Abstract
Megachile (Chalicodoma) parietina (Geoffroy, 1785) is a Palearctic solitary bee included in the Red List of some central European Countries. Females build durable nests, reused year after year, by mixing soil with a salivary secretion. Like for most solitary bees, the resources contained within M. parietina nests attract several other insects which exploit pollen supplies or feed on the immature brood. These associated insects have mainly been studied for mantained bees and considered for their effect on the host reproductive success.
A very large nesting aggregation of M. parietina in Central Tuscany has been studied for three consecutive years. We have identified 32 associated insect species, which certainly are an underestimate of the species present. Among the identified species, only eight had been previously reported for M. parietina. All the species were classified both according to the specificity for the host taxon (Chalicodoma, Megachilidae, Anthophila, Hymenoptera, Others) and to the ecological relationship (cleptoparasites, parasitoids, predators of larvae, food commensal, scavengers, and occasional nest users).
This highlighted both the richness of the ecological network within the nesting aggregation and the value of studying these nesting sites to fill Eltonian shortfalls, i.e. the deficiency in ecology knowledge, of bees and their associated fauna.
Implications for insect conservation.
We suggest that, besides their role in pollination, large and stable bee nesting sites increase the local insect biodiversity, and that attention should be paid to their conservation within actions aimed to support populations of wild pollinators.
Similar content being viewed by others
Introduction
Recent reviews point out the decline of insects worldwide, among which pollinators and in particular wild bees (Hymenoptera, Apoidea, Anthophila) are among the taxa undergoing a faster decline (Sánchez-Bayoa and Wyckhuys 2019; Zattara and Aizen 2021). For Europe, it has been estimated that 9% of the about 2,000 native and naturalized species are threatened with extinction and about 8% are declining at a population level (Nieto et al. 2014). However, as about 60% of the European species still require to be evaluated, due to data deficiency (Rasmont et al. 2017), the actual number of threatened species could be higher.
Since bees are considered the most efficient pollinator taxon, their decrease may affect wild flowering plants and raises concern because of their importance as crop pollinators (Allen-Wardell et al. 1998; Klein et al. 2007). Besides the trophic link with flowering plants, bees are also part of complex ecological networks with other insects, mainly because their immature brood and the food provisioned into the cells are very valuable nutrients exploited by other species (Wcislo and Cane 1996; Falk and Lewington 2018; Scheuchl and Willner 2016). Insects associated with bees are very diverse in their taxonomy and ecology and the literature about insects exploiting bee resources is often fragmentary. This is a clear example of Eltonian shortfalls, i.e. of the shortage of knowledge on species’ interactions that limits a full understanding of the ecological networks (Hortal et al. 2015). These insects often referred to as “natural enemies”, have been mainly investigated for their impact on bees maintained for their pollination service (Krunić et al. 2005), while their effect on populations of wild bees is unknown (Danforth et al. 2019). Besides negative effects, a diverse bee parasite fauna may act as a driver contributing to maintaining a diverse wild bee community, for example by reducing competition from the most abundant species (Hudson et al. 2006; Ashby and King 2017; Brown 2022).
Bee parasites are very diverse both in terms of taxonomy and ecology and the classification of their parasitic relationship with the hosts is far from being univocal. Parasites exploiting resources in bee nests are also found within the same Anthophila, where species of the so-called cuckoo bees are highly specialised in exploiting the nest resources of other bees (Michener 2007; Litman 2019). Cuckoo bees have independently evolved many times (Michener 2007) and are found in four out of the seven bee families (Danforth et al. 2019), representing about 13% of bee species worldwide, and about one-fourth in Europe (Danforth et al. 2019, Bogusch et al. 2006). Concerning host specificity about one-fourth of cuckoo-bees parasitizes one single species, while most of the others invade 2–5 species (Bogusch et al. 2006).
