Abstract
Purpose
Degenerative mitral stenosis (DMS) is associated with a poor prognosis. Although mean transmitral gradient (TMG) has shown a good correlation with outcome, little is known about the association between other echocardiographic parameters and prognosis in patients with DMS. The current study aimed to evaluate the prognostic value of left atrial volume index (LAVI) in patients with DMS.
Methods
A total of 155 patients with DMS (72[63–80] years, 67% female) were included. The population was divided according to LAVI: normal-sized LAVI (LAVI ≤ 34 ml/m2); and enlarged LAVI (> 34 ml/m2).
Results
Patients with enlarged LAVI had a higher left ventricular mass index (120[96–146] vs. 91[70–112] g/m2 p < 0.001), as well as a higher prevalence of significant mitral regurgitation and severe aortic stenosis (23% vs. 10% p = 0.046 and 38% vs. 15% p=0.001, respectively) compared to patients with normal-sized LAVI. During a median follow-up of 25 months, 56 (36%) patients died. Patients with enlarged LAVI had worse prognosis compared to patients with normal-sized LAVI (p = 0.026). In multivariable Cox regression model, an enlarged LAVI was independently associated with all-cause mortality (HR 2.009, 95% CI 1.040 to 3.880, P = 0.038).
Conclusion
An enlarged LAVI (> 34 ml/m2) is significantly associated with excess mortality in patients with DMS. After adjusting for potential confounders, an enlarged LAVI was the only parameter that remained independently associated with prognosis.
Similar content being viewed by others
Introduction
Mitral annular calcification (MAC) is a degenerative process arising from the mitral annulus and can extend to the mitral valve leaflets. This process may cause a reduction in effective mitral valve orifice area and failure of leaflet coaptation, resulting in degenerative mitral stenosis (DMS) [1]. The prognosis of DMS is poor, with a 5-year mortality rate of up to 50% [2]. However, not much is known about the echocardiographic prognostic parameters in patients with DMS. It has been shown that an increased mean transmitral gradient (TMG ≥ 2 mmHg) is associated with worse outcomes [2], with mortality increasing in parallel to the increase in mean TMG [3]. However, in patients with DMS, the mean TMG may not only be related to annular calcification and consequent narrowing of the mitral orifice area. An increased TMG may also be related to other conditions influencing the pressure gradient between the left ventricle and atrium (e.g. reduced left ventricular and/or atrial compliance), especially since patients with DMS are generally older and have multiple comorbidities including hypertension, left ventricular diastolic dysfunction, and atrial fibrillation [4], all of which may impact left atrial (LA) volume. Accordingly, the LA volume index (LAVI) may be an important factor related to prognosis in patients with DMS. Therefore, the aim of the current study is to evaluate whether an enlarged LAVI is associated with worse outcomes in patients with DMS.
Methods
Study population
Patients diagnosed with DMS, between February 2001 and October 2017 from the Leiden University Medical Center (Leiden, The Netherlands) and the National University Health System (Singapore) were selected. DMS was defined as MAC with a mean TMG ≥ 2 mmHg. Patients with previous mitral valve intervention or rheumatic mitral stenosis, were excluded. Demographic and clinical data (including AF, stroke, associated comorbidities, and medical therapy) were collected from hospital electronic patient files. For retrospective analysis of clinically acquired data, the institutional review board of each center waived the need for written patient informed consent. All data used for this study were acquired for clinical purposes and handled anonymously.
Echocardiographic data acquisition and analysis
Transthoracic echocardiography images were recorded using commercially available ultrasound devices. Echocardiography was performed with patients at rest, in the left lateral decubitus position. Electrocardiogram-triggered echocardiographic data were acquired and digitally stored in cine-loop format for offline analysis.
Left ventricular end-diastolic and end-systolic volumes were measured in apical two- and four-chamber views, and the left ventricular ejection fraction (LVEF) was calculated using the biplane Simpson’s method. LA volume was measured on apical 2- and 4-chamber views using the biplane method and indexed to body surface area to calculate LAVI [4]. The population was divided into two groups based on LA size, according to recommendations for cardiac chamber quantification by echocardiography [4]: patients with normal-sized LAVI (≤ 34 ml/m2) and patients with enlarged LAVI (> 34 ml/m2).
