Abstract
Palmaria palmata, commonly referred to as dulse, is a well-known and highly valued red macroalga distributed along the North Atlantic shores within a latitude range of approximately 40 to 80 °N. It is a species of commercial importance with historical records of use as food dating back several centuries to the current harvesting of dulse by hand-picking on the foreshore in Western Europe as well as Canada (New Brunswick and Nova Scotia) and USA (Maine). Because the demand for P. palmata increases and future sustainable commercial developments cannot rely solely on wild-harvested biomass, significant efforts have been made by research and industrial actors to cultivate the species. The low rates of spore release and germination, high mortality and epibiont contaminations remain major bottlenecks and point towards the need for optimized hatchery methods to enable upscaling the biomass production. The present review summarizes the available knowledge related to the biology, including the unique life history of the species among the Rhodophyta, the ecology as well as the nutrient composition and quality of P. palmata as food. Recent advances in taxonomy and cultivation techniques are reported along with a status of regulations for the commercial harvest of wild populations. An outlook on future industrial perspectives using biomass of P. palmata is also given.
Similar content being viewed by others
Morphological description
The name of the red macroalga Palmaria palmata reflects the characteristic shape of the plant consisting of flattened oblong lobes expanding from the middle of the frond as the shape of a hand or a palm. The fronds arise from a disc-shaped holdfast prolonged by a short stipe (Fig. 1). Individuals can grow up to a size of 50 cm-long but usually between 10 and 20 cm. Observations of the species on the North-eastern coast of North America report a length up to 50 cm with wide individual branches that may have marginal proliferations in old damage specimens (Sears 1998). Broader fronds (length and width > 50 cm) may be observed on Eastern Canadian shores (L. Le Gall, pers. observation) compared to those growing on Atlantic European shores.
The texture of the fronds is somewhat leathery when the plant is old. New plants have a distinct fresh red colour while older plants are often darker with a reddish-brown colour. Individuals may turn greenish or even bleach due to higher temperatures and increased sunlight exposure during the early summer months, causing changes in pigment composition. Distinct morphotypes of P. palmata i.e. with finely dissected blades and numerous divisions as shown in Fig. 1, can be observed in more sheltered and silty areas of semi-exposed shores. These morphotypes are known as the varieties sobolifera and sarniensis. However, the validity of this varietal designation is questioned by studies showing the production of viable hybrids from individuals of the sarniensis-sobolifera complex and typical palmata plants (van der Meer 1987), and the low genetic divergence across these entities based on nuclear genes (Kraan and Guiry 2006). These results highlight the morphological plasticity of the species under the influence of environmental factors.
Nomenclature
Scientific name and synonyms
The currently accepted name for the species is Palmaria palmata (Linnaeus) Weber & Mohr 1805. Homotypic synonyms are listed by Guiry and Guiry (2022) and include:
-
Fucus palmatus Linnaeus 1753
-
Ulva palmata (Linnaeus) Withering 1796
-
Ceramium palmatum (Linnaeus) Stackhouse 1797
-
Delesseria palmata (Linnaeus) Lamouroux 1813
-
Sarcophylla palmata (Linnaeus) Stackhouse 1816
-
Halymenia palmata (Linnaeus) Agardh 1817
-
Sphaerococcus palmatus (Linnaeus) Wahlenberg 1826
-
Rhodymenia palmata (Linnaeus) Greville 1830
-
Isomenia palmata (Linnaeus) Bachelot de la Pylaie 1830
Vernacular names
The English common name “dulse” is the most employed to refer to the species also in other languages. This vernacular name as well as the alternative “dillisk” is closely related to “duileasc” (Gaelic) (Mouritsen et al. 2013) and “tellesk” (Breton). Other vernacular names include “creannach” (Gaelic), “søl” or “söl” (Norwegian/Danish and Icelandic) (Østgaard and Indergaard 2017), “goëmon à vache” (French, translated from the Breton “bezhin saout” meaning “cattle’s seaweed”) (Garineaud 2018) and “botelho-comprido” (Portuguese).
Taxonomy and phylogeny
Taxonomy
Palmaria palmata was first described by Linnaeus in 1753 under the name Fucus palmatus. It was later designated as P. palmata by the German naturalists Friedrich Weber and Daniel M.H. Mohr in 1805. In 1830, Greville grouped many red membranaceous species including P. palmata, in the genus Rhodomenia (currently Rhodymenia). The species was then known as Rhodymenia palmata (Linnaeus) Greville, until Guiry (1974) proposed to reinstate the genus Palmaria (over which Rhodymenia had been conserved) after observing morphological, anatomical and reproductive features (e.g. the absence of carposporophytic generation and the presence of a stalk cell involved in the production of the tetrasporangia). He proposed to place Palmaria along with Halosaccion and Leptosarca in a new family (Palmariaceae). A few years later, a new order (Palmariales) was proposed to accommodate the Palmariaceae (Guiry and Irvine 1978). Further studies of the life history (van der Meer and Chen 1979; van der Meer and Todd 1980) and ultrastructural features of the species (Pueschel 1979; Pueschel and Cole 1982), supported Guiry’s revision that was later confirmed by molecular studies (Harper and Saunders 2002). Palmaria palmata was included in two pioneer phylogenies published in 1994, inferred from the analysis of plastid rbcL sequences (encoding the large subunit of the ribulose-1,5-biphosphate carboxylase/oxygenase enzyme or RuBisCo) (Freshwater et al. 1994) and gene sequences encoding small subunit ribosomal RNA (SSU rDNAs) (Ragan et al. 1994). The latter marker established the Palmariales as closely allied to the Nemaliales and Acrochaetiales, which was at the time, an unexpected result. Subsequent phylogenies (e.gHarper and Saunders 2002; Le Gall and Saunders 2007; Saunders et al. 2018) confirmed this relationship.
Recent studies based on molecular-genetic data supported the transfer of related species of P. palmata, namely P. mollis, P. callophylloides, P. marginicrassa and P. stenogona, to the genus Devaleraea (Saunders et al. 2018; Skriptsova and Kalita 2020; Skriptsova et al. 2022). Six species are currently accepted under the Palmaria genus, including Palmaria decipiens (Reinsch) Ricker from the Macquarie Island (Southern Pacific), Palmaria georgica (Reinsch) Ricker from the Southern Atlantic, Palmaria integrifolia Selivanova & Zhigadlova from the Russian Pacific coast, Palmaria hecatensis Hawkes from the Northern Pacific, Palmaria moniliformis (Blinova & Zinova) Perestenko from the Russian Far East and P. palmata from the North Atlantic (Guiry and Guiry 2022). The currently accepted taxonomy for P. palmata is given in Table 1. The monotypic nature of the Palmaria genus, with only one representative in the Northern Atlantic, was hypothesized by Skriptsova and Kalita (2020). Further studies investigating the phylogenetic relationships between P. palmata and the other currently accepted species of the Palmaria genus are required to verify this hypothesis.
Phylogeny and population structure
During the last three decades, genetic-molecular methods allowed for detailed investigations of the phylogenetic relationships of red algae (Freshwater et al. 1994; Ragan et al. 1994). Lindstrom et al. (1996) investigated the radiation of the Palmariaceae based on the comparative analysis of non-coding sequences (ITS) of rDNA from 12 species across the North Atlantic and North Pacific. Four distinct clades across species were identified. Palmaria palmata (type variety) and P. palmata var. sarniensis-sobolifera appeared as an isolated clade and the most divergent from the others. Based on estimates of the divergence rate of ITS sequences, the authors postulated that the species derived from a palmariacean ancestor which occurred in the Pacific during the late Miocene-Pliocene i.e. about 12 million years before present. While several species radiated in the North Pacific, P. palmata dispersed in the Atlantic approximately 3.5 million years ago, corresponding to the opening of the Bering Strait.
A phylogeographic study by Provan et al. (2005), later confirmed by Li et al. (2015), showed that glaciation events of the mid-Pleistocene (0.13–0.78 million years before present) have shaped the biogeography of P. palmata, like other macroalgal species. Populations have persisted in a series of refugia (e.g. the English channel, Western coast of Ireland, North America, Iceland) during periods of unfavourable climatic conditions, then recolonized new areas. Genetic data from Arctic populations (Southern Greenland and Hudson Bay, Canada) revealed that these Arctic collections of P. palmata are extensions of Northwest Atlantic populations (Saunders and McDevit 2013; Bringloe et al. 2020). The analysis of plastid DNA of specimens collected along the coast of Europe revealed relatively large intraspecific variation and more specifically a genetic divergence between Northern/Eastern populations on the one hand and Western populations on the other hand (Provan et al. 2005). The divergence between populations across the Atlantic was inferred from crossing studies of Irish and Canadian isolates producing viable hybrids which however produced poorly viable tetraspores (van der Meer 1987). Both isolates were then regarded as belonging to a single species in the process of splitting into sibling species. Divergence among European and American populations was later confirmed by genetic studies of Provan et al. (2005), Li et al. (2015), Bringloe and Saunders (2018) as well as Saunders et al. (2018) who nevertheless observed genetic similarities in Canadian specimens with European ones, suggesting that migration from the Northeast to the Northwest Atlantic has occurred at least once. A divergence time of approximately 1 million years between populations from both sides of the Atlantic was estimated by Bringloe and Saunders (2018).
Distribution
Geographic distribution
Based on the taxonomic studies cited above, the distribution of P. palmata is primarily confined to the North Atlantic coasts within a latitude range of approximately 40 to 80 °N. The species has been observed from Long Island (Provan et al. 2005) to the Devon Island (GBIF 2022) on the Western Atlantic shores and from Northern Portugal (Fernández-Mendoza et al. 2002; Araújo et al. 2009; GBIF 2022) to the far reaches of the Barents Sea i.e. Spitzbergen and Novaya Zemlya (Gulliksen et al. 1999; Fredriksen et al. 2019; Marambio and Bischof 2021; Mikhaylova 2021) in the Eastern Atlantic. It is also present along the shores of Atlantic islands e.g. Faroe Islands and Iceland (Fig. 2). However, it was explicitly deleted from the list of species found in the Azores, and considered misidentified in previous floristic inventories (Tittley and Neto 2005). Registration of the species in Greenland includes historical identifications (Rosenvinge 1898, 1983; Jónsson 1904) and herbarium specimens in the National History Museum of the University of Copenhagen, as well as more recent observations (Pedersen 1976; Hansen and Johansen 1998; Krause-Jensen et al. 2020), mainly along the West Coast from 60°N (Cape Farewell) to 78°N, but also along the Eastern coast (S. Wegeberg and O. Geertz-Hansen, unpubl. observations). The species has also been reported in the Russian Arctic along the Murman Coast, the White Sea and both Western (Barents Sea) and Eastern coasts (Kara Sea) (Mikhaylova 2021; GBIF 2022). It is even reported in a checklist further East on the Russian Arctic coastline i.e. from the Kara Sea to the Western part of the Chukchi Sea and the Wrangel Island (but not observed in the Laptev Sea) (Vinogradova 1999). Observation records should be verified genetically to confirm the presence of P. palmata in these regions.
Distribution of Palmaria palmata based on observation and collection reports of the species from the Ocean Biodiversity Information System (OBIS 2022) and Global Biodiversity Information Facility (GBIF 2022) databases, distribution sources from AlgaeBase (Guiry and Guiry 2022) as well as records of observations from the cited literature and personal observations from the authors. According to recent taxonomic studies, P. palmata is primarily confined to the North Atlantic. Therefore, records of the species as here presented exclude the relatively few registrations in the Pacific Ocean. See details in the text (“Geographic distribution” subsection)
The morphology of P. palmata is similar to that of closely related species, e.g. Devaleraea mollis (formerly assigned to the Palmaria genus) and P. hecatensis. Therefore, misidentifications may occur. Distribution records of P. palmata in the North Pacific including Southern Alaska, Aleutian Islands, Kuril Islands and Russian shores of the Sea of Japan, are now largely attributed to the confusion with sibling species, as supported by genetic studies (Kraan and Guiry 2006; Skriptsova and Kalita 2020). Recent genetic analyses resolved the previously identified P. palmata from Japan as a new species, namely Devaleraea inkyuleei (Skriptsova et al. 2022). Although some Arctic specimens (from Greenland and the Canadian Arctic) appear to originate from Western Atlantic populations (Saunders and McDevit 2013; Bringloe et al. 2020), the question whether P. palmata is present in the northerly reaches of the Pacific region (Northern Alaska and Russian Far East) remains unanswered. Additional phylogeographic studies are needed to resolve the population genetic structure of P. palmata across the arctic as well as phylogenetic relationships with closely related species.
The currently available distribution records of P. palmata in AlgaeBase (Guiry and Guiry 2022) include relatively recent references to the species in tropical waters including the Galapagos islands, the Pacific coast of Mexico, Ghana, and Southeast Asia. However, reports from these checklists have not been genetically verified. Given the cold-water preference of the species and the induction of fertility at low irradiance (see “Reproduction” section), these reports most likely arise from misidentifications. The distribution range of P. palmata is likely to be affected by the current scenario of climate change resulting in increasing sea temperatures. The species may face local extinctions in areas near its thermal tolerance limit e.g. Northern Portugal, North-western Spain, due to global warming.
Local vertical distribution
Throughout its distribution range, P. palmata is found attached to bedrock and boulders in the intertidal and subtidal zones, in both sheltered and moderately exposed shores. It is also commonly found as an epiphyte on other macroalgae, especially on the stipes of the kelp Laminaria hyperborea (Fig. 3) and Laminaria digitata, and fronds of Fucus serratus and Cystoseira baccata (Faes and Viejo 2003; Garbary et al. 2012). As for other marine photosynthetic organisms, light is a major factor limiting its vertical distribution. Although the species is more abundant in the first 10 m below the surface, it can be observed down to a maximum depth of approximately 20 m in offshore shoals with exceptionally clear waters. In the intertidal zone, exposure to drought stress at low tide as well as grazing by molluscs are the main factors governing the upper distribution limit (Cabioc'h et al. 2006). In subtidal environments, Hawkins and Harkin (1985) observed the rapid colonization of open substrates by P. palmata, in areas where the macroalgal canopy was removed indicating that the competition with kelps restricts this species to a primarily epiphytic habitat in such ecosystems.
Environmental requirements and stressors
Temperature
Temperature is one of the most critical abiotic factors directly affecting photosynthetic performance, growth and reproduction. Palmaria palmata is distributed across Arctic and warm temperate environments with sea temperatures from near 0 °C up to over 20 °C and variable thermic amplitudes (Martínez and Rico 2002; Karsten et al. 2003; Le Gall et al. 2004b). Based on cultivation studies of wild-collected material, the optimal growth temperature ranges between 6 and 12 °C (Morgan and Simpson 1981b; Corey et al. 2012) depending on the origin of the strain. Reduced productivity is reported above 14 °C (Corey et al. 2013) and increased mortality of the biomass at 21 °C (Matos et al. 2006). Palmaria palmata was shown to be less resistant to oxidative stress caused by elevated temperatures (> 20 °C) and hypo- and hyper salinity compared to a proliferative macroalgal species of the subtidal zone i.e. Grateloupia turuturu (Liu and Pang 2010). This was attributed to the lower efficiency of antioxidant enzymatic systems to scavenge reactive oxygen species (ROS) upon stress.