In addition to cuckoo bees, parasitoids developing on or inside bee larvae and/or pupae, predators of bee brood, and competitors for provisioned food are found among several hymenopteran taxa such as in the superfamilies Chalcidoidea, Evanioidea, Chrysidoidea, and in the family Mutillidae, but also among dipterans, e.g., within bee flies (Bombyliidae), satellite flies (Sarcophagidae, Miltogramminae), and Phoridae, in coleopterans (e.g., Meloidae and Cleridae), and strepsipterans. Within these taxa, bee-associated species differ in the ranges of hosts they can exploit (Pärn et al. 2015; Ronchetti and Polidori 2020). Some are generalists over a wide number of bee species, such as some Trichodes species (Coleoptera: Cleridae), whose larvae prey on bee larvae (Danforth et al. 2019). Generalists are also found among parasites laying their eggs in closed cells, as in the case of several Monodontomerus species (Chalcidoidea: Torymidae), which can parasitize bees belonging to several different genera, and, in some cases, also non-bee hosts (Grissell 2007). On the contrary, other species adopting this same parasitic strategy, such as some Leucospis species (Chalcidoidea: Leucospidae) are specialised over one single or a few hosts (Baur and Amiet 2000). Such a strict relationship is certainly due to specialisation in the biology of the parasites, which require synchronisation with their hosts, morphological and physiological adaptations, as well as a very specialised behaviour. Besides parasites that show a certain degree of specialisation, even in the case of generalists, many opportunistic insects and mites may take advantage of the resources contained within the nests of solitary bees (Falk and Lewington 2018; Scheuchl and Willner 2016). Finally, bee cells can be valuable shelters or nests for other insects, including other solitary bees, especially if they are built of durable materials.
In this study, we report the results of a three-year study of the insect fauna associated with a large nesting aggregation of Megachile parietina (Geoffroy, 1785) (previously known as Chalicodoma muraria (Olivier, 1789)). Although the biology of this species is quite well known and previous studies, including the seminal ones by Jean-Henry Fabre, have reported some insect species associated with it (File SF2), the specimens observed on the nest aggregation readily showed several additional species, whose ecological interaction with M. parietina was undescribed. This prompted us to extend our observation over a longer time.
Like in the other species belonging to the subgenus Chalicodoma, the nests of M. parietina built by mixing salivary secretions with clay, sand, and pebbles (Michener 2007; Fabre 1879, 1882), have a “stony” texture (Bonelli and Campadelli 1990) and are very durable. Since we observed that nests are used year after year, M. parietina is an interesting case to study the fauna associated with a species forming persistent aggregations and to acquire new information about the ecological interactions among wild bees and their associated entomofauna. These data contribute to outline a very complex network of ecological relationships centred on M. parietina and filling up knowledge about the insect fauna associated with this and with other solitary bees. Moreover, we describe some previously unreported life-history traits of M. parietina, which allow us to describe the ecology of this species which has been reported as endangered in some European countries.
Materials and methods
Study area
The studied nest aggregation is in a private estate in the countryside in the Council of Montespertoli, in Tuscany, Italy. Most nests are found on a horizontal 11-meter-long metal beam (with an H-shaped section) oriented along the NE-SW direction and supporting the roof of an open barn (43°38’50.9’’ N, 11°01’09.7’’ E); the beam is located 180 cm from the ground. Both faces of the beam are completely covered by nests (Fig. 1).
Several other separated nests are found in the barn and the remains of a second much larger aggregation were found on the walls of another shelter located at about 250 m, which some years earlier was sprayed with insecticides, according to the people living nearby. During spring 2017 the first aggregation was occupied by several dozen of M. parietina females, while we only observed few females on the second one; for this reason, specimen sampling was concentrated on the first nesting aggregation.
Females of this species, which are black and large, are regarded with a certain fear when numerous and close to human activities and attempts to get rid of them are probably not uncommon.
Fieldwork
M. parietina nest association
Observations of M. parietina in the nesting site were performed from April to the end of June in the years 2017, 2018 (at about 7-day intervals) and 2019 (at about 3–4-day intervals), from about 9:30 to 13:30, the time of the day when we observed that insects were most active.
During spring 2019, we individually marked, with a combination of different colours of nail polish, all the females caring for the cells located along a 3.4 m section of the beam; the marked females were then counted on the entire nesting site during the following days, until the end of the flight period. These data were used to estimate the number of females present.
Soil and pollen samples
To describe the building effort and the foraged plants, we collected samples of soil material and of pollen from females (respectively N = 10 and N = 9) returning to their cells after a building or a foraging flight (Fig. 2). Samples were analysed according to the methods reported in File SF1.