Continuous spectral Doppler was used to measure the forward diastolic flow across the mitral valve (mean TMG) in the apical four-chamber view, and was averaged over 3 cardiac cycles in sinus rhythm and 5 cardiac cycles in AF [5]. The severity of mitral regurgitation was assessed following an integrative approach that includes qualitative, semiquantitative and quantitative measures according to contemporary guidelines.
The tricuspid annular plane systolic excursion was used for evaluation of right ventricular systolic function, measured on a right-ventricular focused apical 4-chamber view using M-mode. Concomitant aortic stenosis (AS) severity was determined by the evaluation of the peak aortic velocity, mean transaortic gradient, aortic valve area according to the continuity equation, and dimensionless valve index according to guideline recommendations [6, 7].
Follow-up
The primary outcome of the study was all-cause mortality. Mortality data were collected via the departmental cardiology information system (EPD-Vision 11.8.4.0, Leiden University Medical Center, Leiden, The Netherlands), which is linked to the governmental registry of mortality, and the computerized patient support system (CPSS) in Singapore.
Statistical analysis
Categorical data are presented as frequencies and percentages. Continuous data are expressed as mean ± standard deviation if normally distributed and median and interquartile range (IQR) if non-normally distributed. Categorical data were compared with the χ2 test. Normally distributed variables were compared using the independent samples t-test, while non-normally distributed variables were compared with the Mann–Whitney U test. Cumulative event-free survival for the endpoint of all-cause mortality was evaluated using the Kaplan–Meier method, and the log-rank test was used to compare groups. A multivariable Cox proportional hazards model was constructed to investigate the association of combined enlarged LAVI and mean TMG with all-cause mortality. Covariates considered to have a prognostic association on the basis of epidemiological data, including age, sex, mean TMG, LVEF ≥ 50% and hypertension, were included in the multivariable model. Hazard ratio (HR) and 95% confidence intervals (CI) were reported. Twente random individuals were selected for evaluation of interobserver agreement of LAVI, using intraclass correlation coefficients (ICCs) and Bland–Altman analysis. The second observer was blinded to the measurements of the first observer for interobserver measurements. All tests were two-sided and p-values < 0.05 were considered statistically significant. Statistical analysis was performed using SPSS version 25.0 (IBM Corporation, Armonk, New York) and R version 4.0.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient population
A total of 155 patients (67% women, median age 72 years [IQR 63 to 80]) were included. Of these patients, 69% had hypertension, 32% had AF, and 24% had a history of stroke. A total of 67 patients had a normal-sized LAVI (≤ 34 ml/m2), whereas 88 patients had enlarged LAVI (> 34 ml/m2). Patients with enlarged LAVI had a higher prevalence of arterial hypertension and were using more medications than patients with normal-sized LAVI (Table 1).
Echocardiographic characteristics
The overall study population had preserved left and right ventricular systolic function. A total of 84% had an LVEF ≥ 50% and the median tricuspid annular plane systolic excursion was 22 mm [IQR 19 to 23]. The median TMG was 6 mmHg [IQR 4 to 8], 60 (39%) patients had mean TMG < 5 mmHg, 75 (48%) mean TMG 5–10 mmHg and 20 (13%) mean TMG > 10 mmHg. Concomitant significant (≥ moderate) MR was found in 17% of the study population and 28% had severe AS. The median LAVI was 40 ml/m2 [IQR 28 to 52]. When dividing the population according to the presence of enlarged LAVI, the group of patients with enlarged LAVI had a higher left ventricular mass index as well as a higher prevalence of significant MR and severe AS compared to their counterparts (Table 2). The ICC for LAVI interobserver variability was 0.927(95% CI: 0.817–0.971), p < 0.001).