Salinity
Palmaria palmata is often associated with habitats characterized by relatively stable salinity conditions (30 – 40 PSU). However, it is also found in the intertidal zone where it may be exposed to rapid variations in salinity (due to precipitation). Some populations are exposed to a seasonal decrease in seawater salinity due to e.g. large discharge of ice-melting water in boreal and arctic regions. A strong photosynthesis inhibition and mortality at 15 PSU was reported for P. palmata from Spitzbergen under controlled conditions (Karsten et al. 2003). In contrast, the specific growth rate of individual fronds originating from Danish inner-waters and cultivated in tanks was maximal at 15 PSU compared to 25 and 35 PSU (Schmedes and Nielsen 2020b) suggesting that ecotypes of P. palmata are adapted to a wide range of salinity conditions.
Irradiance
As other plants exposed to high photosynthetically active radiation (PAR) and ultraviolet (UV) radiation, P. palmata can regulate its photosynthetic activity in response to diurnal variations in light conditions. Photoinhibition is a photoprotective mechanism to avoid photodamage resulting from the formation of ROS upon high PAR and UV irradiation (Hanelt and Nultsch 1995; Karsten et al. 2003; Marambio and Bischof 2021). As for other macroalgal species, inhibition of the photosynthetic activity of P. palmata is mainly caused by the UV-B wavelengths (Pakker et al. 2000; Karsten et al. 2001). The physiological adaptation to photooxidative stress involves reversible damage to light-harvesting pigments i.e. phycoerythrin, phycocyanin and chlorophyll a, leading to the dissipation of the absorbed excess energy in the form of heat and enables rapid recovery after the offset of stressful conditions (Hanelt and Nultsch 1995). Chronic photoinhibition entails DNA damage and the alteration of a key subunit of the photosystem II (i.e. D1 protein) requiring de novo synthesis and a longer recovery process (Pakker et al. 2000). Such damage may negatively affect physiological functions and the growth of the plant. The photoreactivation from DNA damage in P. palmata (like in higher plants) involves enzymatic reactions which are highly temperature-dependant.
Seasonal studies of the photosynthetic pigments of P. palmata report a decrease in chlorophyll a and light-harvesting pigments during the summer months (Hanelt and Nultsch 1995; Marambio and Bischof 2021), correlating with field observations of greenish or bleached P. palmata thalli. In culture conditions and under nitrogen depletion, bleaching of P. palmata fronds occurs at irradiance level above 200 μmol photons m−2 s−1 PAR after 2–3 weeks, which can lead to the fragmentation of the thallus (Edwards 2007). However, fronds fully recover their red pigmentation within seven days after adding one pulse of f/2 growth medium under laboratory conditions (Schmedes & Nielsen 2020a, b). Pigment accumulation in P. palmata grown under high nitrogen concentrations is also influenced by light quality. Higher concentrations of chlorophyll a and β-carotene were measured in individuals grown under white or blue light, compared to red light (Parjikolaei et al. 2013).
Light-acclimation by photoinhibition is habitat-specific with a lower ability of subtidal populations to down-regulate photosynthesis. This was highlighted in a comparative study of the photosynthetic performance of several arctic macroalgal species exposed to natural PAR and UV radiation where P. palmata showed greater tolerance to radiation stress compared to species bound to deeper-water habitats (Karsten et al. 2001). Light-acclimation is also affected by other abiotic stress factors such as fluctuations in salinity (Karsten et al. 2003) and temperature (Marambio and Bischof 2021). In addition, P. palmata synthesizes and accumulates photoprotective molecules, namely mycosporine-like amino acids (MAAs) to protect from oxidative stress and degradation of biomolecules (DNA, proteins) from UV radiation (Karsten and Wiencke 1999; Lalegerie et al. 2020).
Nutrients
Macroalgae extract nutrients from their surrounding environment by uptake across the thallus area. Both ammonium (NH4+) and nitrate (NO3−) are nitrogen sources for macroalgal growth although P. palmata has a greater affinity for NH4+ (Morgan and Simpson 1981a; Corey et al. 2013). Under controlled conditions, Lubsch and Timmermans (2020) revealed a synchronized pulse-like pattern of both NO3− and phosphate (PO43−) uptake with intervals of about 7 days which, according to the authors, can be a physiological response to intra- and interspecific competition. Nitrogen, phosphorus and carbon (P. palmata is able to use both CO2 and HCO3−) can be stored to support growth under situations of nutrient limitation. Besides nutrient availability, irradiance plays an important role in nutrient storage in P. palmata. Higher nitrogen and phosphorus levels but lower soluble carbohydrate contents were measured in individuals acclimated to low irradiance compared to plants grown under high irradiance (Morgan and Simpson 1981a; Martínez and Rico 2002). Part of the nitrogen is stored in the form of phycoerythrin (involved in light absorption) to improve photosynthesis under low irradiance and to enhance growth under optimal nutrient conditions (Martínez and Rico 2002).
Biotic factors
The growth of P. palmata and colonization of substrates is limited by competitive interactions with other macroalgae species. Following canopy removal experiments, Hawkins and Harkin (1985) reported P. palmata as one of the early colonizers of open rocky substrate although the species may later be overtopped by more rapidly growing kelp species e.g. L. digitata. Grazing is a major factor structuring macroalgal communities and affecting biomass density (Cabioc'h et al. 2006). Various gastropods e.g. sea hare (Aplysia punctata), sea snail (Littorina littorea), abalone (Haliotis tuberculata), limpets (Patella spp.), as well as sea urchins and amphipods, may feed on adult and juvenile fronds of P. palmata.
Palmaria palmata, especially old individuals, is commonly affected by epiphytes such as filamentous red (e.g. Acrochaetium secundatum) (Sanderson 2015) and brown algae (Ectocarpaceae) (Sanderson et al. 2012; Badis et al. 2019), competing with the host plant for nutrients and light. In addition, colonization by epibionts e.g. encrusting bryozoan (Membranipora membranacea) can result in mechanical stress and tissue damage to the host plant and may reduce the reproductive output. In temperate coastal waters, epibionts typically settle during late spring and early summer. Temperature changes from winter to summer greatly affects the population dynamics of bryozoan and colonization of macroalgal fronds (Saunders et al. 2010).
The cellular invasion of a sibling species i.e. D. mollis, by an oomycete parasite (Petersenia palmariae) was described in detail by Pueschel and van der Meer (1985). Spore infection by an intracellular oomycete was later reported by Sanderson (2015) from a P. palmata culture maintained in tanks. Further investigation of this endoparasite by Badis et al. (2019) using microscopy and molecular methods described the infection of spores and young gametophytes of P. palmata by a novel oomycete species i.e. Olpidiopsis palmariae. Olpidiopsis is an abundant and widespread genus including known pathogens of cultivated red macroalgae (e.g. Pyropia spp.) and causing substantial economic loss in the Asian macroalgae industry (Badis et al. 2020). Such infections may be a major cause of unexplained spore mortality in P. palmata cultures (see the “Cultivation” section).
Reproduction and growth
Life history
Palmaria palmata is considered pseudo-perennial since the fronds grow, reproduce and senesce from a holdfast that survives multiple years. It has a complex heteromorphic diplohaplontic life history (Fig. 4) which is unique among the Rhodophyta and had remained a mystery for phycologists for a long time. Blade-like male gametophytes and tetrasporophytic thalli were described by van der Meer and Chen (1979) but the life history of P. palmata was first revealed by van der Meer and Todd (1980), by crossing a red-pigmented female gametophyte with a mutant green male. After fertilisation and development, a green diploid tetrasporophyte could be observed attached to the red-pigmented female haploid thallus. The life history of P. palmata has been further described in the scientific literature focusing on improving the cultivation of the species (van den Hoek et al. 1995; Werner and Dring 2011a; Grote 2019).
Life cycle of Palmaria palmata as described by van der Meer and Todd (1980). The dashed-line areas on mature sporophyte (with dotted fill) and gametophyte fronds indicate tetrasporangial (i.e. sorus) and spermatangial tissues respectively
According to van der Meer and Todd (1980), haploid tetraspores develop within the sori of the frond of diploid tetrasporophytes through meiosis. The haploid tetraspores germinate into either microscopic female or blade-like male gametophytes of a similar morphology to diploid tetrasporophytes. The female gametophyte develops oogonia with trichogynes (i.e. hair-like structures) to catch spermatia (i.e. non-motile male gametes) released from a mature male gametophyte (at least one year old). The zygote resulting from the fertilization of a female gamete by a spermatium grows into a diploid tetrasporophyte from the encrusting female gametophyte, which is overgrown at an early stage as the tetrasporophyte develops its own discoid holdfast.
The life history of most red algal species (e.g. Gigartinales, Bangiales) often includes different morphologies between the tetrasporophyte and the gametophyte (male and female). Further, as opposed to the commonly observed pattern within the Rhodophyta, the carpogonium (female sexual organ) does not develop into a carposporophyte following fertilization, i.e. a diploid microscopic structure releasing diploid carpospores into the environment from which multiple tetrasporophytes may develop, allowing amplification of the zygote (Fredericq and Hommersand 1990). Instead, the fertilised carpogonium of P. palmata develops into a single tetrasporophyte, which is already attached to the substrate (van den Hoek et al. 1995). Hence, the life history of P. palmata differs from that of most red algae.
Fertility and phenology
Like the majority of macroalgal species, P. palmata relies on the production and release of spores for the reproduction, dissemination and colonization of substrata. To become fertile and induce the maturation of spores, P. palmata has specific environmental requirements regarding temperature and light conditions, which influence the timing of reproduction in situ (phenology).
The development and maturation of tetrasporangial tissue (Fig. 5) is triggered by short day conditions, i.e. 8 h of light per day and a temperature of 10 °C or below (Pang and Lüning 2006; Schmedes and Nielsen 2020a). However, development of tetrasporangia was reported under a photoperiod of 12:12 h (light:dark) but at a lower irradiance, i.e. 5 to 10 µmol photons m−2 s−1, than used in the abovementioned studies (Werner and Dring 2011a). This suggests that a prolonged low exposure to PAR is the actual trigger of fertility induction in P. palmata. This photoperiodic control of the spore development combined with a rather specific temperature requirement narrows the reproductive window, as well as the distribution of the species to temperate and arctic regions which are characterized by a strong seasonality. As argued by Pang and Lüning (2006), this timing may be a strategy to ensure a vigorous vegetative growth in spring under optimal irradiance and daylength conditions.
The seasonality of tetrasporophyte and male gametophyte maturation has been investigated in several studies. In mid-Norway (Trondheimsfjord), mature tetrasporophytes were found from October to May, with the occurrence of mature specimens peaking from December to February (up to 50% of all individuals, Fig. 6). Mature male gametophytes were observed during a slightly shorter time frame, i.e. from November to April, with a peak in January (Bøe 2019; Schmedes 2020). Monthly samples of P. palmata in the Isle of Man revealed a similar pattern of mature tetrasporophytes and male gametophytes being observed from November to July (Kain 1982). This is also supported by observations of mature specimens (tetrasporophytes and male gametophytes) from late autumn to late spring along the Eastern coast of the North Atlantic (Faes and Viejo 2003; Le Gall et al. 2004a; Pang and Lüning 2006; Edwards 2007; Werner and Dring 2011a; Schmedes and Nielsen 2020b) and Greenland (Rosenvinge 1898; Jónsson 1904). However, Bird and McLachlan (1992) describes P. palmata as fertile in summer to late autumn for the Maritime Provinces of Canada, although “well-developed and fertile fronds are found throughout the year”.
Year-round reproductive status of Palmaria palmata thalli collected at Storsteinan (Trondheimsfjord, Norway) between October 2017 and September 2018. The points represent the percentage of occurrence of fertile male gametophytes and tetrasporophytes over n sampled individuals (n ranging from 141 to 266 per sampling date). Data from Bøe (2019) and Schmedes (2020)
Sori and spore development
The formation of sori in P. palmata appears to be governed by meristematic tissues as observed in kelp species (e.g. L. digitata, Saccharina latissima) in which the meristem produces inhibitors of sporangium development during the season of rapid growth (Buchholz and Lüning 1999; Pang and Lüning 2004b). In these species, suboptimal growth conditions under low irradiance and temperature result in the decrease in the production of these inhibitors leading to sori formation. Likewise, isolating the meristem from the rest of the blade induces the development of sporangia (Pang and Lüning 2004b). Titlyanov et al. (2006) observed a similar pattern in P. palmata where the formation of tetraspores in submeristematic fragments was stimulated by isolation from the meristem. However, cutting the marginal edges (meristem) of P. palmata fronds does not systematically result in sorus formation (P. Schmedes, pers. observation). Micrographs of fertile tissue of P. palmata are shown in Fig. 7.
Sporangium formation is initiated by the differentiation of outer cortex cells into sporangial initials i.e. tetrasporocytes, and stalk cells (Pueschel 1979). Tetrasporocytes undergo meiosis in a cruciate cleavage to form four tetraspores. Although the knowledge related to tetrasporangial formation is limited, available information reveals that cleavages in Rhodophyta generally occur either successively or simultaneously i.e. the first cleavage is completed before the next or all cleavages are completed at the same time (Pueschel and Cole 1982). Palmaria palmata, on the other hand, is characterized by an intermediate process in which the second cleavage starts just before the completion of the first division (Pueschel 1979). Following the release of tetraspores, the primary stalk cells form new stalk cells and tetrasporocytes, and the entire process of tetrasporogenesis is then repeated.
High levels of tyramine have been measured in fertile sporophytic tissue compared to the concentrations measured in non-fertile fronds suggesting that this biogenic polyamine is involved in tetrasporogenesis in P. palmata (Schmedes 2020). Low levels of putrescine were measured and both spermine and spermidine were not detected, in contrast with studies reporting the influence of these polyamines in the maturation of reproductive structures in other red algae species (Guzmán-Urióstegui et al. 2002; García-Jiménez and Robaina 2012). More research is needed to properly understand the physiological mechanisms of sorus formation and maturation in P. palmata.
Spore dispersal
Mature spores released to the surrounding environment are rather large (approx. 30 µm) and not motile (Le Gall et al. 2004b). Therefore, their dispersal is governed by hydrodynamic conditions. Norton (1992) suggested that small macroalgal species (compared to e.g. large kelps) release their propagules quite close to the substratum thus limiting the distance of dispersal. Under controlled laboratory conditions, the spores settle and firmly attach to the substratum after 6 h (Le Gall et al. 2004b). However, the settlement process of macroalgal spores and colonization of new substrates may be adversely affected by abiotic factors such as turbidity (Vadas et al. 1992). The drifting of vegetative material of P. palmata as well as organic substrates (e.g. stipes of L. hyperborea) following storm events may allow the dispersion of the species over larger distances. However, due to the single non-motile spore releasing phase in its life cycle, the dispersibility of P. palmata is considered low. Studies highlighting the geographically structured genetic patterns among populations (see “Taxonomy and phylogeny” section and references therein) support the relatively poor dispersion ability of the species.
Growth
The vegetative growth of P. palmata from spore germination has been described in the literature (Le Gall et al. 2004b; Pang and Lüning 2006). Spore settlement is facilitated by the development of a mucilage covering the entire spore and consolidating the attachment to the substrate. The first cell divisions occur in a plane perpendicular to the substratum then in all 3 dimensions resulting in a hemispherical attachment disc from which the frond develops from a single apical cell (Deniaud et al. 2006). As observed in higher plants, the morphogenesis is governed by cortical microtubules during mitosis marking the site of future cellular cleavage and regulating the position of relevant organelles such as the Golgi apparatus, involved in the synthesis of cell-wall polysaccharides (Le Gall et al. 2004c). During germination and the first stages of germling development, the biosynthesis of the cell-wall matrix, mainly composed of xylose, occurs concurrently to the degradation of starch granules (floridean starch) contained in the spores. Young sporeling growing close to each other will often coalesce at an early stage resulting in individuals with several fronds. This may be a strategy from the plant to enhance photosynthetic efficiency and increase germling survival (Deniaud et al. 2006). The vegetative growth of P. palmata (both sporophyte and male gametophyte) is supported by meristematic cells located in marginal tissues of the fronds. New fronds can also grow from marginal proliferations on old thalli (Fig. 8). Clusters of meristematic tissues (wart-like structures) can also be observed scattered in the cortical layer of the thallus (Titlyanov and Titlyanova 2006).