Specimen sampling
To describe the entomological fauna associated with the nest aggregation, specimen collections were performed from April to the end of June in the years 2017, 2018 (at about 7-day intervals) and 2019 (at about 3–4 day intervals). Observations and insect collection were performed mostly from about 9:30 to 13:30. Specimens found on the nest aggregation while inspecting nest cells, laying eggs, flying repeatedly around the cells or lurking close to the cells were collected using forceps or with a net. For the species present in high numbers (i.e., Anthrax anthrax Schrank 1781, Coelioxys aurolimbata Förster, 1853, Leucospis dorsigera Fabricius, 1775, L. gigas Fabricius, 1793, Monodontomerus aeneus (Fonscolombe, 1832), Spogostylum tripunctatum (Wiedemann, 1820), Stelis nasuta (Latreille, 1809)), only some among the present specimens were collected. Each insect was singly inserted in an Eppendorf or Falcon vial and kept at about 10 °C until brought to the lab and frozen. The number of specimens sampled for each species is reported in tab. ST1.
Nest sampling and insect rearing
To describe the nest structure, during spring 2017, 16 isolated nests, with already closed cells, were carefully collected. These nests were transferred to the laboratory and maintained at room temperature within transparent plastic boxes which were checked periodically for emerging insects. Finally, the nest structure was studied and described (File SF1).
Insect identification
An insect collection was prepared from the specimens captured in the field (about 200 specimens) and from those that emerged from the nests maintained in the laboratory (about 260 plus very numerous Melittobia acasta individuals). A few specimens of each species were pinned, for a total of 102 specimens, while others were kept at -20 °C for further studies, including DNA barcoding based on sequencing of the cytochrome c oxidase I (COXI) gene. The collection is housed at the Department of Biology, University of Florence.
Anthophila determination based on morphological characters was made by the authors and by Simone Flaminio. The other specimens were kindly identified by expert taxonomists, namely Mauro Gori for Lauxaniidae, Daniele Sommaggio for Bombyliidae, Michele Zilioli for Chrysididae, Luca Bartolozzi for Cleridae, Morgan Azzoni for Dermestidae, Mario Boni Bartalucci and Guido Pagliano for Leucospidae, Mario Boni Bartalucci for other Hymenoptera.
For most species, one leg of one or more individuals (or the entire individual in the case of very small specimens) was soaked in 0.1 ml of ethanol in a 95 dwell plate and sent to BOLD (http://www.boldsystems.org/) for DNA barcoding. Altogether 37 sequences of the fauna associated with M. parietina have been successfully generated for the project named “PARIE - Megachile parietina associated fauna”.
Ecological and taxonomic relationships
For each identified species literature was carefully searched to clarify the ecological relationship of the identified species with M. parietina and other bees. Associated species were classified into 6 ecological categories (Table 1, ST1, File SF2, Table ST2):
-
Parasitoids. In the first phases of their life, the species develop on or inside one single host individual, and this leads to the host’s death (Eggleton and Elshaw 1992). Both ecto- and endo-parasitoids are found among bee parasitoids (Danforth et al. 2019).
-
Cleptoparasites, i.e., species whose larvae feed on the pollen and nectar provisions of the host. When parasites develop on the food supply of one single host specimen and food consumption leads to the death of the host, such a biological relationship should be more properly named cleptoparasitoidism (Eggleton and Elshaw 1992), while Danforth and coworkers (2019), call such parasites nest depredators. Cleptoparasite species are quite common among different families of Anthophila, including the family Megachilidae, and are referred to as cuckoo bees (or brood-parasite bees). In most of these species, adults differ remarkably from nonparasitic species, for the absence of structures to collect pollen and to build nests (Danforth et al. 2019). Moreover, adult females of some brood-parasitic bees have an armoured exoskeleton entailed with ridges and spines as protection against the attack of host bees. Their larvae may have strong, sharp, and pointed mandibles in species that are also hospicidal. Because brood-parasitic bees generally have one or a few host species (Litman et al. 2013) they can be rare, and more prone to local extinction than non-parasitic species (Danforth et al. 2019). For this reason, parasite bees have been suggested to be good indicators of the entire bee community (Sheffield et al. 2013). Three cuckoo bee species, Stelis nasuta (Latreille, 1809) and Coelioxys aurolimbata Förster, 1853 and Dioxys cincta (Jurine, 1807) are known for M. parietina (Comba 2015).
-
Predators consume adult and immature bees whose death is more or less instantaneous (Danforth et al. 2019). Moreover, predators, contrary to parasitoids, may consume more than one prey (Eggleton and Elshaw 1992). Predation is not necessarily limited to the early stages of the prey. However, since we only sampled specimens on M. parietina nests, while we did not consider the bees while foraging on flowers, we missed all those species capturing adults while away from the nests, such as crab spiders (Tomisidae), hornets (Vespidae) or robber flies (Asilidae).