Patients outcomes
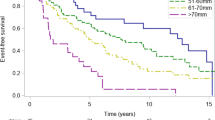
During a median follow-up of 25 months [IQR 6–55], 56 (36%) patients died. The cumulative survival rates at 12, 24, and 60 months’ follow-up were superior for patients with normal-sized LAVI compared to patients with enlarged LAVI (92%, 84%, and 66%, vs. 84%, 70%, and 44%, respectively; p = 0.026; Fig. 1). Univariable Cox regression analysis demonstrated a significant association between enlarged LAVI and all-cause mortality (HR 1.937, 95% CI 1.069 to 3.509, p = 0.029). The ethnicity of the patient did not influence the outcome (p = 0.728). To evaluate the association between LAVI enlargement and outcomes in patients with DMS, a comprehensive multivariable Cox regression was performed. Three different models were constructed; model 1: adjusted enlarged LAVI with demographic variables including age and sex (HR 2.021, 95% CI 1.088 to 3.752, P = 0.026); model 2: included model 1 and echocardiographic variables as mean TMG and LVEF ≥ 50% (HR 2.105, 95% CI 1.128 to 3.930, p = 0.019); and model 3: included model 2 and the clinical variable of hypertension (HR 2.009 95% CI 1.040 to 3.880, p = 0.038). In all three models, enlarged LAVI remained independently associated with all-cause mortality (Table 3). A different model, included aortic valve area is available supplementary material (Table S2).
Discussion
The main findings of this study can be summarized as follows: (1) in patients with DMS, an enlarged LAVI is associated with older age and a higher prevalence of arterial hypertension, heart failure symptoms, heart failure medication and valvular heart disease (concomitant MR and severe AS); (2) patients with an enlarged LAVI have worse prognosis than patients with a normal-sized LAVI; and (3) an enlarged LAVI is independently associated with all-cause mortality after adjusting for demographic, clinical and echocardiographic risk factors.
Clinical and echocardiographic variables associated with MAC formation
DMS is a consequence of MAC, a degenerative process of the mitral valve annulus that can extend to the mitral valve leaflets, thereby increasing the TMG. It has been considered mainly as a cardiac sign of aging [8, 9], with studies in these patients showing a median age > 70 years [2, 3]. Abnormal calcium-phosphorus metabolism, a common finding in patients with chronic renal disease, may contribute to MAC formation [10]. The process also has inflammatory pathophysiological mechanisms [11], Massera et al. showed that patients with MAC have evidence of increased local calcium activity and inflammation [12]. In a multivariable model, disease activity was associated with female sex. It remains unclear why female patients have a higher prevalence of MAC [13], with 67% female patients in the current study population. One hypothesis is the impact of medication to treat osteoporosis on the valve calcification process, which is already being investigated by the Scottish Aortic Stenosis and Lipid Lowering Trial, Impact on Regression (SALTIRE) II in patients with AS. Another theory has to do with sex hormones. Subramanya et al. found that an androgenic hormonal profile is associated with greater coronary artery calcium progression in post-menopausal women [14]. However, the impact of sex hormones on valvular calcification is poorly understood. Several hemodynamic conditions that cause an elevation in left ventricular peak end-systolic pressure can increase mitral annular tension and accelerate the degenerative process. Those conditions are mainly arterial hypertension, AS and left ventricular hypertrophy, and all of them were found to be associated with MAC [15, 16]. Those MAC risk factors have also been identified in our study population: two thirds of the current study population have arterial hypertension, one third severe AS, and the female patients had a high left ventricular mass index (106[78–128]g/m2).
Echocardiographic variables associated with outcomes
For a long time, MAC has been considered as an innocent bystander of cardiac aging. In recent years, however, there has been growing evidence that patients with MAC and DMS have a worse prognosis. In 1004 patients with severe MAC and mean TMG ≥ 2 mmHg, Pasca et al. demonstrated that these patients had a very poor prognosis, with 1- and 5-year survival rates being 78% and 47%, respectively. Prognosis was worse in patients with a more advanced age, as well as in those with chronic kidney disease, AF and concomitant valvular lesions [2]. Another study by Bertrand et al., dividing 5754 patients with MAC into three groups according to the mean TMG, showed that survival rates decreased as the mean TMG increased [3]. Although prognostically relevant, mean TMG is not well validated in patients with DMS. Indeed, studies comparing echocardiographically-derived measurements of TMG with invasive hemodynamic measurements mainly included patients with rheumatic mitral valve stenosis [17]. Moreover, mean TMG is influenced by the pressure difference between the left ventricle and LA, and is therefore also influenced by reduced left ventricular compliance and an elevated LA pressure, conditions that often coexist in patients with DMS. In addition, mean TMG also depends on heart rate and stroke volume, which are affected by the high proportion of supraventricular arrhythmias seen in these patients. In contrast, LAVI is much less dependent on changes in cardiac hemodynamics or supraventricular arrhythmias and may better reflect the severity and duration of volume and pressure overload in patients with DMS or MR. A previous study already showed that patients with DMS had larger LAVI compared to a matched group of patients without DMS [5].