In wild populations the growth rate is maximal between May and July then decreases to eventually become negative in summer because of frond breakage. This is due to a combination of factors such as high irradiance, high temperature, low nutrients, grazing by invertebrates and the presence of epibionts on the surface of the fronds (Faes and Viejo 2003). Plant density (intra- and interspecific competition), grazing and wave impact are important factors for the mortality of young P. palmata plants. Recorded maximal growth rates range from 0.9 to 1.5% day−1 in Northern Ireland and Northern Spain respectively (Faes and Viejo 2003) and was estimated to over 4% day−1 in the Bay of Fundy (Canada) (Lukeman et al. 2012).
Chemical and nutrient composition
Due to the commercial interest for P. palmata mainly in food application, the chemical composition and nutrient content of the species have been studied extensively. Table 2 provides an overview of the proximate composition and levels of important nutritional elements in P. palmata including potentially toxic compounds. Like most macroalgal species, the moisture content of P. palmata is very high and accounts for up to 88% of the fresh biomass. As generally observed in macroalgae, the moisture content of P. palmata is highest during winter and spring (Rødde et al. 2004).
Carbohydrates
Carbohydrates make up to 74% of the dry weight (DW) of P. palmata. Figure 9 illustrates the seasonal variation in the carbohydrate composition of P. palmata. Xylans, which are the primary component of the cell-wall of this species, are essentially composed of β-(1 → 4)- and β-(1 → 3)-linked D-xylose units in a proportion of about 4:1 (Lahaye et al. 1993; Deniaud et al. 2003). Being indigestible, xylans are regarded as the main source of dietary fibres in P. palmata and are present in both water-soluble and insoluble forms (Fig. 9). Minor amounts of cellulose (i.e. insoluble glucans, about 3% DW) are also found as structural carbohydrates. This species also contains water-soluble low molecular weight carbohydrates mainly in the form of floridoside (α-D-galactopyranosyl-(1–2)-glycerol) and to a lesser extent floridean starch (amylopectin-like glucan). Floridoside accounts for below 5% of the DW during winter and up to 25% during the summer months (Rødde et al. 2004). Deniaud et al. (2006) revealed higher carbohydrate levels in sporophytes compared to gametophytes collected at the same period. Floridoside is associated with immune-stimulating activity in response to antigens with potential therapeutic applications (Courtois et al. 2008).
Variation in the carbohydrate composition of Palmaria palmata harvested in Norway (Trondheimsfjord). Data from Rødde et al. (2004)
Proteins and amino acids
The protein content of P. palmata is one of the main reasons for the commercial interest for this species for both food and feed applications. The protein content of P. palmata is high compared to levels reported in most of other edible brown species e.g. Undaria pinnatifida and S. latissima (ca. 10% DW) (Jard et al. 2013; Mæhre et al. 2014; Stévant 2019) while it is comparable to those reported in Ulva sp. (10—26% DW) and lower than levels measured in Pyropia sp. (33 – 47% DW) (Fleurence 1999). Levels up to 35% DW have been reported (Morgan et al. 1980b) although such high values are often derived from a standard nitrogen-to-protein conversion factor of 6.25, overestimating the real protein content due to significant sources of non-protein nitrogenous compounds e.g. nitrate, which can be as high as 18% of the total nitrogen content (Morgan et al. 1980b). A factor of 4.7 was found appropriate to estimate the protein content of P. palmata (7 – 19% DW, Table 2) from the total nitrogen content (Bjarnadóttir et al. 2018). In wild harvested individuals, higher protein contents are found during the winter season (Galland-Irmouli et al. 1999; Rødde et al. 2004) while high levels can be achieved in tank cultivation under low irradiance and high nitrogen supply (Morgan and Simpson 1981a). This is correlated with high levels of proteinaceous water-soluble pigments, i.e. mainly phycoerythrin and to a lesser extent phycocyanin during the winter compared to summer months (Guihéneuf et al. 2018). The relatively high proportion of essential amino acids (EAAs, Table 2) reflects the nutritional potential of proteins from P. palmata. Glutamate and aspartate are the most abundant amino acids in P. palmata. The species also contains substantial amounts of alanine, arginine, glycine, serine, leucine and valine as well as EAA that are often limiting in plant food sources i.e. methionine, lysine, threonine, tryptophan (Morgan et al. 1980b; Galland-Irmouli et al. 1999; Mouritsen et al. 2013; Bjarnadóttir et al. 2018). However, some amino acids are present in low levels or may even be seasonally absent in the profile e.g. cysteine and histidine (Galland-Irmouli et al. 1999). It should be noted that ca. 10% of the total amino acid content of dulse is in the form of free amino acids (Mæhre et al. 2015; Aasen et al. 2022). Peptides and MAAs from P. palmata are suggested as natural antioxidants in various commercial applications following reported bioactivity in the scientific literature (Yuan et al. 2009; Harnedy et al. 2017).
Lipids
In P. palmata, lipids are distributed among neutral, polar and free fatty acids (FA) as respectively 36%, 37.5% and 26.5% of the total FA (Foseid et al. 2020). Despite a low total lipid content compared to typical plant lipid sources, mono- and polyunsaturated fatty acids (MUFAs and PUFAs) are highly represented as described in Table 2. The lipid fraction of P. palmata is rich in long-chain ω-3 PUFA, namely eicosapentaenoic acid (EPA), which may account for over 50% of the total lipids (Mouritsen et al. 2013; Lopes et al. 2019). However, it does not contain any significant amounts of docosahexaenoic acid (DHA) making P. palmata an interesting raw material for the production of high purity EPA concentrates (Mishra et al. 1993). Besides, antioxidant activity was reported from the EPA-rich polar lipid fraction of P. palmata (Lopes et al. 2019). Geographical location, age of the plant as well as storage of the harvested material greatly affect the lipid profile of the species (Mouritsen et al. 2013). As for other phytochemical constituents, lipids are varying according to seasons or environmental conditions. Schmid et al. (2017) measured significantly higher total FA content in P. palmata harvested in May (1.3% DW) compared to October (0.7% DW). The same authors also reported a notably low ω-6/ω-3 ratio varying from 0.08 to 0.24 across seasons (Schmid et al. 2017). A high ω6/ω3 ratio typical of Western diets (in a range between 15 and 17) is associated with the occurrence of chronic diseases including cardiovascular diseases, cancer, inflammatory and autoimmune diseases. Edible macroalgae, that are generally characterized by a ratio of 1 or below, and particularly P. palmata, have the potential to provide a more balanced ω-6/ω-3 ratio as recommended by international authorities.
Minerals and ash content
The species is a rich source of minerals (reflected by the ash content) including both macro (Na, K, Ca and Mg) and microelements (e.g. Fe, I, Mn). The ash content is generally lowest during the summer and autumn months and highest during winter and spring (Rødde et al. 2004). Palmaria palmata contains higher levels of K compared to Na which is interesting in a nutritional perspective given that diets rich in Na are associated with health risks such as high blood pressure and cardiovascular diseases. It should be noted that the iodine content of P. palmata is in the upper range of values reported from red macroalgal species but lower than those of kelp species (Duinker et al. 2020). Reports of the iodine content of commercially available macroalgae, particularly from the Laminariaceae (e.g. S. latissima, L. digitata) have recently raised concerns from national food safety authorities regarding potentially negative health effects of excessive iodine intakes. Although the iodine content of P. palmata may reach values within the range of those measured in brown macroalgae (i.e. up to 790 mg kg−1 DW measured from screening 26 samples across Norway; Duinker et al. (2020)), average values are lower than the iodine levels found in commercial kelp species and the weekly consumption of a few meals containing P. palmata will not expose the consumer to excessive iodine intakes.
Vitamins
There are several available reports of the vitamin content of dulse (see references in Table 2) but studies comparing the vitamin levels of edible macroalgal species with dietary reference intakes and with other food sources are scarce. In a meta-analysis of available data on the vitamin C content of macroalgae, Nielsen et al. (2021) concluded that most species (including P. palmata) are not a rich source of vitamin C compared to other plants (e.g. rosehip, parsley, broccoli) and that normal consumption will not contribute significantly to the recommended nutrient intake for this compound. This contrasts with historical records of dulse being consumed by Norwegian Vikings during expeditions at sea to protect against scurvy (Mouritsen et al. 2013). It is therefore more likely that the combined consumption of seafood rich in vitamin C (e.g. shellfish, fish roe) effectively prevented this widespread disease among seafarers rather than the consumption of dulse alone. On the other hand, dulse appears to be a good source of vitamin B, particularly B1 (thiamine), B2 (riboflavin) and B12 (cobalamin) (MacArtain et al. 2007; Kraan 2013). As animal products are the most common dietary sources of vitamin B12, vegetarians and vegans may therefore be at risk of vitamin B12 deficiency. Based on maximum levels measured in P. palmata i.e. 240 µg kg−1 DW (Table 2), the daily consumption of 1 g dried material will cover 10% of the recommended nutrient intake of 2.4 µg day−1 established by the World Health Organization (WHO 2005). Dulse may therefore represent an alternative dietary source of vitamin B12. Among the fat-soluble vitamins (i.e. A, D, E and K), P. palmata is particularly rich in β-carotene (up to 456 mg kg−1 DW; Kraan 2013), known as provitamin A (precursor to vitamin A) as well as vitamin K (Mouritsen et al. 2013). To the authors’ knowledge there are no records of the vitamin D content of P. palmata.
Potentially toxic compounds (PTEs)
Palmaria palmata may accumulate potentially toxic elements (PTEs) such as heavy metals present in the environment either naturally or from anthropogenic activities. Prasher et al. (2004) revealed a greater affinity of P. palmata for lead (Pb) and cadmium (Cd) compared to other metals, but the reported concentrations of Pb and Cd in the literature for this species are generally lower than the maximum limit allowed in food supplements in the European Union (EU) (3.0 mg kg−1 DW for both metals, EU 1881/2006) (Duinker et al. 2020). However, Desideri et al. (2016) reported a Pb level of 4.4 mg kg−1 DW in a commercial P. palmata sample (of unknown origin) indicating that elevated levels may be achieved. Similarly, there are numerous reports of relatively low levels of mercury (Hg) in edible macroalgae including P. palmata, below the threshold value of 0.1 mg kg−1 DW (maximum level in food supplements, EU 1881/2006) (Biancarosa et al. 2018; Duinker et al. 2020). Roleda et al. (2019) investigated seasonal, interannual and geographical variations in contaminants in several edible macroalgal species. Despite an average Hg content in P. palmata (0.063 mg kg−1 DW) below the threshold, the authors reported maximum levels (0.314 mg kg−1 DW) exceeding the limit. Hence, further attention must be given to monitor the contaminant levels of commercially sold products from P. palmata. Inorganic arsenic (iAs) is another PTE that can be found in marine macroalgae. The levels found in P. palmata are below the French recommendation of 3 mg kg−1 DW (ANSES 2018) and therefore considered safe in the context of using this species in food applications. Kainic acid is an amino acid which is structurally similar to glutamate, but which can be neurotoxic at high doses. It can be found in variable concentrations in P. palmata typically from below the detection limit to relatively low levels (up to 560 mg kg−1 DW, Table 2). Although these levels are much lower than concentrations inducing damages to neurons, Ramsey et al. (1994) measured exceptionally high levels i.e. exceeding 10,000 mg kg−1 DW in a dwarf mutant of P. palmata (not a natural strain). Further research is therefore needed to establish human safety standards and identify the factors affecting the biosynthesis and accumulation of this compound in P. palmata.
Exploitation of wild stocks
Historical uses
Dulse is one of the few macroalgal species that is associated with ancient eating traditions in Europe and North America, with documented uses from centuries back. The use of duileasc as food was mentioned in the Brehon laws of Ireland from the fifth century (Rhatigan 2009). Later records from the twelfth century and onward describe the collection, drying and trading of dulse by coastal communities in Ireland, mostly from the Northern and Western coasts, and Scotland (Delaney et al. 2016; Mac Monagail et al. 2017). The species was considered a seasonal staple food, often used as a condiment served with e.g. bread, whey milk and butter, or traded at local markets and fairs. In coastal Norway, søl could be eaten either fresh or dried, together with dried fish (Østgaard and Indergaard 2017). Similar uses were reported from historical records from Iceland from the eighth century (Kristjánsson 1980). The fronds were handpicked during the summer, rinsed and spread over the fields for sun-drying. They were then stored in closed barrels to be consumed the rest of the year. Dulse was even used as a trading commodity and medieval legal texts established rights to dulse-rich shores in Iceland (Mouritsen et al. 2013) reflecting the importance of this species during ancient times. There are only limited written records of ancient traditions of eating P. palmata in Brittany but the similarity of Breton vernacular names (e.g. tellesk, terlesk) with Irish names (duileasc, dillisk) suggest a common origin (Garineaud 2018). Another Breton name i.e. bezhin saout (cattle’s seaweed) reflects its use as fodder for livestock. Palmaria palmata has also been used as food for over a century by populations along the Northwest Atlantic coast including the state of Maine (USA) and the Canadian Maritime Provinces. Although the species may have been eaten by the Indigenous People of the Maritimes, it is not mentioned in available written records. Tradition for eating this species in North America would most likely originate from its use in the British Isles.
Dulse was known for its bioactive properties and besides being consumed as a sea vegetable, it was also used for specific purposes. Preparations based on boiled dulse were given to children and animals in e.g. Ireland and Wales as a treatment against worms. Anthelmintic effect was later correlated to kainic acid content in P. palmata. More generally, dulse provided nutrients to local communities in Northern Atlantic Europe, especially in periods of food scarcity. This has led to some extent to negative associations of eating macroalgae with poverty and famine. There has been in recent decades a renewed interest for using edible species, and particularly P. palmata, as a natural, sustainable, healthy and tasty food ingredient with a great potential in culinary applications.
Harvesting and regulatory frameworks
The current commercial exploitation of P. palmata both in Europe and North America largely relies on harvesting wild populations (Chopin and Ugarte 1998; Mac Monagail et al. 2017). On both sides of the Atlantic, the species is collected at low tide in a way that a portion of the frond is left on its substrate to ensure regrowth and perennity of this activity. It is then mainly dried locally to be sold either in the form of whole blades, flakes or powder by small local businesses or by wholesalers. This activity is seasonal i.e. from late spring to autumn (Chopin and Ugarte 1998; Rhatigan 2009) and generally characterized by a low level of mechanization limiting the quantity available to the market. In most cases, harvesting P. palmata on the shore is combined with other activities e.g. fishing, farming, foraging shellfish or other macroalgal species (Chopin and Ugarte 1998; Mesnildrey et al. 2012). The amount of harvestable biomass is also limited by interannual variations in population stocks (which may be low due to unfavourable natural conditions) and the accessibility of harvesting sites either by land or by boat. Besides, increasing the commercial use of P. palmata based on wild catches may increase pressure on natural populations. These limitations are currently hampering market developments based on wild P. palmata biomass.