-
Commensal, i.e., species living, at least occasionally, within the nest whose use of host resources has a limited effect on host fitness (Danforth et al. 2019).
-
Scavengers are species that consume the remains of immature hosts after they have developed into adults (Danforth et al. 2019).
-
Occasional nest users, i.e., species using nests of other insects. Among bees, the use of other species’ nests could also correspond to interspecific usurpation (facultative interspecific parasitism), but limited observations have been reported in this regard (Danforth et al. 2019).
Moreover, each species was also classified according to the specificity to one of the following host taxa: subgenus Chalicodoma, Megachilidae, Anthophila, Hymenoptera, other taxa.
Ecological network
A network of the ecological relationships between the species identified during the present study was constructed using Cytoscape (Shannon et al. 2003). The network drawing represents: the ecological category; the number of other species with which each species interacts (either as an active partner, e.g. as a predator or as a passive partner, e.g. as a prey); the frequency of observations; the kind of knowledge about the interaction between any couple of species, i.e. observed in the study, described in the literature, or supposed from the literature but never specifically described. The network nodes have been first spatially clustered according to ecological category and then ordered to maximise clarity of visualisation.
A further network was constructed by removing the focal species M. parietina and the insects having M. parietina or the Chalicodoma subgenus as the only or main host.
Results
M. parietina nesting association
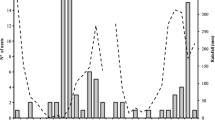
Females and males emerge in the second half of April, and the first females caring for the nests are observed about a week later when males are no more present (Fig. 3). Only a few females are present at the end of June. Remarkably, each year the flight season corresponded to the flowering period of Sulla (Italian sainfoin Hedysarum coronarium L.), a Mediterranean fodder plant grown on clayey soils, which was present next to the nests. The Sulla turned out to be the main plant visited by M. parietina (Fig. 2C) which contributed in a relevant manner to its fruit set (file SF1, fig. S4 therein). Most plants were withered in the last week of June when only a few M. parietina were still active on their nests (Fig. 3).
Number of Megachile parietina females active on a limited surface (80 dm2) of the nesting site during Spring 2019 (see text) and of the main parasites sampled in front of the nests. For the more abundant parasites (see text) only a few specimens were sampled; therefore, the actual number of these insects was much higher than shown. Single-headed solid arrows show the dates when some species were observed to emerge from M. parietina nests, the dotted arrow indicates the date when most Sulla plants were withered; the double-headed arrow indicates the time when most cells had been already closed and M. parietina females build an external layer over the cells
During spring 2019, a total of 193 M. parietina females were individually marked on an 0.80 m2 surface of the beam. Since the cells were evenly distributed on the surface, we could estimate that about 660 adult females were active on the barn nests (2.70 m2) over the flight period of the species. All females were observed to re-use old cells and only a few empty cells were present at the end of the flight season. Once cells were sealed, new ones were built on top or close to the old ones. Distances between cells used by different females ranged from 1 to 5 cm. In the same year, no new isolated nests were observed, although a few newly funded isolated nests were seen in the previous two years.
The highest number of females was found at the beginning of May with a density over the nesting surface of 1.6 females/dm2. The number of females decreased quite rapidly during the flight season; we assumed that a large fraction of females either dispersed or died. In fact, we observed females on their cells for a period ranging from 2 to 52 days (Fig. 3). Most cleptoparasites and parasitoids were present at the end of May and in June when they were more numerous than the host.
During the second half of June, most M. parietina females were observed to lay a thick layer of building material to cover the already closed cells.
Nest structure
The structure of single nests is described in file SF1. By considering the weight of the soil brought during each building flight, we estimated that the construction of a single nest, with the possible contribution of females over more years, may require about 3.000 flights.
Identification of associated insect species
32 different species were identified among the specimens collected on the nesting site and those that emerged from the nests brought to the laboratory (460 specimens in total, plus very numerous Melittobia acasta individuals). The list of the species, the specificity to the host taxa, and their classification according to the biological categories, as discussed above, are reported in Table 1, while table ST1 and file SF2 also report details about their identification and biology. All the identifications based on morphological traits were confirmed through the comparison of COXI, with a few exceptions.
Ecological and taxonomic relationships
Although some parasites and possible nest users were already known for M. parietina (Table ST1), more than two-thirds of the identified species (24 out of 32) have never been reported before. This can be due to the large nest aggregation which attracts other insects that build their nests on dry vertical surfaces and in turn attract their parasites, further increasing the number of present species. However, some species reported by previous authors were not found in the present study (Table ST1).