In addition, LA dilatation has been shown to have a strong association with cardiovascular events and worse outcomes in different cardiovascular diseases [18, 19]. In 611 patients with rheumatic mitral stenosis, enlarged LAVI was independently associated with poor outcomes, although this association was less strong in patients with very severe mitral stenosis (mitral valve area ≤ 1 cm2) [20]. In the present study, LAVI enlargement (> 34 ml/m2) and aortic valve area were the only echocardiographic parameter that were associated with outcomes on univariable Cox regression analysis (Supplementary data – Table S1). LAVI remained independently associated with all-cause mortality after adjustment for other relevant confounders. Compared to the group with normal-sized LAVI, patients with enlarged LAVI had a significantly higher prevalence of comorbidities associated with MAC prognosis, including arterial hypertension, higher left ventricular mass index and severe AS (Fig. 2). This illustrates that comorbidities in patients with DMS may impact on LAVI. It remains difficult to distinguish between the impact of DMS and other cofactors on LA dilatation. The similar mean TMG in both groups supports the assumption that DMS probably contributes less to LAVI enlargement than the other comorbidities.
Assessment of the mitral valve area in patients with DMS is challenging. The different methods of measuring mitral valve stenosis are extrapolated from studies of rheumatic mitral stenosis and are less validated in patients with DMS. Moreover, there is a lack of information regarding the correlation of valve stenosis and mean TMG or prognosis in this patient population. Because LAVI is less influenced by instantaneous changes in heart rate or flow, and better integrates clinical and hemodynamic changes noted in patients with DMS, it probably better reflects the complexity of cardiac co-morbidities observed in these patients. Measurements of LAVI should therefore be included in the risk-stratification of patients with DMS.
Study limitations
This is a retrospective, two centers study, with inherent limitations. Calcium burden and staging of the disease were not systematically available. Only one study center had strain analysis available, so this was not provided in the current study. The exact contribution of mitral valve area to LAVI enlargement could not be ascertained. Heart failure symptoms and coronary artery disease information were not included in the database. The study data was unsuitable for using the Charlson comorbidity index. Since the cause of death was not systematically ascertained, all-cause mortality was chosen as the endpoint. The study population was of limited size and larger studies are needed to confirm our observations.
Conclusion
Greater LAVI is independently associated with all-cause mortality in patients with DMS. LAVI measurements may therefore be incorporated in the current risk stratification of these patients. Whether treatment of DMS will reduce LA dimensions and improve prognosis merits further investigation.
References
Movva R, Murthy K, Romero-Corral A, Seetha Rammohan HR, Fumo P, Pressman GS (2013) Calcification of the mitral valve and annulus: systematic evaluation of effects on valve anatomy and function. J Am Soc Echocardiogr 26(10):1135–1142
Pasca I, Dang P, Tyagi G, Pai RG (2016) Survival in patients with degenerative mitral stenosis: results from a large retrospective cohort study. J Am Soc Echocardiogr 29(5):461–469
Bertrand PB, Churchill TW, Yucel E, Namasivayam M, Bernard S, Nagata Y et al (2020) Prognostic importance of the transmitral pressure gradient in mitral annular calcification with associated mitral valve dysfunction. Eur Heart J 41(45):4321–4328
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L et al (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28(1):1-39 e14
Qamruddin S, Pressman GS (2020) Determining severity of mitral stenosis due to annular calcification. Am J Cardiol 125(1):161–162
Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP et al (2009) Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr 22(1):1–23
Lancellotti P, Tribouilloy C, Hagendorff A, Popescu BA, Edvardsen T, Pierard LA et al (2013) Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 14(7):611–644