There is currently no systematic reporting of the harvested quantities of dulse from wild populations to the Food and Agriculture Organization (FAO). This is most likely due to different regulations for harvesting macroalgae on the foreshore across countries. In Canada, P. palmata is commercially harvested in the Bay of Fundy, mainly along the shores of Grand Manan Island in New Brunswick. Annual landings in New Brunswick during the period 1982 – 2002 ranged from 38 to 100 dry tonnes (i.e. approximately 380 to 1000 t wet weight, WW) (Chopin and Ugarte 1998). Lower amounts (2 dry tonnes or ca. 20 wet tonnes) were also harvested on the South-eastern cost of Nova Scotia (Garbary et al. 2012). Unlike the industrial exploitation of wild bladder wrack (Ascophyllum nodosum) beds which is subject to harvesting licences and monitoring of the resource, there is at present no regulation applied to the commercial wild harvest of P. palmata in Canada. Based on a resource assessment before and after harvest and modelling the growth of P. palmata during the summer and spring seasons (> 4% growth per day), the removal of 50% of the standing crop on harvesting sites each month was considered sustainable for the resource (Lukeman et al. 2012). In the state of Maine, USA, harvesting macroalgal biomass from wild beds for commercial use is subject to a specific permit issued by the state’s Department of Marine Resources (DMR). Harvesters must report their activity to the DMR monthly including harvesting areas, species and landings (State of Maine DMR 2003). However, only the landings for combined macroalgae species are available from DMR’s database. The DMR is in the process of establishing a management plan for Maine’s harvested macroalgae including P. palmata. Until such a plan is implemented, the Maine Seaweed Council (MSC, network of commercial and recreational harvesters) created guidelines outlining the best practices for a sustainable harvest. Based on the knowledge of its members, the council advises that a maximum of 75% of the P. palmata biomass at a given site may be sustainably harvested (above the holdfast) to allow for regrowth of the stock (MSC 2022).
In Ireland, the 1933 Foreshore Act prohibits the removal of beach material; hence, a licence from the Department of Housing, Local Government and Heritage is required to harvest macroalgae on the foreshore. Although no data is available regarding annual quantities of P. palmata collected, these volumes are assumed to be minimal (< 30 t WW, L. Watson, pers. comm.). In Scotland and Norway access to the macroalgal resource may be given by landowners upon a lease or a fee (NETALGAE 2012). Palmaria palmata is also commercially harvested in Iceland but the newly established Icelandic regulatory framework does not address the exploitation of this species (Maack 2019). Official monitoring of the resource does not currently exist in these countries.
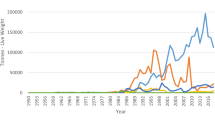
In contrast, harvesting is more strictly regulated in France. Dulse is collected mainly in North-western Brittany (Finistère), where fronds grow up to 60 cm whereas fronds are smaller and thinner further East (sobolifera-sarniensis morphotype in Côte d’Armor) and regarded less attractive for food applications. Harvesting of P. palmata is allowed from April to December. However, it is not collected during the summer months due to sun-bleaching giving the fronds a white-yellow tint. The commercial harvest of this species, as for other hand-picked macroalgal species, is subject to an annual authorization delivered by local county authorities. Specific decrees may be issued by these authorities to adjust e.g. harvesting areas for resource management purposes as well as minimum cutting heights (normally set to 25 cm) (Mesnildrey et al. 2012; Philippe 2013). Information including the quantities harvested and the location must be reported monthly to the authority by professionals holding an authorization (Philippe 2013). The obtained data for hand-picked dulse in France reveals large interannual variations in the harvested volumes ranging from 138 to 458 t (WW) (Fig. 10) reflecting variations in biomass availability due to abiotic and biotic factors such as grazing from gastropods. Ongoing work in collaboration with research institutions, local authorities and professionals evaluates the biology and the distribution of commercially exploited species including P. palmata as well as the effects of the harvesting activities on local standing stocks to define and implement management measures and optimize the harvesting effort (CRPMEM 2021). According to a study of the European market for sea vegetables, France alone produced 90% of the dulse consumed in Europe in 2013, while the remaining 10% came from Ireland, Iceland and North America (BIM 2014).
Systematic reporting of annual landings of wild-harvested P. palmata at a local scale (i.e. harvesting sectors) and national level is a prerequisite for establishing management schemes for the sustainable exploitation of this resource. Meanwhile on both sides of the Atlantic, the demand for dulse from industrial retailers is currently outstripping the supply. Therefore, significant efforts have been made during the past decades to cultivate the species to allow for an increased and more consistent supply of P. palmata than available from wild populations.
Cultivation
Palmaria palmata can be cultivated either in tanks under controlled conditions in land-based facilities, or at sea following the deployment of seeded substrates (e.g. twine ropes, nets) on cultivation rigs in open-waters. A detailed description of the culture conditions from relevant studies until 2017 is available in a comprehensive review by Grote (2019). The present section provides a summary of the experimental research conducted since 1980 as well as insights on the current status of dulse aquaculture and recent improvements of cultivation techniques (i.e. after 2017). The main bottlenecks to achieve commercial production are also identified. Key features for the cultivation of P. palmata following different strategies i.e. vegetative propagation or sporeling production, and the optimal conditions for the successive steps of the process based on the state-of-the-art knowledge, are summarized in the Fig. 11.
Summary of different methods and optimal conditions for the cultivation of Palmaria palmata based on published studies. Light conditions are given as the photoperiod (light:dark) and the irradiance (PAR, in µmol photons m−2 s−1). References: [1] Pang and Lüning (2006); [2] Morgan and Simpson (1981a); [3] Morgan and Simpson (1981b); [4] Titlyanov et al. (2006); [5] Corey et al. (2014); [6] Pang and Lüning (2004a); [7] Titlyanov and Titlyanova (2006); [8] Edwards and Dring (2011); [9] Le Gall et al. (2004b); [10] Werner and Dring (2011a); [11] Schmedes et al. (2019); [12] Schmedes (2020); [13] Schmedes and Nielsen (2020a); [14] Corey et al. (2012); [15] Corey et al. (2013); [16] Sagert and Schubert (2000); [17] Martínez et al. (2006); [18] Kim et al. (2013); [19] Levinsen (2020); [20] Grote (2019); [21] Schmedes and Nielsen (2020b); [22] Evans and Langdon (2000); [23] Matos et al. (2006); [24] Sanderson et al. (2012)
Cultivation techniques
Small scale experiments of P. palmata cultivation have been carried out in tanks based on the vegetative propagation of juvenile fronds (Morgan et al. 1980a), whole adult thalli (Pang and Lüning 2004a; Corey et al. 2014), apical meristematic thallus pieces (Morgan and Simpson 1981a, b; Pang and Lüning 2006), marginal proliferations (Titlyanov et al. 2006; Schmedes and Nielsen 2020b) and isolated meristematic fragments (Titlyanov and Titlyanova 2006) as starting material for land-based cultivation. While acceptable growth rates have been achieved under experimental settings e.g. 8–12% day−1 (Morgan and Simpson 1981b; Pang and Lüning 2004a; Sanderson 2015; Manríquez-Hernández et al. 2016), the main bottlenecks for establishing a commercially viable cultivation based on vegetative propagation are related to the difficulty of building up high stocking densities of biomass free of epiphytes on a large scale and maintaining such growth rates over time while avoiding losses of biomass from unintended sporulation events. The vegetative growth of P. palmata has also been tested at sea by inserting thalli into cultivation ropes (Browne 2001; Bak 2019) although this method is time-consuming and individuals may detach from the action of waves and currents, limiting its viability on a commercial scale. Alternatively, fronds can be placed into mesh bags attached to vertical ropes as reported by Martínez et al. (2006) but self-shading under high stocking densities will limit growth rates.
Other methods for the cultivation of P. palmata requires the seeding of growth substrates either using released tetraspores (Browne 2001; Le Gall et al. 2004b; Sanderson 2006; Edwards 2007; Werner and Dring 2011a; Schmedes 2020) or by direct seeding using germinated gametophytes (Schmedes et al. 2019). These methods have gained increasing attention since they may allow the production of biomass on a larger scale through a hatchery step, as for the cultivation of other species of commercial interest, e.g. S. latissima. However, the biology of P. palmata and cultivation technology are more complex than those of kelp species (Redmond et al. 2014), which limits the development of commercial cultivation operations despite an increasing demand for this species from several markets including the food sector.
Experimental open-water cultivation of P. palmata has been demonstrated using vertical droppers or nets attached to longlines suspended below the surface (Sanderson 2006; Edwards 2007; Edwards and Dring 2011; Sanderson et al. 2012; Schmedes 2020). Data from the literature reports appropriate seedling densities for open-water cultivation of P. palmata, ranging from 9 to 50 individuals cm−1 substrate (Werner and Dring 2011a; Schmedes 2020). Besides, deploying well-grown seedlings (minimum size 3–8 mm) (Werner and Dring 2011b; Sanderson et al. 2012) during the autumn or early winter (instead of summer) (Werner and Dring 2011b) are strategies promoting the growth of P. palmata against epiphytes and fouling competitors such as other macroalgae (e.g. Ectocarpus spp., Ulva spp., S. latissima) and hydroids. Epiphytes affect not only the growth but may also compromise the quality of the biomass to be used in commercial applications (e.g. food) and should therefore be avoided. Tank cultivation on land allows for a greater control over epiphytes and other competitors provided that the supplied seawater is treated (mechanical filtration, UV-treatment).
Variable biomass yields from P. palmata cultivation at sea have been reported in the literature but differences in seedling density and size at deployment, local growth conditions, cultivation time and farming configuration makes the comparison of these figures difficult. Based on the overview of reported numbers by Grote (2019), 0.5 to 1 kg WW per meter longline appears a realistic baseline estimate of the expected biomass yield of P. palmata cultivated at sea. The use of high-density substrates such as nets or textile sheets, extended hatchery time and multiple sequential harvests will result in higher yields per unit area (Werner and Dring 2011a; Schmedes 2020). Furthermore, relatively high growth performance of P. palmata may be achieved in integrated multitrophic aquaculture (IMTA) systems where the species is grown using nutrient effluents from finfish production either in land-based facilities (Matos et al. 2006; Kim et al. 2013; Corey et al. 2014; Grote 2016; Levinsen 2020) or aquaculture sites at sea (Sanderson et al. 2012) highlighting the bioremediation potential of the species.
Bottlenecks related to spore availability and mortality
Without control on the fertility of the raw material, the seeding of growth substrates and subsequent sporelings growth is restricted by the seasonal occurrence of mature fertile tetrasporophytes from wild stocks (Fig. 6). In some areas, e.g. regions with low tidal range where P. palmata is mainly found subtidal, the low accessibility to fertile raw material may represent an additional challenge. Besides, the spore yield of P. palmata is relatively low. Edwards (2007) revealed the variability of spore production from a natural Irish population and estimated the number of spores per individual between 7 and 36 × 106 which is several orders of magnitude lower than other macroalgal species e.g. L. digitata, Saccharina longicruris releasing 9 to 20 × 109 spores per square meter colonized substrate (Chapman 1984). Thus, a relatively large amount of wild-harvested fertile material is necessary to seed substrates and achieve an appropriate density of seedlings for successful out-growth. Furthermore, the results from a sampling campaign of mature tetrasporophytes in Norway confirm the variability in spore yield from natural populations and show that the number of spores obtained from tetrasporangial tissue is affected by the time of collection of fertile material (Fig. 12). This suggests that the availability of potent spore donors is somehow limited even though fertile individuals can be found during an extended period.
Spore release from fertile tetrasporophytes of Palmaria palmata collected during the autumn 2017 near Storsteinan (Trondheimsfjord, Norway) as a function of the duration of sporulation (days). Small pieces of cleaned sori were dissected and submerged into well-plates containing 5 mL filtered seawater and daily transferred to new wells prior to spores counting. Values are given as mean ± standard deviation (n = 6). Data from P. Schmedes (unpublished data)
Another major hurdle to the mass cultivation of P. palmata sporelings is spore mortality typically ranging from 60 to 90% (Werner and Dring 2011a, c). Unexplained seeding failures may be attributed to the infection of spores and young gametophytes by the intracellular oomycete O. palmariae (Sanderson 2015; Badis et al. 2019), highlighting the possibility of substantial yield-limiting epidemic outbreaks in commercial cultivation of P. palmata. Besides, the contamination of cultures in tanks by endophytes, epiphytic filamentous brown (Ectocarpus spp.) and red algae (Acrochaetium secundatum) as well as microalgae is common and may affect the growth and survival of seedlings (Werner and Dring 2011a; Sanderson 2015). Cleaning procedures consisting in 1-min bath treatments in sodium hypochlorite (NaOCl, from 0 to 1.2%) were not suitable for the disinfection of wild-collected reproductive material due to tissue damage (at 1.2%) or limited contaminant removal at 0.6% and lower concentrations (Bak 2019). In a study of Kientz et al. (2011), efficient removal of P. palmata epibionts was achieved following exposure to a mixture of 50% ethanol and 1% NaOCl for 30 s. However, the effect of this procedure on spores and seedlings survival was not investigated. Other methods using e.g. potassium iodide (KI) may also be suited and should be tested systematically.
Collecting fertile tetrasporophytes at the peak of sorus occurrence (January to March, Fig. 6) for spore release and further seeding of substrate implies a temporal overlap and shortening of the growth season because the optimal time for deploying seeded substrates in open-sea cultivation sites ranges from the autumn to late winter and harvesting the following spring (Werner and Dring 2011a, b; Schmedes 2020). Some successful attempts to induce fertility and subsequent spore release in P. palmata have been reported by isolating submeristematic thalli fragments exposed to short days (8 h:16 h, L:D) and 10 °C with a maturation time varying from 1.5 to 2.5 months (Pang and Lüning 2006; Titlyanov et al. 2006). Recent work documented the sequential effects of short days (8 h:16 h) and high nutrient on the induction of tetrasporogenesis (Schmedes 2020). In this study, spore formation occurred only at 5 °C and was not observed at 10 °C suggesting that local populations require specific combinations of environmental factors triggering the onset of sporogenesis. Furthermore, the response time to obtain mature sori from vegetative wild individuals highly depends on time of collection and sporogenesis may become evident after 0.5 to 3 months, but the emergence of mature sori may extend up to 4 to 6 months (Schmedes 2020). Gaining control over the fertility of P. palmata would allow the year-round production of spores and seedlings as well as flexibility to accommodate the hatchery phase according to optimal deployment time.
Recent improvements of existing protocols
Optimizing hatchery conditions to promote spore release, dispersal then attachment to the substrate is critical for the viable commercial cultivation of P. palmata. In a recent study, Schmedes and Nielsen (2020a) developed a new system based on conical seeding tanks in a flow-through system in which fertile material is placed above the substrate (nets) and equipped with a secondary tank to recover unattached spores. The consecutive use of sorus material (5 to 15 g WW); i.e. 3 times 3-days seeding period, successfully seeded nets (equivalent to 54 m linear rope) resulting in an average seedling density of 9 individuals per centimetre. The authors observed that an average of only 16% of the released spores attached to the substrate while the remaining were recovered in the secondary tank and germinated into male and female gametophytes after settlement. Homogenisation of the propagule suspension (i.e. the mixture of aggregated spores and young seedlings) and transfer to agitated seeding tanks allowed the gametophytes to establish discoid reattachment and spores to settle on the substrate in a secondary seeding trial. The procedure is referred to as the GMA-method (germinate, macerate, agitate) (Schmedes et al. 2019). Moreover, 50% of all released spores in a single batch will germinate into female gametophytes which will not develop further unless fertilized. An additional step consisting in mixing male gametes to the propagule suspension allows to fertilize the female gametophytes resulting in a doubling of the number of seedlings on a seeded substrate. These combined methods provide substantial improvements in spore-use efficiency compared to the high requirements of previous protocols for fertile material, i.e. 150 g of sorus tissue for seeding the equivalent of 82 m linear twine (Werner and Dring 2011a).