Briefly, based on literature information, the specificity to the host taxa could not be assigned for 14 species (Table 1), and among these, eight, mainly other megachilid bees, were considered occasional nest users. Although interspecific nest usurpation among bee species with overlapping flight periods is also possible, we never observed a direct interaction between females of these species and M. parietina and, to the best of our knowledge, such observations have never been reported. Two species can be considered emblematic of the shortage of ecological information, the bombyliid species Spogostylum tripunctatum (Wiedemann, 1820), which to the best of our knowledge has only been reported for M. parietina (Bonelli and Campadelli 1990), and the lauxaniid fly Minettia tabidiventris (Papp, 1877) whose biology is unknown and that we classified as a scavenger. Out of the six species classified as predators of larvae and food commensals, only one had been previously reported for our focal bee species. Respectively seven and six species were classified as parasitoids and cleptoparasites, whose hosts are mainly megachilid bees.
Figure 4.a shows the ecological relationships between the species identified in the present study, as resulting from our observations and the available literature. Black and grey arrows, indicating respectively relationships we have reported for the first time and those already known, show the richness and complex ecological network supported by M. parietina. Such richness is considerably increased by the six no parasitic megachilid species using M. parietina nests. Interestingly these bees, together with other species classified as occasional nest users (Table 1 and ST2), are in the same number as cleptoparasites and parasitoids and this supports our hypothesis that even the “empty nests” are indeed a very valuable resource. According to the literature information, most of the species using organic resources (pollen, immature brood, and insect remains), may interact with more species of the network (e.g., Coelioxys aurolimbata, that may find two different hosts species in the nesting site), increasing connectance and also the possibility of tri-trophic interactions (e.g., Leucospis dorsigera, that may parasitise bees classified as occasional nest users).
Ecological network within the studied M. parietina nesting association (A); the ecological network after removing M. parietina and the species having it as the specific or main host (B). The node shape represents the ecological category; the node size, the number of species with which each species interacts; the node greyscale, the frequency of the observation; the greyscale of the arrow, the state of art about the relationship (see text)
The importance of the bare nests for the ecological network is shown in Fig. 4.b where, after removing M. parietina and species having it or the Chalicodoma subgenus as hosts, 85.2% of the ecological relationships were maintained.
Discussion
In the present work, we report and describe the insect fauna associated with a large Megachile parietina nesting aggregation and we describe some aspects of the foraging biology and the nest structure and building behaviour of this species. The studied nest aggregation had been first noticed in 2017 but given the number of adult females (approximately 660 females in spring 2019), the beam has likely been successfully colonised for several years, thanks also to the abundance of Sulla (Hedysarum coronarium L.), which we found to be the favoured plant for pollen foraging, and the abundance of loose soil, used as nesting material (File SF1).
In addition to M. parietina, 32 other insect species were present; most of them were identified both based on the morphology and through barcoding based on the COXI gene sequence. To the best of our knowledge, this is the first barcoding project where a solitary bee and its associated fauna have been studied. The reported number of species is certainly lower than those present, as it is also proved from organic remains found in the collected nests for which the species could not be identified. M. parietina nests are very hard and durable and are therefore a valuable and costly resource that is reused year after year not only by this same species but also by several additional bees and other insects. Based on the available information on their biology, including diet, hosts, and environment, only four species were classified as occasional visitors, while for all the others a biological relation could be proposed. Several species, among which six other megachilid bees, have been found and were considered occasional nest users. Their presence likely increases the local entomological biodiversity since we also identified cleptoparasites and parasitoids associated with these bees.
Interestingly, only eight of the identified species have been previously reported for M. parietina. This shows that also for a quite common and well-known bee species, there are shortfalls (Hortal et al. 2015) in the ecological relationships with the “natural enemies” and, more generally, with the associated entomofauna. The taxonomic diversity and the several different ecological roles we observed highlighted the complexity and, for some species, the redundancy of the relationships within the nesting association (Fig. 4a).
Based on these results, long-lasting associations of bee nests appear as point-like hotspots of local insect biodiversity and seem to offer very favourable conditions to increase knowledge on pollinator ecology and their associated fauna.
