Abstract
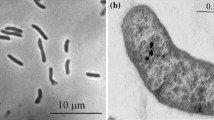
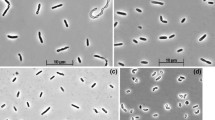
A psychrotrolerant acetate-oxidizing sulfate-reducing bacterium (strain akvbT) was isolated from sediment from the northern part of The North Sea with annual temperature fluctuations between 8 and 14 °C. Of the various substrates tested, strain akvbT grew exclusively by the oxidation of acetate coupled to the reduction of sulfate. The cells were motile, thick rods with round ends and grew in dense aggregates. Strain akvbT grew at temperatures ranging from −3.6 to 26.3 °C. Optimal growth was observed at 20 °C. The highest cell specific sulfate reduction rate of 6.2 fmol cell−1 d−1 determined by the ^{35}\hbox{SO}_{4}^{2-} method was measured at 26 °C. The temperature range of short-term sulfate reduction rates exceeded the temperature range of growth by 5 °C. The Arrhenius relationship for the temperature dependence of growth and sulfate reduction was linear, with two distinct slopes below the optimum temperatures of both processes. The critical temperature was 6.4 °C. The highest growth yield (4.3–4.5 g dry weight mol−1 acetate) was determined at temperatures between 5 and 15 °C. The cellular fatty acid composition was determined with cultures grown at 4 and 20 °C, respectively. The relative proportion of cellular unsaturated fatty acids (e.g. 16:1ω7c) was higher in cells grown at 4 °C than in cells grown at 20 °C. The physiological responses to temperature changes showed that strain akvbT was well adapted to the temperature regime of the environment from which it was isolated. Phylogenetic analysis showed that strain akvbT is closest related to Desulfobacter hydrogenophilus, with a 16S rRNA gene sequence similarity of 98.6%. DNA–DNA-hybridization showed a similarity of 32% between D. hydrogenophilus and strain akvbT. Based on phenotypic and DNA-based characteristics we propose that strain akvbT is a member of a new species, Desulfobacter psychrotolerans sp.␣nov.
Similar content being viewed by others
References
Abildgaard L., Ramsing N.B. and Finster K. (2004). Characterization of the marine propionate-degrading, sulfate-reducing bacterium Desulfofaba fastidiosa sp nov and reclassification of Desulfomusa hansenii as Desulfofaba hansenii comb. nov. Int. J. Syst. Evol. Microbiol. 54: 393–399
Bak F. 1988. Sulfatreduzierende bakterien und ihre aktivität im litoralsediment der unteren Güll (Überlinger See) Ph.D. Thesis. In: Mikrobieele Ökologie, Konstanz
Bakermans C. and Nealson K.H. (2004). Relationship of critical temperature to macromolecular synthesis and growth yield in Psychrobacter cryopegella. J. Bacteriol. 186: 2340–2345
Brandt K.K. and Ingvorsen K. (1997). Desulfobacter halotolerans sp nov, a halotolerant acetate-oxidizing sulfate-reducing bacterium isolated from sediments of Great Salt Lake, Utah. Syst. Appl. Microbiol. Syst Appl Microbiol. 20: 366–373
Brysch K., Schneider C., Fuchs G. and Widdel F. (1987). Lithoautotrophic growth of sulfate-reducing bacteria, and description of Desulfobacterium autotrophicum gen-nov, sp-nov. Arch. Microbiol. 148: 264–274
Cashion P., Holder-Franklin M.A., Mccully J. and Franklin M. (1977). Rapid method for base ratio determination of bacterial DNA. Anal. Biochem. 81: 461–466
Christensen D. (1984). Determination of substrates oxidized by sulfate reduction in intact cores of marine-sediments. Limnol. Oceanogr. 29: 189–192
Deley J., Cattoir H. and Reynaert A. 1970. Quantitative measurement of DNA hybridization from renaturation rates. Eur. J. Biochem. 12: 133–142
Delille D. and Perret E. (1989). Influence of temperature on the growth-potential of Southern Polar marine-bacteria. Microbial. Ecol. 18: 117–123
Denich T.J., Beaudette L.A., Lee H. and Trevors J.T. (2003). Effect of selected environmental and physico-chemical factors on bacterial cytoplasmic membranes. J. Microbiol. Meth. 52: 149–182