Commercial developments
There is currently no large-scale cultivation of P. palmata. A Portuguese company (AlgaPlus Ltd) runs a semi-commercial land-based production of P. palmata (max. 2 t WW year−1, H. Abreu, pers. comm.) from the vegetative propagation of acclimated strains since 2013. Other land-based pre-commercial growth trials are being conducted in Eastern Canada (e.g. Acadian Seaplants Ltd). A few other companies e.g. in USA (Springtide Ltd, Maine Fresh Sea Farm Ltd) and Denmark (OceanWide Seaweed ApS) are currently developing methodologies for the commercial production of spores and seedlings and subsequent deployment at sea. Further efforts are needed to develop a sustainable and profitable industry based on P. palmata cultivation. These include securing stable seed supplies, improve spores and seedlings survival and identify the conditions maintaining a high productivity in land- and sea-based cultivation operations. Technical adjustments of the cultivation settings e.g. turning off tank aeration during the dark hours, may provide substantial reductions in energy costs without affecting the growth of P. palmata (Caines et al. 2014). Cultivation protocols and technology adapted to this species must be developed to reduce the costs per ton produced (Werner and Dring 2011a) and to make the production of P. palmata an economically viable activity in Europe and North America.
Commercial applications
Direct food applications and sensory profile
Food is the main commercial application for P. palmata. In fact, it is one of the most popular species among Western consumers and high-end chefs. The increased interest for dulse and edible macroalgae in general is supported by current trends promoting local, sustainable, healthy and vegetarian/vegan foods. Besides its remarkable nutritional profile (see section on “Chemical and nutrient composition and Table 2), dulse is highly appreciated for its unique flavour profile and is used either directly as a snack or in various food preparations (Rhatigan 2009; Mouritsen et al. 2013; Chapman et al. 2015). It is often sold to the consumer in dry form (whole fronds, flakes) or as an ingredient in products (spreads, herbal tea mixes, breads, seaweed salt, mustard). It may also be found in fresh salted form.
Umami is the most typical flavour reported in the literature from sensory descriptions of P. palmata (Mouritsen et al. 2012; Chapman et al. 2015). Umami is the fifth basic taste (along with sweet, salty, sour and bitter) and is described as a savoury, long-lasting flavour of broth and meat. It is elicited primarily by free glutamate and to a lesser extent free aspartate, which are abundant in typical umami-rich foods e.g. dried and aged Japanese kelp kombu (Saccharina japonica), cured meat, soy sauce and aged cheese (Ninomiya 1998). The levels of free glutamate measured in P. palmata (1 to 4 mg g−1 DW) vary depending on the origin of the raw material and physiological status of the plant (Mouritsen et al. 2012). Although these levels are lower than those found in kombu (up to 16 mg g−1 DW), the umami response does not depend solely on the free glutamate content but also on other flavour-active compounds e.g. 5’-ribonucleotides (guanylate, inosinate and adenylate), small peptides and glutamate derivatives (Zhao et al. 2016) which have not yet been studied in P. palmata. In culinary applications, umami is found to enhance the flavour of other ingredients that do not contain appreciable amounts of glutamate such as vegetables. Several collaborations between scientists and chefs have distinguished P. palmata as a promising ingredient providing not only flavour but also a pleasing visual appearance to a wide range of dishes such as ice cream, bread and pasta (Mouritsen et al. 2012, 2019; Chapman et al. 2015) (Fig. 13).
Examples of food dishes containing Palmaria palmata prepared during a cooking workshop in Norway described in Chapman et al. (2015). Top: dulse tagliatelle with chicken breast. Bottom: clip fish (i.e. salted and dried cod) brandade with dulse. Photographs by Pierrick Stévant
As for other foods, the organoleptic quality of P. palmata is greatly affected by post-harvest processing steps, storage conditions and preparation methods. Freshly harvested dulse, often considered less palatable than dried material, is described by fresh marine aromas while it is associated with green aromas following freezing then thawing (Le Pape et al. 2002) and with fishy and fish meal notes in its dry form (Michel et al. 1997; Stévant et al. 2020b). The light rehydration of dried P. palmata fronds and subsequent storage for several weeks or months develop distinct sweet, rich and complex flavours including umami and notes of honey and liquorice (Chapman et al. 2015; Stévant et al. 2020b). This flavour development is correlated with a higher level and diversity of volatile compounds in lightly rehydrated (referred to as semi-dried) dulse compared to the dry material (Stévant et al. 2020b), and with the hydrolysis of soluble proteins into smaller peptides (Stévant et al. 2020a). A more tender texture of the fronds was also reported from this maturation process, reflecting structural alteration from endogenous (e.g. enzymatic) degradation of cell-wall polysaccharides (xylans). A specific preparation, consisting of deep-frying dried and smoked dulse fronds, has recently received media attention since it was found to produce flavours and texture similar to pork bacon (Mouritsen et al. 2019). This highlights the potential of dulse in culinary innovations to create distinct flavours and textures using different processing methods.
Market value
The renewed interest from Western consumers for macroalgae and particularly P. palmata has led to an increasing demand for this species, primarily from food manufacturers, exceeding the current production based on wild-harvested biomass. This can explain the relatively high bulk retail value (business-to-business) of dried dulse which was reported to range from 16 to 19 € kg−1 approximately ten years ago (Walsh and Watson 2011; Werner and Dring 2011a) and is now estimated between 40 and 75 € kg−1 (H. Abreu, pers. comm.). As a comparison, statistics from the Norwegian directorate of Fisheries (2022) for the national macroalgae production reports a sales value of 2 to 4 € kg−1 for the cultivated kelps S. latissima and A. esculenta between 2019 and 2021 (original values expressed in NOK, 1 NOK = 0.099 €). Based on an online search of dried dulse products, the current median consumer retail price of dried dulse is estimated to 13.40 € 100 g−1 (n = 50, Fig. 14). Despite large variations (min = 4.68, max = 47.60 € 100 g−1) which may reflect different marketing strategies and differences in consumers’ willingness-to-pay for such products across countries, this estimate is in the upper range of reported prices for dried edible macroalgae including several species (Le Bras et al. 2015).
Boxplot and overlaid scatter plot of the consumer retail value of dehydrated Palmaria palmata based on an online search (conducted in September 2022) including 50 products consisting of dried raw material (whole, flaked or powdered) from France (n = 9), Ireland (n = 9), Canada (n = 7), United Kingdom (n = 7), USA (n = 6), Norway (n = 3), Portugal (n = 2), Denmark (n = 2), Iceland (n = 2), Spain (n = 2) and from unknown origin (n = 1). 68% of the products were labelled “organic”. Original prices in USD, CAD, GBP, DKK, ISK and NOK were converted in € according to exchange rates in September 2022
Ingredient in human and animal nutrition
There are few written historical records of dulse being used to feed livestock during ancient times, e.g. in Iceland (Hallsson 1964). The relatively high protein content of P. palmata, reaching levels of typical protein-rich plant material (e.g. pulse, beans and soy), and a favourable amino acid profile (high proportion of EAA, Table 2) has driven the interest for its commercial exploitation in animal nutrition. However, the results from in vitro assays revealed the rather low digestibility of proteins from P. palmata, both from algal powder and water-soluble extracts (Galland-Irmouli et al. 1999; Bikker et al. 2020). This is due to the ionic interactions of cell-wall polysaccharides (xylans) with proteins and the high viscosity of aqueous extracts, reducing the protein bioaccessibility to proteolytic enzymes. Results from a mink feeding trial supported the findings from in vitro assays and revealed the low apparent amino acid digestibility (especially histidine) of dried and milled P. palmata fronds added to the diet, and a lower body weight of the animals (minks) fed with this diet compared to a control containing fish meal (Krogdahl et al. 2021). In contrast, the inclusion of up to 15% P. palmata into salmon smolt diets did not significantly affect fish growth (feed conversion ratio, specific growth rate) or health parameters (hematological, immunological and hepatic functions) (Wan et al. 2016).
Processing steps such as boiling (Mæhre et al. 2015), freshwater-soaking and fermentation using a Trichoderma strain (Marrion et al. 2003) has been shown to improve the digestibility of proteins from P. palmata, as a results of the partial removal or degradation of xylans. Water extraction procedures are typically used to recover proteins from dulse. Since xylans also impair the aqueous extraction of proteins, several protocols including an enzymatic pre-treatment step using xylanase have been tested and resulted in higher extraction yields (Joubert and Fleurence 2008; Harnedy and FitzGerald 2013; Bjarnadóttir et al. 2018; Naseri et al. 2020). Bjarnadóttir et al. (2018) and Naseri et al. (2020) obtained a higher protein concentration and proportion of EAA (approximately between 40 and 50%) in the solid phase from the extraction of enzyme-treated P. palmata samples compared to levels found in the aqueous phase. A protein concentrate obtained from a similar protocol was shown to have a higher apparent digestibility in minks compared to diets containing whole P. palmata (Krogdahl et al. 2021). The authors concluded that such a protein concentrate may have a potential as animal feed ingredient, although it should be combined with other protein-rich sources in feed formulations to compensate for some limiting amino acids. A pilot-scale protein extraction of dulse using xylanase increased the protein content from 12% (initial raw material) to 28% DW in the final product, i.e. the solid fraction after extraction (Aasen et al. 2022). Since existing feed protein ingredients (e.g. from soybean) have a relatively low price, the economic viability of a feed protein concentrate from P. palmata will greatly depend on associated production and processing costs. The use of high quantities of enzymes in an industrial setting may not be viable economically. The recovery of valuable compounds (e.g. minerals, pigments, soluble carbohydrates) from side streams (e.g. liquid fraction) can add value to the harvested biomass and create an economic incentive for the use of dulse in animal nutrition.
Schiener et al. (2017) proposed the simultaneous recovery of the protein- and lipid- enriched solid residue on the one hand, and liquid extract containing carbohydrates (mainly xylans) and minerals on the other hand, after enzymatic treatment of P. palmata under acidic conditions. No commercial application was suggested for the latter extract, but it is likely to contain water-soluble flavour compounds, e.g. free amino acids, peptides (Stévant et al. 2020b) and phycoerythrin pigment (Dumay et al. 2013), and may have potential as a flavour extract or food colorant. However, commercial development of commodity products from dulse (e.g., feed or food ingredients) are limited by raw material availability, as well as production and process efficiency, to be competitive with existing alternatives. Fermentation (ensiling), even without the addition of a commercial start culture, appeared to be an efficient method for the conservation and fractionation of P. palmata biomass, in which nearly up to 50% of the initial fresh weight containing up to one third of the total dry matter can be separated into a liquid fraction (Gallagher et al. 2021). The effluent formation from this process can be improved by additional processing steps such as screw-pressing, wilting or chopping. The commercial potential of the solid and liquid components of fermented dulse in human and animal nutrition have not yet been explored.
Other industrial applications
Dulse is characterized by relatively high levels of phycoerythrin pigment, especially during the winter season (Table 2). This water-soluble pigment has industrial applications as colorant in food and cosmetic products and as fluorescent marker in immuno-assays (Sekar and Chandramohan 2008). Microalgae such as Porphyridium cruentum are the main commercial source of phycoerythrin. Protocols have also been developed for the optimized extraction and purification of the phycoerythrin from P. palmata (Galland-Irmouli et al. 2000; Dumay et al. 2013) but have not been implemented commercially. The results from a drying experiment of P. palmata indicates that the phycoerythrin is rather sensitive to degradation upon processing of the biomass, as illustrated by the reduced absorption spectra of extracts from air-dried compared to freeze-dried samples (Fig. 15). Appropriate post-harvest treatments and storage methods that maintain the integrity of the compound and maximize extraction yields must be established for future commercial exploitation of phycoerythrin from P. palmata.
Absorption peaks of phycoerythrin from Palmaria palmata extracts obtained from air-dried samples at 40 and 70 °C and freeze-dried (FD) samples. Data from Stévant (2019)
The antioxidant activity of aqueous and ethanol-soluble extracts from P. palmata and the potential of these extracts as natural antioxidant has been studied extensively. Antioxidant activity is associated with various molecules such as phenolic compounds (Yuan et al. 2005; Suwal et al. 2019), although found at lower levels in P. palmata compared to brown macroalgae (Roleda et al. 2019), as well as peptides (Wang et al. 2010; Harnedy et al. 2017) and polysaccharides (Suwal et al. 2019). The bioactivity of extracts can be enhanced by enzymatic treatments using proteases (Wang et al. 2010; Harnedy et al. 2017) or polysaccharidase (i.e. hemicellulose) under high hydrostatic pressure (Suwal et al. 2019). A protein hydrolysate from P. palmata associated with renin inhibitory bioactivity (i.e. reducing the risk of hypertension) was successfully added to a bread which retained bioactivity following the baking process (Fitzgerald et al. 2014). In a recent study, Lopes et al. (2019) revealed the antioxidant activity of a polar lipid-rich extract from dulse. These results highlight the potential of P. palmata and derived products to be used as functional ingredient to extend the shelf-life and improve the health value of foods. On the other hand, the results from a human intervention study suggest that the daily consumption of 5 g dulse (incorporated into a bread) for 4 weeks stimulates inflammation, increases serum triglycerides and alters thyroid function (Allsopp et al. 2016). However, these metabolic changes remained within the normal clinical range and were unlikely to impact health. Further studies of this kind including larger sample sizes and longer interventions may confirm these results and provide information on the medium to long-term impact of dulse consumption.
Palmaria palmata is also rich in photoprotective molecules, namely MAAs (see “environment requirements and stressors”) showing antioxidant activity (Yuan et al. 2009) and anti-proliferative properties on human cells (Athukorala et al. 2016) when isolated in aqueous methanol extracts. Moreover, these compounds are suggested as a biocompatible alternative to the use of synthetic UV radiation filters in sunscreens and cosmetic formulations, which are associated with negative impacts on marine ecosystems (Lawrence et al. 2018). Besides, the antioxidant properties of dulse can also be applied to edible films and coatings for the food industry. Albertos et al. (2019) successfully incorporated 1% powdered dulse in a chitosan-based edible films, delaying the oxidation and microbial spoilage of fish burgers.
Technical solutions based on low-cost technologies must be developed for the production, preservation and processing of P. palmata to enable future commercial developments with this species. Applying circular principles such as cultivation in IMTA systems, use of industrial surplus heat for biomass processing, valorisation of waste streams in biorefinery processes, will increase the profitability and the environmental sustainability of new products based on dulse.
Conclusions
Palmaria palmata is a common red macroalgae species of the Northern Atlantic shores. Owing to remarkable nutritional features and unique flavours, dulse has traditionally been harvested and used as food among coastal communities in Western Europe and North America. The recent interest from Western consumers for natural foods and the (re)discovery of macroalgae as a source of nutrients, bioactive compounds and flavours has shed light on the potential of dulse in gastronomy and as a functional ingredient in the food industry. Other than food, P. palmata may enter the composition of commercial formulations e.g. animal feed, cosmetic products, as an alternative to ingredients associated with negative environmental impacts (e.g. soy protein concentrate, synthetic antioxidants). Hence, the increasing demand for dulse biomass from industrial actors.
