lagoons Hydrobiologia 686 15 28 https://doi.org/10.1007/s10750-012-1001-8
Turpie J (2007) Cape Action for People and the Environment (C.A.P.E.) Estuarine Management Guideline 9: Maximising the economic value of estuaries. Anchor Environmental Consultants, Cape Town. (Available at https://www.overstrand.gov.za/en/documents/town-planning/legislation/national-environmental-management-integrated-coastal-management-act-24-of-2008/1281-no-9-guideline-economic-value-oct-2007/file).
Turpie J, Clark B (2007) Development of a conservation plan for temperate South African estuaries on the basis of biodiversity importance, ecosystem health and economic costs and benefits. Final Report. Anchor Environmental Consultants / C.A.P.E. Regional Estuarine Management Programme, Cape Town. (Available at https://anchorenvironmental.co.za/sites/default/files/2017-11/Cape%20Estuaries%20Cons%20Plan%20Final%20Report.pdf).
C Villiers de A Hodgson A Forbes 1999 Studies on estuarine macroinvertebrates B Allanson D Baird Eds Estuaries of South Africa Cambridge University Press Cambridge
MR Vinson MA Baker 2008 Poor growth of rainbow trout fed New Zealand mud snails Potamopyrgus antipodarum N Amer J Fish Manag 28 701 708 https://doi.org/10.1577/M06-039.1
J Wasserman L Claassens JB Adams 2020 Mapping subtidal estuarine habitats with a remotely operated underwater vehicle (ROV) Afr J Mar Sci 42 123 128 https://doi.org/10.2989/1814232X.2020.1731598
M Waycott CM Duarte TJB Carruthers SL Williams 2009 Accelerating loss of seagrasses across the globe threatens coastal ecosystems Proc Natl Acad Sci USA 106 12377 12381 https://doi.org/10.1073/pnas.0905620106
MA Whalen RDB Whippo JJ Stachowicz JE Duffy 2020 Climate drives the geography of marine consumption by changing predator communities P Natl Acad Sci USA 117 28160 28166 https://doi.org/10.1073/pnas.2005255117
N Whanpetch M Nakaoka H Mukai T Suzuki S Nojima T Kawai C Aryuthaka 2010 Temporal changes in benthic communities of seagrass beds impacted by a tsunami in the Andaman Sea, Thailand Estuar Coast Shelf Sci 87 246 252 https://doi.org/10.1016/j.ecss.2010.01.001
AK Whitfield 1988 The fish community of the Swartvlei estuary and the influence of food availability on resource utilization Estuaries 11 160 170 https://doi.org/10.2307/1351968
AK Whitfield 2017 The role of seagrass meadows, mangrove forests, salt marshes and reed beds as nursery areas and food sources for fishes in estuaries Rev Fish Biol Fish 27 75 110 https://doi.org/10.1007/s11160-016-9454-x
AK Whitfield 2020a Littoral habitats as major nursery areas for fish species in estuaries: a reinforcement of the reduced predation paradigm Mar Ecol Progr Ser 649 219 234
AK Whitfield 2020b Fish food webs in a South African estuary: a spatial and temporal assessment Environ Biol Fish https://doi.org/10.1007/s10641-020-01042-y,inpress
AK Whitfield LE Beckley BA Bennett GM Branch HM Kok IC Potter RP Elst van der 1989 Composition, species richness and similarity of ichthyofaunas in eelgrass Zostera capensis beds of southern Africa S Afr J Mar Sci 8 251 259 https://doi.org/10.2989/02577618909504565
AK Whitfield SJM Blaber 1978 Resource segregation among iliophagous fish in Lake St Lucia, Zululand Environ Biol Fish 3 293 296 https://doi.org/10.1007/BF00001455
AK Whitfield HM Kok 1992 Recruitment of juvenile marine fishes into permanently open and seasonally open estuarine systems on the southern coast of South Africa Ichthyol Bull JLB Smith Inst 57 1 39
Whitfield AK, Baliwe NG (2013) A century of science in South African estuaries: Bibliography and review of research trends. South African Network for Coastal and Oceanic Research Occasional Report No. 7:1–289.
RH Whittaker 1972 Evolution and measurement of species diversity Science 147 250 260 https://doi.org/10.2307/1218190
van Niekerk L, Adams JB, Lamberth SJ, MacKay CF, Taljaard S, Turpie JK, Weerts SP, Raimondo DC (eds) (2019) South African National Biodiversity Assessment 2018: Technical Report. Volume 3: Estuarine Realm. South African National Biodiversity Institute, Pretoria, Report SANBI/NAT/NBA2018/Vol3/A.
Acknowledgements
I am most grateful to SANParks area office, Knysna, and SANParks Scientific Services, Rondevlei, for permission to conduct research on the Knysna system over the last decade or so; to Rhodes University Research Committee for their support; to my collaborators at various times, sequentially Farnon Ellwood, Morvan Barnes, Ian Hendy and Louw Claassens, for their contribution to the study; to Brian Allanson for his copious encouragement and support; and to an anonymous reviewer for saving me from error.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
RSKB conceived, designed, and executed this study and wrote the manuscript. No other person is entitled to authorship.
Corresponding author
Ethics declarations
Conflict of interest
The author declares no conflict of interest.
Consent for publication
The author consents to publication.
Ethical approval
All applicable international, national, and/or institutional guidelines for sampling, care, and experimental use of organisms for the study were followed, and all necessary permissions and approvals were obtained in respect of the original collections of the data.
Additional information
Handling Editor: Télesphore Sime-Ngando
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Barnes, R.S.K. Patterns of seagrass macrobenthic biodiversity in the warm-temperate Knysna estuarine bay, Western Cape: a review. Aquat Ecol 55, 327–345 (2021). https://doi.org/10.1007/s10452-021-09848-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-021-09848-3