Dowling N.J.E., Widdel F. and White D.C. (1986). Phospholipid ester-linked fatty-acid biomarkers of acetate-oxidizing sulfate reducers and other sulfide-forming bacteria. J. Gen. Microbiol. 132: 1815–1825
DSMZ 2005. Deutche Sammlung von Mikroorganismen und Zellkulturen GmbH, DSMZ http://www.dsmz.de/media.htm
Fossing H. and Jørgensen B.B. (1989). Measurement of bacterial sulfate reduction in sediments: evaluation of a single-step chromium reduction method. Biogeochem. 8: 205–222
Guillou C. and Guespin-Michel J.F. (1996). Evidence for two domains of growth temperature for the psychrotrophic bacterium Pseudomonas fluorescens MF0. Appl. Environ. Microb. 62: 3319–3324
Harder W. and Veldkamp H. (1968). Physiology of an obligately psychrophilic marine Pseudomonas species. J. Appl. Bacteriol. 31: 12–23
Harder W. and Veldkamp H. 1971. Competition of marine psychrophilic bacteria at low temperatures. Antonie van Leuwenhoek 37: 51– 63
Hébraud M. and Potier P. 1999. Cold shock response and low temperature adaptation in psychrotrophic bacteria. J. Mol. Microbiol. Biotechnol. 1: 211–219
Hicks R.E., Amann R.I. and Stahl D.A. (1992). Dual staining of natural bacterioplankton with 4’,6-diamidino-2-phenylindole and fluorescent oligonucleotide probes targeting kingdom-level 16s Ribosomal-RNA sequences. Appl. Environ. Microb. 58: 2158–2163
Huss V.A.R., Festl H. and Schleifer K.H. (1983). Studies on the spectrophotometric determination of DNA hybridization from renaturation rates. Syst. Appl. Microbiol. 4: 184–192
Isaksen M.F. and Teske A. (1996). Desulforhopalus vacuolatus gen nov, sp nov, a new moderately psychrophilic sulfate-reducing bacterium with gas vacuoles isolated from a temperate estuary. Arch. Microbiol. 166: 160–168
Isaksen M.F. and Jørgensen B.B. (1996). Adaptation of psychrophilic and psychrotrophic sulfate-reducing bacteria to permanently cold marine environments. Appl. Environ. Microbiol. 62: 408–414
Jørgensen B.B. (1978). A comparison of methods for the quantification of bacterial sulfate reduction in coastal marine sediments I. Measurement with radiotracer techniques. Geomicrobiol. J. 1: 11–27
Jørgensen B.B. (1982). Mineralization of organic-matter in the sea bed - the role of sulfate reduction. Nature. 296: 643–645
Knoblauch C. and Jørgensen B.B. (1999). Effect of temperature on sulfate reduction, growth rate and growth yield in five psychrophilic sulfate-reducing bacteria from Arctic sediments. Environ. Microbiol. 1: 457–467
Knoblauch C., Sahm K. and Jørgensen B.B. (1999). Psychrophilic sulfate-reducing bacteria isolated from permanently cold Arctic marine sediments: description of Desulfofrigrus oceanense gen. nov., sp nov., Desulfofrigus fragile sp nov., Desulfofaba gelida gen. nov., sp nov., Desulfotalea psychrophila gen. nov., sp nov and Desulfotalea arctica sp nov. Int. J. Syst. Bacteriol. 49: 1631–1643
Kohring L.L., Ringelberg D.B., Devereux R., Stahl D.A., Mittelman M.W. and White D.C. (1994). Comparison of phylogenetic-relationships based on phospholipid fatty-acid profiles and ribosomal-RNA sequence similarities among dissimilatory sulfate-reducing bacteria. Fems Microbiol. Lett. 119: 303–308
Könneke M. and Widdel F. (2003). Effect of growth temperature on cellular fatty acids in sulfate-reducing bacteria. Environ. Microbiol. 5: 1064–1070
Kuever J., Konneke M., Galushko A. and Drzyzga O. (2001). Reclassification of Desulfobacterium phenolicum as Desulfobacula phenolica comb. nov and description of strain Sax(T) as Desulfotignum balticum gen. nov., sp nov. Int. J. Syst. Evol. Micr. 51: 171–177
Kuykendall L.D., Roy M.A., O’Neill J.J. and Devine T.E. (1988). Fatty-acids, antibiotic-resistance, and deoxyribonucleic-acid homology groups of Bradyrhizobium-japonicum. Int. J. Syst. Bacteriol. 38: 358–361
Lane D.J. (1991). 16/23S rRNA Sequencing. In: Stackebrandt E., Goodfellow M. (eds). Nucleic acid techniques in bacterial systematics. Wiley, Chichester, England.