Large and stable nesting aggregations of solitary bees have been reported for many species, mainly ground-nesting, belonging to the families Andrenidae, Apidae, Halictidae and Melittidae (Danforth et al. 2019). The conditions favouring such large aggregations on a limited space are probably the coexisting presence of a suitable soil texture and feeding plants supporting a large number of larvae. Compared to most bee species nesting in aggregations, M. parietina is quite unique in that it can be considered an ecosystem engineer since its persistent stony-like nests can modify the very local environment and create new microhabitats suitable for other insect species. Similarly to what we have observed, large nesting sites of other bees are expected to attract a rich entomofauna. Therefore, investigating them could both reveal unreported associations between species and help to understand the role of solitary bees within ecological networks, besides their role as pollinators. Moreover, sampling from bee nests or cells is a straightforward way to fill up Eltonian shortfalls for bees and associated insects in that information could also be gained from DNA barcoding or metabarcoding of the organic remains, of both animal and vegetal origin.
So far, bee “natural enemies” have been mainly investigated either to describe the biology of some target species or to investigate the impact of parasites on bees maintained for their pollination services (Krunić et al. 2005), while there has been limited interest in the diversity of these associated faunas, that as it happens in general for parasites, is far from being considered charismatic (Carlson et al. 2020; Rubio-Godoy and Pérez-Ponce de León 2023). This attitude reflects a lack of consideration for parasites in conservation biology (Carlson et al. 2020).
However, a species-specific relationship can have a dramatic effect on the parasites in the case of host decline and eventually lead to co-extinction. Although according to the IUCN, M. parietina is classified in the Least Concern (LC) of extinction risk category (Dewulf and Praz 2014), this species is included in the National Red List (or Red Data Books) of Switzerland (VU – Vulnerable; Amiet 1994), Czech Republic (EN – In Danger; Farkac et al. 2005), Norway (RE – extinct in the region, Kålås et al. 2010) and Germany (CR – Critically endangered; Westrich et al. 2011), and a remarkable decline has been documented in France, due to the reduction of Fabaceae cultivation (Müller et al. 2006; Rasmont et al. 2003; Patiny et al. 2009; Vereecken et al. 2010). Therefore, in these countries, also the most strictly associated insect parasites (Stelis nasuta, Coelioxys aurolimbata, Dioxys cincta, Leucospis gigas, Meloe erythrocnemus, Zonitis nana) are expected to follow a similar trend.
Many programs and initiatives aimed to support local populations of wild bees and favour the awareness of their ecological importance have been recently undertaken in several countries. We propose that, within these programs, more attention should be paid to the protection and conservation of bee nesting sites, in particular for gregarious species, given their high biodiversity potential. Knowing the fauna associated with bee nest aggregations could also help to understand how wild bees contribute to local biodiversity.
So, we suggest this as a holistic approach to be considered both in the investigation of bee biology as well as in management and maintenance actions for pollinator communities.
Data accessibility
Barcoding data are accessible on Boldsystem.org, at the project “PARIE - Megachile parietina associated fauna”.
References
Allen-Wardell G et al (1998) The potential consequences of Pollinator declines on the conservation of Biodiversity and Stability of Food Crop yields. Conserv Biol 12:8–17. https://doi.org/10.1046/j.1523-1739.1998.97154.x
Amiet F (1994) Liste rouge des abeilles menacées de Suisse. Listes rouges des espèces animales menacées de Suisse. In: Duelli P (ed) Office fédéral de l’environnement, des forêts et du paysage. Berne, pp 38–44