This review highlights the current lack of adapted resource management schemes for the exploitation of wild biomass in relevant countries, including the reporting of annual landings to the FAO. Increasing our understanding of the biology, taxonomy and distribution of the species will provide a basis for effective decision-making for conservation purposes and sustainable harvest. However, significantly increasing the harvesting pressure on wild populations is not considered sustainable. Instead, P. palmata may be cultivated in land-based facilities and/or at sea, either vegetatively or by seeding growth substrates with tetraspores and germinated gametophytes. However, there are major bottlenecks limiting the commercial cultivation of dulse, including the low rate of released spores, high mortality and contamination from epiphytes and endophytes. Establishing cultivation protocols for the industrial production of P. palmata as well as efficient biomass processing methods is the key to enable commercial developments in food and other applications in the future.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Aasen IM, Sandbakken IS, Toldnes B, Roleda MY, Slizyte R (2022) Enrichment of the protein content of the macroalgae Saccharina latissima and Palmaria palmata. Algal Res 65:102727
Albertos I, Martin-Diana AB, Burón M, Rico D (2019) Development of functional bio-based seaweed (Himanthalia elongata and Palmaria palmata) edible films for extending the shelflife of fresh fish burgers. Food Packag Shelf Life 22:100382
Allsopp P, Crowe W, Bahar B, Harnedy PA, Brown ES, Taylor SS, Smyth TJ, Soler-Vila A, Magee PJ, Gill CIR, Strain CR, Hegan V, Devaney M, Wallace JMW, Cherry P, FitzGerald RJ, Strain JJ, O’Doherty JV, McSorley EM (2016) The effect of consuming Palmaria palmata-enriched bread on inflammatory markers, antioxidant status, lipid profile and thyroid function in a randomised placebo-controlled intervention trial in healthy adults. Eur J Nutr 55:1951–1962
ANSES (2018) Opinion of the French Agency for Food, Environmental and Occupational Health & Safety on the risk of excess iodine intake from the consumption of seaweed in foodstuffs. https://www.anses.fr/en/system/files/NUT2017SA0086EN.pdf
Araújo R, Bárbara I, Tibaldo M, Berecibar E, Tapia PD, Pereira R, Santos R, Pinto IS (2009) Checklist of benthic marine algae and cyanobacteria of northern Portugal. Bot Mar 52:24–46
Athukorala Y, Trang S, Kwok C, Yuan YV (2016) Antiproliferative and antioxidant activities and mycosporine-like amino acid profiles of wild-harvested and cultivated edible canadian marine red macroalgae. Molecules 21:E119
Badis Y, Klochkova TA, Strittmatter M, Garvetto A, Murúa P, Sanderson JC, Kim GH, Gachon CMM (2019) Novel species of the oomycete Olpidiopsis potentially threaten European red algal cultivation. J Appl Phycol 31:1239–1250
Badis Y, Klochkova TA, Brakel J, Arce P, Ostrowski M, Tringe SG, Kim GH, Gachon CMM (2020) Hidden diversity in the oomycete genus Olpidiopsis is a potential hazard to red algal cultivation and conservation worldwide. Eur J Phycol 55:162–171
Bak UG (2019) Seaweed cultivation in the Faroe Islands: An investigation of the biochemical composition of selected macroalgal species, optimised seeding technics, and open-ocean cultivation methods from a commercial perspective. PhD thesis, Technical University of Denmark (DTU), Kongens Lyngby, Denmark. https://backend.orbit.dtu.dk/ws/portalfiles/portal/183321338/THESIS_URD_FINAL_sendt.pdf
Biancarosa I, Belghit I, Bruckner CG, Liland NS, Waagbo R, Amlund H, Heesch S, Lock EJ (2018) Chemical characterization of 21 species of marine macroalgae common in Norwegian waters: benefits of and limitations to their potential use in food and feed. J Sci Food Agric 98:2035–2042
Bikker P, Stokvis L, van Krimpen MM, van Wikselaar PG, Cone JW (2020) Evaluation of seaweeds from marine waters in Northwestern Europe for application in animal nutrition. Anim Feed Sci Technol 263:114460
BIM (2014) The European Market for Sea Vegetables https://bim.ie/wp-content/uploads/2021/02/The,European,Market,for,Sea,Vegetables,-,2015.pdf.
Bird C, McLachlan J (1992) Seaweed flora of the maritimes. 1. Rhodophyta - the red algae. Biopress Ltd, Bristol, UK
Bjarnadóttir M, Aðalbjörnsson BV, Nilsson A, Slizyte R, Roleda MY, Hreggviðsson GÓ, Friðjónsson ÓH, Jónsdóttir R (2018) Palmaria palmata as an alternative protein source: enzymatic protein extraction, amino acid composition, and nitrogen-to-protein conversion factor. J Appl Phycol 30:2061–2070
Bøe R (2019) Investigation of important steps in Palmaria palmata cultivation. MSc thesis, Norwegian University of Science and Technology (NTNU), Trondheim, Norway. https://ntnuopen.ntnu.no/ntnu-xmlui/bitstream/handle/11250/2625856/no.ntnu:inspera:43674632:47126134.pdf?sequence=1
Le Bras Q, Lesueur M, Lucas S, Gouin S (2015) Etude du marché français des algues alimentaires. Catalogue et analyse des produits existants. vol 37. Les publications du Pôle halieutique AGROCAMPUS OUEST, Programme IDEALG Phase 2, Rennes, France. https://hal.archives-ouvertes.fr/hal-01344025/
Bringloe TT, Saunders GW (2018) Mitochondrial DNA sequence data reveal the origins of postglacial marine macroalgal flora in the Northwest Atlantic. Mar Ecol Prog Ser 589:45–58
Bringloe TT, Verbruggen H, Saunders GW (2020) Unique biodiversity in Arctic marine forests is shaped by diverse recolonization pathways and far northern glacial refugia. Proc Natl Acad Sci USA 117:22590–22596
Browne K (2001) Mariculture of the edible red alga Palmaria palmata. PhD thesis, Queen's University of Belfast (QUB), Belfast, UK
Buchholz C, Lüning K (1999) Isolated, distal blade discs of the brown alga Laminaria digitata form sorus, but not discs, near to the meristematic transition zone. J Appl Phycol 11:579–584
Cabioc'h J, Floc'h J-Y, Le Toquin A, Boudouresque C-F, Meinesz A, Verlaque M (2006) Guide des algues des mers d'Europe. Delachaux et Niestlé, Paris, France
Caines S, Manríquez-Hernández JA, Duston J, Corey P, Garbary DJ (2014) Intermittent aeration affects the bioremediation potential of two red algae cultured in finfish effluent. J Appl Phycol 26:2173–2181
Chapman ARO (1984) Reproduction, recruitment and mortality in two species of Laminaria in southwest Nova Scotia. J Exp Mar Biol Ecol 78:99–109
Chapman AS, Stévant P, Larssen WE (2015) Food or fad? Challenges and opportunities for including seaweeds in a Nordic diet. Bot Mar 58:423–433
Chopin T, Ugarte R (1998) The seaweed resources of Eastern Canada. In: Critchley A, Ohno M (eds) Seaweed resources of the world. Japan International Cooperation Agency, Yokosuka, Japan
Corey P, Kim JK, Garbary DJ, Prithiviraj B, Duston J (2012) Bioremediation potential of Chondrus crispus (Basin Head) and Palmaria palmata: effect of temperature and high nitrate on nutrient removal. J Appl Phycol 24:441–448
Corey P, Kim JK, Duston J, Garbary DJ, Prithiviraj B (2013) Bioremediation potential of Palmaria palmata and Chondrus crispus (Basin Head): effect of nitrate and ammonium ratio as nitrogen source on nutrient removal. J Appl Phycol 25:1349–1358
Corey P, Kim JK, Duston J, Garbary DJ (2014) Growth and nutrient uptake by Palmaria palmata integrated with Atlantic halibut in a land-based aquaculture system. Algae 29:35–45
Courtois A, Simon-Colin C, Boisset C, Berthou C, Deslandes E, Guézennec J, Bordron A (2008) Floridoside extracted from the red alga Mastocarpus stellatus is a potent activator of the classical complement pathway. Mar Drugs 6:407–417
CRPMEM (2020) Evaluation et gestion de la biomasse exploitable en algues de rive. Rapport de synthese du programme biomasse algues. Comité Régional des Pêches Maritimes et des Élevages Marins de Bretagne. http://www.bretagne-peches.org/modules/kameleon/upload/rapport_biomasse_algues_public_vf.pdf
CRPMEM (2021) AGRID- Amélioration des connaissances sur les algues de rives et leur récolte pour une gestion durable. Comité Régional des Pêches Maritimes et des Élevages Marins de Bretagne. https://www.bretagne-peches.org/?titre=agrid--amelioration-des-connaissances-sur-les-algues-de-rives-et-leur-recolte-pour-une-gestion-durable&mode=autres-projets&archive=1&id=3715. Accessed 13.12 2021
Delaney A, Frangoudes K, Ii SA (2016) Society and seaweed: Understanding the past and present. In: Fleurence J, Levine I (eds) Seaweed in Health and Disease Prevention. Academic Press, San Diego, pp 7–40
Deniaud E, Quemener B, Fleurence J, Lahaye M (2003) Structural studies of the mix-linked β-(1→3)/β-(1→4)-d-xylans from the cell wall of Palmaria palmata (Rhodophyta). Int J Biol Macromol 33:9–18
Deniaud E, Gall LL, Rusig A-M, Lahaye M (2006) Initial observations on glycoside deposition in cell walls of Palmaria palmata (L.) Kuntze (Rhodophyta) during spore germination. Bot Mar 49:266–269
Desideri D, Cantaluppi C, Ceccotto F, Meli MA, Roselli C, Feduzi L (2016) Essential and toxic elements in seaweeds for human consumption. J Toxicol Environ Health, A 79:112–122
Duinker A, Kleppe M, Fjære E, Biancarosa I, Heldal HE, Dahl L, Lunestad BT (2020) Knowledge update on macroalgae food and feed safety - based on data generated in the period 2014–2019 by the Institute of Marine Research, Norway. IMR reports, vol 2020–44. Institute of Marine Research, Bergen, Norway. https://www.hi.no/hi/nettrapporter/rapport-fra-havforskningen-en-2020-44
Dumay J, Clément N, Morançais M, Fleurence J (2013) Optimization of hydrolysis conditions of Palmaria palmata to enhance R-phycoerythrin extraction. Bioresour Technol 131:21–27
Edwards MD, Dring MJ (2011) Open-sea cultivation trial of the red alga, Palmaria palmata from seeded tetraspores in Strangford Lough, Northern Ireland. Aquaculture 317:203–209
Edwards MD (2007) The cultivation of the edible red alga, Palmaria palmata for aquaculture. PhD thesis, The Queen’s University of Belfast (QUB), Belfast, UK
EU (2006) Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32006R1881
Evans F, Langdon CJ (2000) Co-culture of dulse Palmaria mollis and red abalone Haliotis rufescens under limited flow conditions. Aquaculture 185:137–158
Faes VA, Viejo RM (2003) Structure and dynamics of a population of Palmaria palmata (Rhodophyta) in Northern Spain. J Phycol 39:1038–1049
Fernández-Mendoza F, Gómez D, Pérez-Ortega S, Molares A, Dopazo M, Anta F, Barreno E, Fos S, López-Prada M, Fernández-Toirán L, Ágreda Cabo T, Vázquez J, Castro M, Lago M, Blanco-Dios J, Rodríguez M, Peña V, Calvo S, Dosil J, Cremades J (2002) Fragmenta chorologica occidentalia, Algae 7776–7892. Ann Jard Bot Madrid 59:289–291
Fitzgerald C, Aluko RE, Hossain M, Rai DK, Hayes M (2014) Potential of a renin inhibitory peptide from the red seaweed Palmaria palmata as a functional food ingredient following confirmation and characterization of a hypotensive effect in spontaneously hypertensive rats. J Agr Food Chem 62:8352–8356
Fleurence J (1999) Seaweed proteins: biochemical, nutritional aspects and potential uses. Trends Food Sci Technol 10:25–28
Foseid L, Natvik I, Devle H, Ekeberg D (2020) Identification of fatty acids in fractionated lipid extracts from Palmaria palmata, Alaria esculenta and Saccharina latissima by off-line SPE GC-MS. J Appl Phycol 32:4251–4262
Fredericq S, Hommersand M (1990) Sexual reproduction and cystocarp development. In: Cole K, Sheath R (eds) Biology of the red algae. Cambridge University Press, Cambridge, pp 305–345
Fredriksen S, Karsten U, Bartsch I, Woelfel J, Koblowsky M, Schumann R, Moy SR, Steneck RS, Wiktor JM, Hop H, Wiencke C (2019) Biodiversity of benthic macro- and microalgae from Svalbard with special focus on Kongsfjorden. In: Hop H, Wiencke C (eds) The ecosystem of Kongsfjorden, Svalbard. Springer, Cham. pp 331–371
Freshwater DW, Fredericq S, Butler BS, Hommersand MH, Chase MW (1994) A gene phylogeny of the red algae (Rhodophyta) based on plastid rbcL. Proc Natl Acad Sci USA 91:7281–7285
Le Gall L, Deniaud E, Rusig AM (2004a) Phenological study of the reproduction of the Rhodophyceae Palmaria palmata along the French coast of the English Channel. Cah Biol Mar 45:269–275
Le Gall L, Rusig A-M, Cosson J (2004c) Organisation of the microtubular cytoskeleton in protoplasts from Palmaria palmata (Palmariales, Rhodophyta). Bot Mar 47:231
Gallagher JA, Adams JMM, Turner LB, Kirby ME, Toop TA, Mirza MW, Theodorou MK (2021) Bio-processing of macroalgae Palmaria palmata: metabolite fractionation from pressed fresh material and ensiling considerations for long-term storage. J Appl Phycol 33:533–544
Galland-Irmouli AV, Fleurence J, Lamghari R, Luçon M, Rouxel C, Barbaroux O, Bronowicki JP, Villaume C, Guéant JL (1999) Nutritional value of proteins from edible seaweed Palmaria palmata. J Nutr Biochem 10:353–359
Galland-Irmouli AV, Pons L, Luçon M, Villaume C, Mrabet MT, Guéant JL, Fleurence J (2000) One-step purification of R-phycoerythrin from the red macroalga Palmaria palmata using preparative polyacrylamide gel electrophoresis. J Chromatogr B 739:117–123
Garbary DJ, Beveridge LF, Flynn AD, White KL (2012) Population ecology of Palmaria palmata (Palmariales, Rhodophyta) from harvested and non-harvested shores on Digby Neck, Nova Scotia. Canada Algae 27:33–42
García-Jiménez P, Robaina RR (2012) Effects of ethylene on tetrasporogenesis in Pterocladiella capillacea (Rhodophyta). J Phycol 48:710–715
Garineaud C (2018) Récolter la mer : des savoirs et des pratiques des collecteurs d’algues à la gestion durable des ressources côtières dans le Finistère (Bretagne). PhD thesis, Museum national d’histoire naturelle - MNHN, Paris. https://tel.archives-ouvertes.fr/tel-01745164
GBIF (2022) Global Biodiversity Information Facility. GBIF Occurrence Download . . GBIF.org. Accessed 14.02 2022
Grote B (2016) Bioremediation of aquaculture wastewater: evaluating the prospects of the red alga Palmaria palmata (Rhodophyta) for nitrogen uptake. J Appl Phycol 28:3075–3082
Grote B (2019) Recent developments in aquaculture of Palmaria palmata (Linnaeus) (Weber & Mohr 1805): cultivation and uses. Rev Aquac 11:25–41
Guihéneuf F, Gietl A, Stengel DB (2018) Temporal and spatial variability of mycosporine-like amino acids and pigments in three edible red seaweeds from western Ireland. J Appl Phycol 30:2573–2586
Guiry MD (1974) A preliminary consideration of the taxonomic position of Palmaria palmata (Linnaeus) Stackhouse = Rhodymenia palmata (Linnaeus) Greville. J Mar Biol Ass UK 54:509–528
Guiry MD, Guiry GM (2022) AlgaeBase. World-wide electronic publication. National University of Ireland, Galway. http://www.algaebase.org. Accessed 23.04.2021
Gulliksen B, Palerud R, Brattegard T, Sneli J (1999) Distribution of marine benthic macro-organisms at Svalbard (including Bear Island) and Jan Mayen Research Report for DN, vol 1999–4. Directorate for Nature Management, Trondheim, Norway. https://www.miljodirektoratet.no/publikasjoner/publikasjoner-fra-dirnat/dn-rapporter/distribution-of-marine-benthic-macro-organisms-at-svalbard-including-bear-island-and-jan-mayen-/
Guzmán-Urióstegui A, García-Jiménez P, Marián F, Robledo D, Robaina R (2002) Polyamines influence maturation in reproductive structures of Gracilaria cornea (Gracilariales, Rhodophyta). J Phycol 38:1169–1175
Hallsson SV (1964) The uses of seaweeds in Iceland. In: De Virville AD, Feldmann J (eds) Proceedings of the Fourth International Seaweed Symposium, Biarritz, September 1961. Pergamon Press, Oxford, pp 398–405
Hanelt D, Nultsch W (1995) Field studies of photoinhibition show non-correlations between oxygen and fluorescence measurements in the Arctic red alga Palmaria palmata. J Plant Physiol 145:31–38
Hansen L, Johansen S (1998) Makrofauna og makroalger i littoralzonen udvalgte steder på Disko, NV Grønland. Fagprojekt, Arktisk Kursus. 37 pp.