Lien T. and Beeder J. (1997). Desulfobacter vibrioformis sp. nov., a sulfate reducer from a water-oil separation system. Int. J. Syst. Bacteriol. 47: 1124–1128
Ludwig W., Strunk O., Westram R., Richter L., Meier H., Yadhukumar, Buchner A., Lai T., Steppi S., Jobb G., Forster W., Brettske I., Gerber S., Ginhart A.W., Gross O., Grumann S., Hermann S., Jost R., Konig A., Liss T., Lussmann R., May M., Nonhoff B., Reichel B., Strehlow R., Stamatakis A., Stuckmann N., Vilbig A., Lenke M., Ludwig T., Bode A. and Schleifer K.H. 2004. ARB: a software environment for sequence data. Nucleic Acids Res. 32: 1363–1371, http://www.arb-home.de/
Mesbah M., Premachandran U. and Whitman W.B. (1989). Precise measurement of the G+C content of deoxyribonucleic-acid by High-Performance Liquid-Chromatography. Int. J. Syst. Bacteriol. 39: 159–167
Miller L.T. (1982). Single derivatization method for routine analysis of bacterial whole-cell fatty-acid methyl-esters, including hydroxy-acids. J. Clin. Microbiol. 16: 584–586
Mohr P.W. and Krawiec S. (1980). Temperature characteristics and Arrhenius plots for nominal psychrophiles, mesophiles and thermophiles. J. Gen. Microbiol. 121: 311–317
Morita R.Y. (1975). Psychrophilic bacteria. Bacteriol. Rev. 39: 144–167
Muyzer G., Dewaal E.C. and Uitterlinden A.G. (1993). Profiling of complex microbial-populations by Denaturing Gradient Gel-Electrophoresis analysis of Polymerase Chain Reaction-amplified genes-coding for 16s Ribosomal-RNA. Appl. Environ. Microb. 59: 695–700
Nedwell D.B., Walker T.R., Ellis-Evans J.C. and Clarke A. (1993). Measurements of seasonal rates and annual budgets of organic-carbon fluxes in an Antarctic coastal environment at Signy Island, South Orkney Islands, suggest a broad balance between production and decomposition. Appl. Environ. Microb. 59: 3989–3995
Bundesamt für Seeschifffahrt und Hydrographie. 2004. North-Sea. Sea surface temperatures http://www.bsh.de/en/Marine%20data/Observations/Sea%20surface%20temperatures/anom.jsp
Oude Elferink S.J.W.H., Maas R.N., Harmsen H.J.M. and Stams A.J.M. (1995). Desulforhabdus amnigenus Gen-Nov Sp-Nov, a sulfate reducer isolated from anaerobic granular sludge. Arch. Microbiol. 164: 119–124
Oude Elferink S.J.W.H., Akkermans-van Vliet W.M., Bogte J.J. and Stams A.J.M. (1999). Desulfobacca acetoxidans gen. nov., sp, nov., a novel acetate- degrading sulfate reducer isolated from sulfidogenic granular sludge. Int. J. Syst. Bacteriol. 49: 345–350
Parkes R.J., Gibson G.R., Mueller-Harvey I., Buckingham W.J. and Herbert R.A. (1989). Determination of the substrates for sulfate-reducing bacteria witin marine and estuarine sediments with different rates of sulfate reduction. J. Gen. Microbiol. 135: 175–187
Rabus R., Hansen T. and Widdel F. 2000. The dissimilatory sulfate- and sulfur-reducing Prokaryotes. In: Dworkin M. et al. (eds), The Prokaryotes: An Evolving Electronic Resource for the Microbiological Community, 3rd edition, release 3.7, November 2, 2001. Springer-Verlag, New York, http://link.springer-ny.com/link/service/books/10125/
Rabus R., Bruchert V., Amann J. and Konneke M. (2002). Physiological response to temperature changes of the marine, sulfate-reducing bacterium Desulfobacterium autotrophicum. Fems Microbiol. Ecol. 42: 409–417
Russell N.J. (1990). Cold adaptation of microorganisms. Phil. Trans. Roy. Soc. B. 326: 595–611
Russell N.J. and Hamamoto T. 1998. Psychrophiles. In: K.␣Horikoshi and W.D. Grant (eds), Extremophiles: Microbial Life in Extreme Environments. John Wiley & Sons, New York, pp. 25–45
Rütters H., Sass H., Cypionka H. and Rullkotter J. (2001). Monoalkylether phospholipids in the sulfate-reducing bacteria Desulfosarcina variabilis and Desulforhabdus amnigenus. Arch. Microbiol. 176: 435–442