Ashby B, King KC (2017) Friendly foes: the evolution of host protection by a parasite. Evol Lett 1:211–221. https://doi.org/10.1002/evl3.19
Baur H, Amiet F (2000) Die Leucospidae (Hymenoptera: Chalcidoidea) der Schweiz, mit einem Bestimmungsschliissel und Daten zu den europaischen arten. Rev Suisse Zool 107:359–388. https://doi.org/10.5962/bhl.part.80135
Bogusch P, Kratochvíl L, Straka J (2006) Generalist cuckoo bees (Hymenoptera: Apoidea: Sphecodes) are species-specialist at the individual level. Behav Ecol Sociobiol 60:422–429. https://doi.org/10.1007/s00265-006-0182-4
Bonelli B, Campadelli G (1990) Note biologiche su Chalicodoma parietina Geoffr. (Hymenoptera Megachilidae). Boll Ist Ent < < G. Grandi > > Univ Bologna 44:1–9
Brown M (2022) Complex networks of parasites and pollinators: moving towards a healthy balance. Proc Royal Soc B. https://doi.org/10.1098/rstb.2021.0161
Carlson CJ, Hopkins S, Bell KC, Doña J, Godfrey SS, Kwak ML, Lafferty KD, Moir ML, Speer KA, Strona G, Torchin M, Wood CL (2020) A global parasite conservation plan. Biol Conserv 250:108596. https://doi.org/10.1016/j.biocon.2020.108596
Comba M (2015) Hymenoptera: Apoidea: Anthophila of Italy, URL: http://digilander.libero.it/Mario.comba/ [accessed 15 July, 2015]
Danforth B (2019) In: Minckley R, Neff J (eds) The Solitary Bees. Biology, Evolution, Conservation, first edn. Princeton University Press, Princeton, New Jersey
Dewulf A, Praz C (2014) Megachile parietina. The IUCN Red List of Threatened Species 2014 eT19199035A50141970. https://doi.org/10.2305/IUCN.UK.2014-3.RLTS.T19199035A50141970.en. Downloaded on 16 August 2021
Eggleton P, Elshaw R (1992) Insect parasitoids: an evolutionary overview. Philos Trans R Soc B 337:1–20. https://doi.org/10.1098/rstb.1992.0079
Fabre J-H (1879) Les Chalicodomes. Souvenirs entomologiques- Serie I, Paris
Fabre J-H (1882) Nouvelles Recherches Sur Les Chalicodomes. Souvenirs entomologiques - Serie II, Paris
Falk S, Lewington R (2018) Field Guide to the bees of Great Britain and Ireland. British Wild Fields Guides. Bloomsburry Publishing, London
Farkac J, Král D, Škorpík M (2005) List of threatened species in Czech Republic. Invertebrates. URL http://www.nationalredlist.org/site.aspx?&species=18217&pageid=116
Grissell EE (2007) Torymidae (Hymenoptera: Chalcidoidea) associated with bees (Apoidea), with a list of chalcidoid bee parasitoids. J Hymenopt Res 16:234–265
Hortal J, de Bello F, Diniz-Filho JAF, Lewinsohn TM, Lobo JM, Ladle RJ (2015) Seven shortfalls that Beset large-scale knowledge of Biodiversity. Annu Rev Ecol Evol Syst 46:523–549. https://doi.org/10.1146/annurev-ecolsys-112414-054400
Hudson PJ, Dobson AP, Lafferty KD (2006) Is a healthy ecosystem one that is rich in parasites? Trends Ecol Evol 21:381–385. https://doi.org/10.1016/j.tree.2006.04.007
Kålås JA, Viken Ã, Henriksen S, Skjelseth S (2010) The 2010 norwegian red list for species. Norwegian Biodiversity Information Centre, Trondheim
Klein AM, Vaissière B, Cane JH, SteVan-Dewenter I, Cunningham SA, Kremen C, Tscharntke T (2007) Importance of crop pollinators in changing landscapes for world crops. Proc Royal Soc B 274:303–313. https://doi.org/10.1098/rspb.2006.3721
Krunić M, Stanisavljević L, Pinzauti M, Felicioli A (2005) The accompanying fauna of Osmia cornuta and Osmia rufa and effective measures of protection. Bull Insectology 58:141–152. http://www.bulletinofinsectology.org/pdfarticles/vol58-2005-141-152krunic.pdf
Litman JR (2019) Under the radar: detection avoidance in brood parasitic bees. Philos Trans R Soc B 374:20180196. https://doi.org/10.1098/rstb.2018.0196
Litman JR, Praz CJ, Danforth BN, Griswold TL, Cardinal S (2013) Origins, evolution, and diversification of cleptoparasitic lineages in long-tongued bees. Evolution 67:2982–2998. https://doi.org/10.1111/evo.12161
Michener CD (2007) The bees of the world. Johns Hopkins University Press, Baltimore, MD
Müller A, Diener S, Schnyder S, Stutz K, Sedivy C, Dorn S (2006) Quantitative pollen requirements of solitary bees: implications for bee conservation and the evolution of bee-flower relationships. Biol Conserv 130:604–615. https://doi.org/10.1016/j.biocon.2006.01.023