Harnedy PA, FitzGerald RJ (2013) Extraction of protein from the macroalga Palmaria palmata. LWT-Food Sci Technol 51:375–382
Harnedy PA, O’Keeffe MB, FitzGerald RJ (2017) Fractionation and identification of antioxidant peptides from an enzymatically hydrolysed Palmaria palmata protein isolate. Food Res Int 100:416–422
Harper JT, Saunders GW (2002) A re-classification of the Acrochaetiales based on molecular and morphological data, and establishment of the Colaconematales ord. nov. (Florideophyceae, Rhodophyta). Eur J Phycol 37:463–476
Hawkins SJ, Harkin E (1985) Preliminary canopy removal experiments in algal dominated communities low on the shore and in the shallow subtidal on the Isle of Man. Bot Mar 28:223–230
Jard G, Marfaing H, Carrère H, Delgenes JP, Steyer JP, Dumas C (2013) French Brittany macroalgae screening: Composition and methane potential for potential alternative sources of energy and products. Bioresour Technol 144:492–498
Jónsson H (1904) The marine algae of East Greenland. Meddelelser om Grønland 30:1–73 (as Rhodymenia palmata, p 12)
Jørgensen K, Olesen PT (2018) Kainic acid in the seaweed Palmaria palmata (dulse). Food Addit Contam B 11:198–200
Joubert Y, Fleurence J (2008) Simultaneous extraction of proteins and DNA by an enzymatic treatment of the cell wall of Palmaria palmata (Rhodophyta). J Appl Phycol 20:55–61
Kain JM (1982) The reproductive phenology of nine species of Rhodophyta in the subtidal region of the Isle of Man. Br Phycol J 17:321–331
Karsten U, Wiencke C (1999) Factors controlling the formation of UV-absorbing mycosporine-like amino acids in the marine red alga Palmaria palmata from Spitsbergen (Norway). J Plant Physiol 155:407–415
Karsten U, Bischof K, Wiencke C (2001) Photosynthetic performance of Arctic macroalgae after transplantation from deep to shallow waters. Oecologia 127:11–20
Karsten U, Dummermuth A, Hoyer K, Wiencke C (2003) Interactive effects of ultraviolet radiation and salinity on the ecophysiology of two Arctic red algae from shallow waters. Polar Biol 26:249–258
Kientz B, Thabard M, Cragg SM, Pope J, Hellio C (2011) A new method for removing microflora from macroalgal surfaces: an important step for natural product discovery. Bot Mar 54:457–469
Kim JK, Duston J, Corey P, Garbary DJ (2013) Marine finfish effluent bioremediation: effects of stocking density and temperature on nitrogen removal capacity of Chondrus crispus and Palmaria palmata (Rhodophyta). Aquaculture 414–415:210–216
Kraan S, Guiry M (2006) A genetic investigation of two morphotypes of Palmaria palmata (Palmariales, Rhodophyceae) using Rubisco spacer and ITS 1 and ITS 2 sequences. Cryptogam Algol 27:17–30
Kraan S (2013) Pigments and minor compounds in algae. In: Domínguez H (ed) Functional ingredients from algae for foods and nutraceuticals. Woodhead Publishing, NY pp 205–251
Krause-Jensen D, Archambault P, Assis J, Bartsch I, Bischof K, Filbee-Dexter K, Dunton KH, Maximova O, Ragnarsdóttir SB, Sejr MK, Simakova U, Spiridonov V, Wegeberg S, Winding MHS, Duarte CM (2020) Imprint of climate change on pan-Arctic marine vegetation. Front Mar Sci 7:617324
Kristjánsson L (1980) Íslenskir sjávarhættir. Menningarsjóðs B, Bókaútgáfa Menningarsjóðs, Reykjavík, Iceland
Krogdahl Å, Jaramillo-Torres A, Ahlstrøm Ø, Chikwati E, Aasen I-M, Kortner TM (2021) Protein value and health aspects of the seaweeds Saccharina latissima and Palmaria palmata evaluated with mink as model for monogastric animals. Anim Feed Sci Technol 276:114902
Lahaye M, Michel C, Barry JL (1993) Chemical, physicochemical and in-vitro fermentation characteristics of dietary fibres from Palmaria palmata (L.) Kuntze. Food Chem 47:29–36
Lalegerie F, Stiger-Pouvreau V, Connan S (2020) Temporal variation in pigment and mycosporine-like amino acid composition of the red macroalga Palmaria palmata from Brittany (France): hypothesis on the MAA biosynthesis pathway under high irradiance. J Appl Phycol 32:2641–2656
Lawrence KP, Long PF, Young AR (2018) Mycosporine-like amino acids for skin photoprotection. Curr Med Chem 25:5512–5527
Le Gall L, Saunders GW (2007) A nuclear phylogeny of the Florideophyceae (Rhodophyta) inferred from combined EF2, small subunit and large subunit ribosomal DNA: Establishing the new red algal subclass Corallinophycidae. Mol Phylogenet Evol 43:1118–1130
Le Gall L, Pien S, Rusig AM (2004b) Cultivation of Palmaria palmata (Palmariales, Rhodophyta) from isolated spores in semi-controlled conditions. Aquaculture 229:181–191
Le Pape MA, Grua-Priol J, Demaimay M (2002) Effect of two storage conditions on the odor of an edible seaweed, Palmaria palmata, and optimization of an extraction procedure preserving its odor characteristics. J Food Sci 67:3135–3139
Levinsen J (2020) Cultivation of Palmaria palmata in land based integrated multi trophic aquaculture systems - from spore to harvestable resource. MSc thesis, Technical University of Denmark (DTU), Kongens Lyngby, Denmark
Li J-J, Hu Z-M, Duan D-L (2015) Genetic data from the red alga Palmaria palmata reveal a mid-Pleistocene deep genetic split in the North Atlantic. J Biogeogr 42:902–913
Lindstrom SC, Olsen JL, Stam WT (1996) Recent radiation of the Palmariaceae (Rhodophyta). J Phycol 32:457–468
Liu F, Pang SJ (2010) Stress tolerance and antioxidant enzymatic activities in the metabolisms of the reactive oxygen species in two intertidal red algae Grateloupia turuturu and Palmaria palmata. J Exp Mar Biol Ecol 382:82–87
Lopes D, Melo T, Meneses J, Abreu MH, Pereira R, Domingues P, Lillebø AI, Calado R, Domingues MR (2019) A new look for the red macroalga Palmaria palmata: A seafood with polar lipids rich in EPA and with antioxidant properties. Mar Drugs 17:533
Lubsch A, Timmermans KR (2020) Phosphate and nitrate uptake dynamics in Palmaria palmata (Rhodophyceae): Ecological and physiological aspects of nutrient availability. J Phycol 56:1184–1195
Lukeman RJ, Beveridge LF, Flynn AD, Garbary DJ (2012) A mathematical model of the commercial harvest of Palmaria palmata (Palmariales, Rhodophyta) on Digby Neck, Nova Scotia, Canada. Algae 27:43–54
Maack Á (2019) Wild Seaweed Harvesting: "The Next Big Industry in Iceland”? ways to encourage sustainable harvesting and improve the regulatory framework on seaweed. MSc thesis, Lund University, Lund, Sweden
Mac Monagail M, Cornish L, Morrison L, Araújo R, Critchley AT (2017) Sustainable harvesting of wild seaweed resources. Eur J Phycol 52:371–390
MacArtain P, Gill CIR, Brooks M, Campbell R, Rowland IR (2007) Nutritional value of edible seaweeds. Nutr Rev 65:535–543
Mæhre HK, Malde MK, Eilertsen KE, Elvevoll EO (2014) Characterization of protein, lipid and mineral contents in common Norwegian seaweeds and evaluation of their potential as food and feed. J Sci Food Agric 94:3281–3290
Mæhre HK, Edvinsen GK, Eilertsen K-E, Elvevoll EO (2015) Heat treatment increases the protein bioaccessibility in the red seaweed dulse (Palmaria palmata), but not in the brown seaweed winged kelp (Alaria esculenta). J Appl Phycol 28:581–590
Manríquez-Hernández JA, Duston J, Garbary DJ (2016) Effect of irradiance on bioremediation capacity of the red algae Chondrus crispus and Palmaria palmata. Aquac Int 24:39–55
Marambio J, Bischof K (2021) Differential acclimation responses to irradiance and temperature in two co-occurring seaweed species in Arctic fjords. Polar Res 40:5702
Marrion O, Schwertz A, Fleurence J, Guéant JL, Villaume C (2003) Improvement of the digestibility of the proteins of the red alga Palmaria palmata by physical processes and fermentation. Nahrung 47:339–344
Martínez B, Rico JM (2002) Seasonal variation of P content and major N pools in Palmaria palmata (Rhodophyta). J Phycol 38:1082–1089
Martínez B, Viejo RM, Rico JM, Rødde RH, Faes VA, Oliveros J, Álvarez D (2006) Open sea cultivation of Palmaria palmata (Rhodophyta) on the northern Spanish coast. Aquaculture 254:376–387
Matos J, Costa S, Rodrigues A, Pereira R, Sousa Pinto I (2006) Experimental integrated aquaculture of fish and red seaweeds in Northern Portugal. Aquaculture 252:31–42
Mesnildrey L, Jacob C, Frangoudes K, Reunavot M, Lesueur M (2012) La filière des macroalgues en France. Les publications du Pôle halieutique AGROCAMPUS OUEST, vol no. 39. Rennes, France. https://hal-agrocampus-ouest.archives-ouvertes.fr/hal-00840526/document
Michel F, Priol J, Galaup P, Demaimay M, Bigot C (1997) Effet de deux techniques de séchage sur les composés volatils de deux algues alimentaires Ulva sp et Palmaria palmata. Sci Aliment 17:601–617
Mikhaylova T (2021) A comprehensive bibliography, updated checklist, and distribution patterns of Rhodophyta from the Barents Sea (the Arctic Ocean). Bot Mar 64:211–220
Mishra VK, Temelli F, Ooraikul B, Shacklock PF, Craigie JS (1993) Lipids of the red alga, Palmaria palmata. Bot Mar 36:169
Morgan KC, Wright JLC, Simpson FJ (1980b) Review of chemical constituents of the red alga Palmaria palmata (dulse). Econ Bot 34:27–50
Morgan KC, Simpson FJ (1981a) The cultivation of Palmaria palmata. Effect of light intensity and nitrate supply on growth and chemical composition. Bot Mar 24:273
Morgan KC, Simpson FJ (1981b) The Cultivation of Palmaria palmata. Effect of light intensity and temperature on growth and chemical composition. Bot Mar 24:547–552
Morgan K, Shacklock P, Simpson F (1980a) Some aspects of the culture of Palmaria palmata in greenhouse tanks. Bot Mar 23:765–770
Mouritsen OG, Williams L, Bjerregaard R, Duelund L (2012) Seaweeds for umami flavour in the New Nordic Cuisine. Flavour 1:4
Mouritsen OG, Dawczynski C, Duelund L, Jahreis G, Vetter W, Schröder S (2013) On the human consumption of the red seaweed dulse (Palmaria palmata (L.) Weber & Mohr). J Appl Phycol 25:1777–1791
Mouritsen OG, Rhatigan P, Pérez-Lloréns JL (2019) The rise of seaweed gastronomy: phycogastronomy. Bot Mar 62:195–209
MSC (2022) Maine Seaweed Council Harvester's Guidelines. Maine Seaweed Council. https://www.seaweedcouncil.org/msc-harvester-information/. Accessed 28.01 2022
Munda IM, Gubenšek F (1976) The amino acid composition of some common marine algae from Iceland. Bot Mar:85–92
Naseri A, Marinho GS, Holdt SL, Bartela JM, Jacobsen C (2020) Enzyme-assisted extraction and characterization of protein from red seaweed Palmaria palmata. Algal Res 47:101849
NETALGAE (2012) Seaweed industry in Europe. https://www.seaweed.ie/irish_seaweed_contacts/doc/Filieres_12p_UK.pdf
Nielsen CW, Rustad T, Holdt SL (2021) Vitamin C from seaweed: A review assessing seaweed as contributor to daily intake. Foods 10:198
Ninomiya K (1998) Natural occurrence. Food Rev Int 14:177–211
Norton TA (1992) Dispersal by macroalgae. Br Phycol J 27:293–301
Norwegian Directorate of Fisheries (2022) Akvakulturstatistikk (tidsserier)-Alger. Fiskeridirektoratet. https://www.fiskeridir.no/Akvakultur/Tall-og-analyse/Akvakulturstatistikk-tidsserier/Alger. Accessed 14 Sept 2022
OBIS (2022) Ocean Biodiversity Information System. www.obis.org. Accessed 21 Feb 2022
Østgaard K, Indergaard M (2017) Vår historiske bruk av tang og tare. Naturen 141:194–206
Pakker H, Martins RST, Boelen P, Buma AGJ, Nikaido O, Breeman AM (2000) Effects of temperature on the photoreactivation of ultraviolet-B–induced DNA damage in Palmaria palmata (Rhodophyta). J Phycol 36:334–341
Pang S, Lüning K (2004a) Tank cultivation of the red alga Palmaria palmata: Effects of intermittent light on growth rate, yield and growth kinetics. J Appl Phycol 16:93–99
Pang SJ, Lüning K (2004b) Breaking seasonal limitation: year-round sporogenesis in the brown alga Laminaria saccharina by blocking the transport of putative sporulation inhibitors. Aquaculture 240:531–541
Pang SJ, Lüning K (2006) Tank cultivation of the red alga Palmaria palmata: Year-round induction of tetrasporangia, tetraspore release in darkness and mass cultivation of vegetative thalli. Aquaculture 252:20–30
Parjikolaei BR, Kloster L, Bruhn A, Rasmussen M, Fretté X, Christensen K (2013) Effect of light quality and nitrogen availability on the biomass production and pigment content of Palmaria palmata (Rhodophyta). Chem Eng Trans 32:967–972
Pedersen P (1976) Marine, benthic algae from southernmost Greenland. Meddelelser om Grønland. Nyt nordisk forlag, Copenhagen
Philippe M (2013) Récolte des algues de rive, guide de bonnes pratiques. Projet ALGAMARBIO. Inter Bio-Bretagne, Rennes, France. https://www.bio-bretagne-ibb.fr/wp-content/uploads/IBB-Guide-recolte-algues-29122013.pdf
Prasher SO, Beaugeard M, Hawari J, Bera P, Patel RM, Kim SH (2004) Biosorption of heavy metals by red algae (Palmaria palmata). Environ Technol 25:1097–1106