Barasch E, Gottdiener JS, Larsen EK, Chaves PH, Newman AB, Manolio TA (2006) Clinical significance of calcification of the fibrous skeleton of the heart and aortosclerosis in community dwelling elderly. The Cardiovascular Health Study (CHS). Am Heart J 151(1):39–47
Roberts WC, Shirani J (1998) Comparison of cardiac findings at necropsy in octogenarians, nonagenarians, and centenarians. Am J Cardiol 82(5):627–631
Fox CS, Larson MG, Vasan RS, Guo CY, Parise H, Levy D et al (2006) Cross-sectional association of kidney function with valvular and annular calcification: the Framingham heart study. J Am Soc Nephrol 17(2):521–527
Fox CS, Guo CY, Larson MG, Vasan RS, Parise H, O’Donnell CJ et al (2006) Relations of inflammation and novel risk factors to valvular calcification. Am J Cardiol 97(10):1502–1505
Massera D, Trivieri MG, Andrews JPM, Sartori S, Abgral R, Chapman AR et al (2019) Disease activity in mitral annular calcification. Circ Cardiovasc Imaging 12(2):e008513
Kanjanauthai S, Nasir K, Katz R, Rivera JJ, Takasu J, Blumenthal RS et al (2010) Relationships of mitral annular calcification to cardiovascular risk factors: the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 213(2):558–562
Subramanya V, Zhao D, Ouyang P, Ying W, Vaidya D, Ndumele CE et al (2019) Association of endogenous sex hormone levels with coronary artery calcium progression among post-menopausal women in the Multi-Ethnic Study of Atherosclerosis (MESA). J Cardiovasc Comput Tomogr 13(1):41–47
Fulkerson PK, Beaver BM, Auseon JC, Graber HL (1979) Calcification of the mitral annulus: etiology, clinical associations, complications and therapy. Am J Med 66(6):967–977
Nestico PF, Depace NL, Morganroth J, Kotler MN, Ross J (1984) Mitral annular calcification: clinical, pathophysiology, and echocardiographic review. Am Heart J 107(5 Pt 1):989–996
Nishimura RA, Rihal CS, Tajik AJ, Holmes DR Jr (1994) Accurate measurement of the transmitral gradient in patients with mitral stenosis: a simultaneous catheterization and Doppler echocardiographic study. J Am Coll Cardiol 24(1):152–158
Tsang TS, Abhayaratna WP, Barnes ME, Miyasaka Y, Gersh BJ, Bailey KR et al (2006) Prediction of cardiovascular outcomes with left atrial size: is volume superior to area or diameter? J Am Coll Cardiol 47(5):1018–1023
Moller JE, Hillis GS, Oh JK, Seward JB, Reeder GS, Wright RS et al (2003) Left atrial volume: a powerful predictor of survival after acute myocardial infarction. Circulation 107(17):2207–2212
Cho IJ, Jeong H, Chang HJ (2021) Prognostic value of left atrial volume index in patients with rheumatic mitral stenosis. Clin Cardiol 44(3):364–370
Funding
The Department of Cardiology of the Leiden University Medical Center received research grants from Medtronic, Biotronik, Boston Scientific, GE Healthcare, and Edwards Lifesciences. Steele C Butcher received funding from European Society of Cardiology [Grant Number 000080404]. Idit Yedidya received a fellowship grant from Rabin Medical Center, Israel. Ching-Hui Sia was supported by the National University of Singapore Yong Loo Lin School of Medicine’s Junior Academic Faculty Scheme.
Author information
Authors and Affiliations
Contributions
IY, SCB, JS, PB—Conception or design of the work; acquisition, analysis, and interpretation of data for the work; drafting the work; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. JNN, TL, RL, CHS, NWSC, WKFK, KKP—Conception or design of the work; revising the article critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. RK, NAM, VD, JJB—Conception or design of the work; revising the article critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
Victoria Delgado received speaker fees from Abbott Vascular, Edwards Lifesciences, GE Healthcare, MSD, Novartis and Medtronic. Nina Ajmone Marsan and Jeroen J Bax received speaker fees from Abbott Vascular. The other authors report no conflicts.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yedidya, I., Butcher, S.C., Stassen, J. et al. Prognostic value of left atrial volume index in degenerative mitral stenosis. Int J Cardiovasc Imaging 38, 2687–2693 (2022). https://doi.org/10.1007/s10554-022-02691-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-022-02691-z