Sagemann J., Jørgensen B.B. and Greeff O. (1998). Temperature dependence and rates of sulfate reduction in cold sediments of Svalbard, Arctic Ocean. Geomicrobiol. J. 15: 85–100
Sass H., Berchtold M., Branke J., Konig H., Cypionka H. and Babenzien H.D. (1998). Psychrotolerant sulfate-reducing bacteria from an oxic freshwater sediment, description of Desulfovibrio cuneatus sp. nov. and Desulfovibrio litoralissp. nov. Syst. Appl. Microbiol. 21: 212–219
Sørensen J., Christensen D. and Jørgensen B.B. (1981). Volatile fatty-acids and hydrogen as substrates for sulfate-reducing bacteria in anaerobic marine sediment. Appl. Environ. Microbiol. 42: 5–11
Tamaoka J. and Komagata K. (1984). Determination of DNA-base composition by reversed-phase High-Performance Liquid-Chromatography. Fems. Microbiol. Lett. 25: 125–128
Taylor J. and Parkes R.J. (1985). Identifying different populations of sulfate-reducing bacteria within marine sediment systems, using fatty-acid biomarkers. J. Gen. Microbiol. 131: 631–642
Wayne L.G., Brenner D.J., Colwell R.R., Grimont P.A.D., Kandler O., Krichevsky M.I., Moore L.H., Moore W.E.C., Murray R.G.E., Stackebrandt E., Starr M.P. and Trüper H.G. (1987). Report of the Ad-Hoc-Committee on reconciliation of approaches to bacterial systematics. Int. J. Syst. Bacteriol. 37: 463–464
Wheeler D.L., Church D.M., Lash A.E., Leipe D.D., Madden, T.L., Pontius J.U., Schuler G.D., Schriml L.M., Tatusova T.A., Wagner L. and Rapp B.A. (2002). Database resources of the National Center for Biotechnology Information: 2002 update. Nucleic Acids Res. 30: 13–16
Widdel F. (1987). New types of acetate-oxidizing, sulfate-reducing Desulfobacter species, D-hydrogenophilus sp-nov, D-latus sp-nov, and D-curvatus sp-nov. Arch. Microbiol. 148: 286–291
Widdel F. and Pfennig N. (1977). New anaerobic, sporing, acetate-oxidizing, sulfate-reducing bacterium, Desulfotomaculum(emend) acetoxidans. Arch. Microbiol. 112: 119–122
Widdel F. and Pfennig N. (1981). Studies on dissimilatory sulfate-reducing bacteria that decompose fatty-acids .1. Isolation of new sulfate-reducing bacteria enriched with acetate from saline environments - description of Desulfobacter-postgatei gen-nov, sp-nov. Arch. Microbiol. 129: 395–400
Widdel F. and Bak F. (1992). Gram-negative mesophilic sulfate-reducing bacteria. In: Balows H., Trüper H.G., Dworkin M., Harder W. and Schleifer K.H. (eds). The prokaryotes. Springer-Verlag, New York
Wilkinson S.G. (1988). Gram-negative bacteria. In: Ratledge C., Wilkinson S.G. (eds). Microbial Lipids. Academic Press, London, pp. 299–457
Zheng D.D., Alm E.W., Stahl D.A. and Raskin L. (1996). Characterization of universal small-subunit rRNA hybridization probes for quantitative molecular microbial ecology studies. Appl. Environ. Microb. 62: 4504–4513
Acknowledgements
The study was supported by SNF Grant No. 21-00-0309. Kai Finster thanks the crew of the research vessel R/V Heincke for a very fruitful sampling cruise. We thank Tove Wiegers for her skilful technical assistance, Kasper Kjeldsen for help with the phylogentic analysis and Rodney Herbert for a critical review of the manuscript. The constructive criticism of 3 anonymous reviewers is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tarpgaard, I.H., Boetius, A. & Finster, K. Desulfobacter psychrotolerans sp. nov., a new psychrotolerant sulfate-reducing bacterium and descriptions of its physiological response to temperature changes. Antonie Van Leeuwenhoek 89, 109–124 (2006). https://doi.org/10.1007/s10482-005-9014-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-005-9014-1