Provan J, Wattier RA, Ca M (2005) Phylogeographic analysis of the red seaweed Palmaria palmata reveals a Pleistocene marine glacial refugium in the English Channel. Mol Ecol 14:793–803
Pueschel CM (1979) Ultrastructure of tetrasporogenesis in Palmaria palmata (Rhodophyta). J Phycol 15:409–424
Pueschel CM, Cole KM (1982) Rhodophycean pit plugs: An ultrastructural survey with taxonomic implications. Am J Bot 69:703–720
Pueschel CM, Meer JPvd (1985) Ultrastructure of the fungus Petersenia palmariae (Oomycetes) parasitic on the alga Palmaria mollis (Rhodophyceae) Can J Bot 63:409-418
Ragan MA, Bird CJ, Rice EL, Gutell RR, Murphy CA, Singh RK (1994) A molecular phylogeny of the marine red algae (Rhodophyta) based on the nuclear small-subunit rRNA gene. Proc Natl Acad Sci USA 91:7276–7280
Ramsey UP, Bird CJ, Shacklock PF, Laycock MV, Wright JLC (1994) Kainic acid and 1′-hydroxykainic acid from Palmariales. Nat Toxins 2:286–292
Redmond S, Green L, Yarish C, Kim J, Neefus C (2014) New England seaweed culture handbook, nursery systems. vol CTSG‐14‐01. Connecticut Sea Grant. http://seagrant.uconn.edu/publications/aquaculture/handbook.pdf
Rhatigan P (2009) Irish seaweed kitchen. Booklink, Co., Down, Ireland
Rødde RSH, Vårum KM, Larsen BA, Myklestad SM (2004) Seasonal and geographical variation in the chemical composition of the red alga Palmaria palmata (L.) Kuntze. Bot Mar 47:125–133
Roleda MY, Marfaing H, Desnica N, Jónsdóttir R, Skjermo J, Rebours C, Nitschke U (2019) Variations in polyphenol and heavy metal contents of wild-harvested and cultivated seaweed bulk biomass: Health risk assessment and implication for food applications. Food Control 95:121–134
Rosenvinge L (1898) Deuxiéme mémoire sur les algues du Groenland. Meddelelser om Grønland 20: 1–125 (as Rhodymenia palmata, p 128)
Rosenvinge L (1983) Grønlands Havalger. Meddelelser om Grønland 3, 765–981 (as Rhodymenia palmata, p. 812). Bianco Lunos Kgl. Hof-Bogtrykkeri (F. Dreyer), Copenhagen, Denmark
Ryzhik IV, Кlindukh MP, Dobychina EO (2021) The B-group vitamins in the red alga Palmaria palmata (Barents Sea): Composition, seasonal changes and influence of abiotic factors. Algal Res 59:102473
Sagert S, Schubert H (2000) Acclimation of Palmaria palmata (Rhodophyta) to light intensity: Comparison between artificial and natural light fields. J Phycol 36:1119–1128
Sánchez-Machado DI, López-Cervantes J, López-Hernández J, Paseiro-Losada P (2004) Fatty acids, total lipid, protein and ash contents of processed edible seaweeds. Food Chem 85:439–444
Sanderson JC, Dring MJ, Davidson K, Kelly MS (2012) Culture, yield and bioremediation potential of Palmaria palmata (Linnaeus) Weber & Mohr and Saccharina latissima (Linnaeus) C.E. Lane, C. Mayes, Druehl & G.W. Saunders adjacent to fish farm cages in northwest Scotland. Aquaculture 354–355:128–135
Sanderson CJ (2006) Reducing the environmental impact of seacage fish farming through cultivation of seaweed. PhD thesis, The Open University, Milton Keynes, UK
Sanderson CJ (2015) Maximising production of Palmaria palmata (Linnaeus) Weber & Mohr, 1805. Final report to MASTS under visiting fellowship scheme 2015. Scottish Association for Marine Sciences (SAMS) & Kai Ho Tasmanian Sea Vegetables. https://masts.ac.uk/wp-content/uploads/attachments/hughes-sanderson-visiting-fellowship-report.pdf
Saunders GW, McDevit DC (2013) DNA barcoding unmasks overlooked diversity improving knowledge on the composition and origins of the Churchill algal flora. BMC Ecol 13:9
Saunders MI, Metaxas A, Filgueira R (2010) Implications of warming temperatures for population outbreaks of a nonindigenous species (Membranipora membranacea, Bryozoa) in rocky subtidal ecosystems. Limnol Oceanogr 55:1627–1642
Saunders GW, Jackson C, Salomaki ED (2018) Phylogenetic analyses of transcriptome data resolve familial assignments for genera of the red-algal Acrochaetiales-Palmariales Complex (Nemaliophycidae). Mol Phylogenet Evol 119:151–159
Schiener P, Zhao S, Theodoridou K, Carey M, Mooney-McAuley K, Greenwell C (2017) The nutritional aspects of biorefined Saccharina latissima, Ascophyllum nodosum and Palmaria palmata. Biomass Convers Biorefin 7:221–235
Schmedes PS, Nielsen MM (2020a) New hatchery methods for efficient spore use and seedling production of Palmaria palmata (dulse). J Appl Phycol 32:2183–2193
Schmedes PS, Nielsen MM (2020b) Productivity and growth rate in Palmaria palmata affected by salinity, irradiance, and nutrient availability—the use of nutrient pulses and interventional cultivation. J Appl Phycol 32:4099–4111
Schmedes PS, Nielsen MM, Petersen JK (2019) Improved Palmaria palmata hatchery methods for tetraspore release, even settlement and high seedling survival using strong water agitation and macerated propagules. Algal Res 40:101494
Schmedes P (2020) Investigating hatchery and cultivation methods for improved cultivation of Palmaria palmata. PhD thesis, Technical University of Denmark (DTU), Nykøbing Mors, Denmark
Schmid M, Guihéneuf F, Stengel DB (2017) Plasticity and remodelling of lipids support acclimation potential in two species of low-intertidal macroalgae, Fucus serratus (Phaeophyceae) and Palmaria palmata (Rhodophyta). Algal Res 26:104–114
Sears JR (1998) NEAS keys to the benthic marine algae of the northeastern coast of North America from Long Island sound to the strait of Belle Isle. Northeast Algal Society Dartmouth, MA, USA
Sekar S, Chandramohan M (2008) Phycobiliproteins as a commodity: trends in applied research, patents and commercialization. J Appl Phycol 20:113–136
Skriptsova AV, Suzuki M, Semenchenko AA (2022) Morphological variation in Northwest Pacific Devaleraea mollis and description of D. inkyuleei sp. nov. (Palmariaceae, Rhodophyta). Phycologia 61:606–615
Skriptsova AV, Kalita TL (2020) A re-evaluation of Palmaria (Palmariaceae, Rhodophyta) in the North-West Pacific. Eur J Phycol 55:266–274
State of Maine DMR (2003) Department of Marine Resources Regulations, Chapter 8: Landings Program. https://www.maine.gov/dmr/laws-regulations/regulations/index.html.
Stévant P, Ólafsdóttir A, Déléris P, Dumay J, Fleurence J, Ingadóttir B, Jónsdóttir R, Ragueneau É, Rebours C, Rustad T (2020a) Data on the sensory characteristics and chemical composition of the edible red seaweed dulse (Palmaria palmata) after dry and semi-dry storage. Data Brief 33:106343
Stévant P, Ólafsdóttir A, Déléris P, Dumay J, Fleurence J, Ingadóttir B, Jónsdóttir R, Ragueneau É, Rebours C, Rustad T (2020b) Semi-dry storage as a maturation process for improving the sensory characteristics of the edible red seaweed dulse (Palmaria palmata). Algal Res 51:102048
Stévant P (2019) Seaweeds in food applications: effects of processing on product quality. Ph.D thesis, Norwegian University of Science and Technology (NTNU), Trondheim, Norway
Suwal S, Perreault V, Marciniak A, Tamigneaux É, Deslandes É, Bazinet L, Jacques H, Beaulieu L, Doyen A (2019) Effects of high hydrostatic pressure and polysaccharidases on the extraction of antioxidant compounds from red macroalgae, Palmaria palmata and Solieria chordalis. J Food Eng 252:53–59
Titlyanov EA, Titlyanova TV (2006) Meristem clusters in cortical layer of thallus cells of the cultured red alga Palmaria palmata. Russ J Mar Biol 32:333
Titlyanov EA, Titlyanova TV, Kadel P, Lüning K (2006) New methods of obtaining plantlets and tetraspores from fragments and cell aggregates of meristematic and submeristematic tissue of the red alga Palmaria palmata. J Exp Mar Biol Ecol 339:55–64
Tittley I, Neto AI (2005) The marine algal (seaweed) flora of the Azores: additions and amendments. Bot Mar 48:248–255
Vadas RL, Johnson S, Norton TA (1992) Recruitment and mortality of early post-settlement stages of benthic algae. Br Phycol J 27:331–351
van den Hoek C, Mann D, Jahns H, Jahns M (1995) Algae: An introduction to phycology. Cambridge University Press, Cambridge
van der Meer JP (1987) Experimental hybridization of Palmaria palmata (Rhodophyta) from the northeast and northwest Atlantic Ocean. Can J Bot 65:1451–1458
van der Meer JP, Chen LC-M (1979) Evidence for sexual reproduction in the red algae Palmaria palmata and Halosaccion ramentaceum. Can J Bot 57:2452–2459
van der Meer JP, Todd ER (1980) The life history of Palmaria palmata in culture. A new type for the Rhodophyta. Can J Bot 58:1250–1256
Vinogradova KL (1999) De distribution algarum-macrophytorum in maribus Rossiae arcticis notula. Nov Sist Nizshikh Rast 33:14–24
Walsh M, Watson L (2011) A market analysis towards the further development of seaweed aquaculture in Ireland. Irish Sea Fish Board, Dublin, Ireland. https://www.seaweed.ie/irish_seaweed_contacts/doc/A%20Market%20Analysis%20towards%20the%20Further%20Development%20of%20Seaweed%20Aquaculture%20in%20Ireland.pdf
Wan AHL, Soler-Vila A, O’Keeffe D, Casburn P, Fitzgerald R, Johnson MP (2016) The inclusion of Palmaria palmata macroalgae in Atlantic salmon (Salmo salar) diets: effects on growth, haematology, immunity and liver function. J Appl Phycol 28:3091–3100
Wang T, Jónsdóttir R, Kristinsson HG, Hreggvidsson GO, Jónsson JÓ, Thorkelsson G, Ólafsdóttir G (2010) Enzyme-enhanced extraction of antioxidant ingredients from red algae Palmaria palmata. LWT 43:1387–1393
Werner A, Dring MJ (2011a) Cultivating Palmaria palmata. Aquaculture Explained, vol 27. BIM
Werner A, Dring MJ (2011b) Recommendations for optimal ongrowing and harvesting techniques for Palmaria palmata in different Irish sites with indications of yield. Aquaculture Explained. BIM. https://bim.ie/wp-content/uploads/2021/02/Palmaria,palmata,-,Recommendations,for,optimal,ongrowing,and,harvesting,techniques.pdf
Werner A, Dring MJ (2011c) Recommendations for optimal techniques for obtaining spores of Palmaria palmata, settling and maintaining them prior to outplanting at sea. Aquaculture Explained. BIM. https://bim.ie/wp-content/uploads/2021/02/Palmaria,palmata,-,Recommendations,for,optimal,techniques,for,obtaining,spores,of,Palmaria,palmata,,settling,and,maintaining,them,prior,to,outplanting,at,sea.pdf
WHO (2005) Vitamin and mineral requirements in human nutrition, 2nd edn. World Health Organization, Geneva
Yuan YV, Bone DE, Carrington MF (2005) Antioxidant activity of dulse (Palmaria palmata) extract evaluated in vitro. Food Chem 91:485–494
Yuan YV, Westcott ND, Hu C, Kitts DD (2009) Mycosporine-like amino acid composition of the edible red alga, Palmaria palmata (dulse) harvested from the west and east coasts of Grand Manan Island, New Brunswick. Food Chem 112:321–328
Zhao CJ, Schieber A, Gänzle MG (2016) Formation of taste-active amino acids, amino acid derivatives and peptides in food fermentations – A review. Food Res Int 89:39–47
Acknowledgements
The authors gratefully acknowledge the following contributors for sharing their knowledge on P. palmata and valuable data related to their respective fields of expertise: Jean-Baptiste Wallaert & Françoise Duchemin (Chambre Syndicale des Algues et Végétaux Marins, France), Helena Abreu (AlgaPlus, Portugal), Lucy Watson (Bord Iascaigh Mhara, Ireland), Stefan Kraan (The Seaweed Company, Ireland), Raul Ugarte (Acadian Seaplant, Canada), Tatiana Mikhaylova (Komarov Botanical Institute, Russia), Cecilie Bay Wirenfeldt (Technical University of Denmark), Annelise Chapman (Tango Seaweed AS, Norway) and Sanna Matsson & Bjørn Tore Nystrand (Møreforsking AS). The authors are also grateful to anonymous reviewers for their valuable comments and contribution to improving the manuscript.
Funding
This work was financed by the Møre & Romsdal County Council (grant number 2021–0164), the Research Council of Norway (SusKelpFood project, RCN grant number 326803) and Møreforsking AS.
Author information
Authors and Affiliations
Contributions
P.S. wrote the main manuscript text (all chapters), prepared Figs. 1, 2, 4, 6 and 9–15, and edited Figs. 3, 5, 7 and 8; P.S.S. contributed to chapters 4–9 and to Fig. 11, prepared Figs. 5, 7 and 8 and provided data to produce Figs. 6 and 12; L.L.G. contributed to chapters 1, 3 and 6; S.W. contributed to chapters 3–6; J.D. contributed to chapters 7 and 10; C.R. contributed to chapter 8; All authors reviewed the entire manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stévant, P., Schmedes, P.S., Le Gall, L. et al. Concise review of the red macroalga dulse, Palmaria palmata (L.) Weber & Mohr. J Appl Phycol 35, 523–550 (2023). https://doi.org/10.1007/s10811-022-02899-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-022-02899-5