Nieto A et al (2014) European red list of bees. Publication Office of the European Union, Luxembourg. https://doi.org/10.2779/77003
Pärn M, Soon V, Vallisoo T, Hovi K, Luig J (2015) Host specificity of the tribe Chrysidini (Hymenoptera: Chrysididae) in Estonia ascertained with trap-nesting. Eur J Entomol 112:91–99. https://doi.org/10.14411/eje.2015.012
Patiny S, Rasmont P, Michez D (2009) A survey and review of the status of the wild bees in the West- Palaeartic region. Apidologie 40:313–331. https://doi.org/10.1051/apido/2009028
Rasmont P, Barbier Y, Iserbyt S, Wahis R, Terzo M (2003) Jean-Henry Fabre pourrait-il observer aujourd’hui tous ces insect? in: Jean-Henri Fabre, un autre regard sur l’insecte. Actes Colloq Int sur l’entomologie 18–19 octobre 2002, Saint Léons en Lévézou (France, Aveyron), Conseil general de l’Aveyron, Rodez: 209–220
Rasmont P, Devalez J, Pauly A, Michez D, Radchenko VG (2017) Ajouts à la liste de l’UICN des Abeilles sauvages d’Europe (Hymenoptera: Apoidea). Ann Soc Entomol Fr 53:17–32. https://doi.org/10.1080/00379271.2017.1307696
Ronchetti F, Polidori C (2020) A sting affair: a global quantitative exploration of bee, wasp and ant hosts of velvet ants. PLoS ONE 15:e0238888. https://doi.org/10.1371/journal.pone.0238888
Rubio-Godoy M, Pérez-Ponce de León G (2023) Equal rights for parasites: Windsor 1995, revisited after ecological parasitology has come of age. Biol Conserv 284:110174. https://doi.org/10.1016/j.biocon.2023.110174
Sánchez-Bayoa F, Wyckhuys KAG (2019) Worldwide decline of the entomofauna: a review of its drivers. Biol Conserv 232:8–27. https://doi.org/10.1016/j.biocon.2019.01.020
Scheuchl E, Willner W (2016) Taschenlexikon der Wildbienen Mitteleuropas: Alle Arten im Porträt. Quelle e Meyer ed, Wiebelsheim
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T (2003) Cytoscape: a oftware environment for integrated models of biomolecular interaction networks. Genome Res 13:2498–2504. https://doi.org/10.1101/gr.1239303
Sheffield C, Pindar A, Packer L, Kevan P (2013) The potential of cleptoparasitic bees as indicator taxa for assessing bee communities. Apidologie 44:501–510. https://doi.org/10.1007/s13592-013-0200-2
Vereecken NJ, Niolu P, Dufrêne E, Le Goff G (2010) Observations sur les nids de deux chalicodomes et leurs occupants en Sardaigne (Italie). Osmia 4:15–19. https://doi.org/10.47446/OSMIA4.4
Wcislo WT, Cane JH (1996) Floral resource utilization by solitary bees (Hymenoptera: Apoidea) and exploitation of their stored foods by natural enemies. Ann Rev Entomol 41:257–286. https://doi.org/10.1146/annurev.en.41.010196.001353
Westrich P, Frommer U, Mandery K, Riemann H, Ruhnke H, Saure C, Voith J (2011) Rote Liste und Gesamtartenliste der Bienen (Hymenoptera, Apidae) Deutschlands – (5. Fassung, Dezember 2011) [Red List and complete species list of bees in Germany]. In: Bundesamt für Naturschutz (ed.), Rote Liste der gefährdeter Tiere, Pflanzen und Pilze Deutschlands. Band 3: Wirbellose Tiere (Teil 1) [Red List of threatened animals, plants and fungi of Germany], pp. 371–416, Bonn
Zattara EE, Aizen MA (2021) Worldwide occurrence records suggest a global decline in bee species richness. One Earth 4:114–123. https://doi.org/10.1016/j.oneear.2020.12.005
Acknowledgements
We thank Mrs. Francesca Balducci for having reported the presence of M. parietina and Mr. Francesco Balducci to allow us to access his property. We thank Morgan Azzoni, Luca Bartolozzi, Mario Boni Bartalucci, Mauro Gori, Simone Flaminio, Guido Pagliano, Daniele Sommaggio, Michele Zilioli, for specimen determination and Oana Catalina Moldoveanu and Martino Maggioni for their help in the field. FRD acknowledges the support of NBFC to the University of Florence, funded by the Italian Ministry of University and Research, PNRR, Missione 4 Componente 2, “Dalla ricerca all’impresa”, Investimento 1.4, Project CN00000033.
Funding
Open access funding provided by Università degli Studi di Firenze within the CRUI-CARE Agreement. Funding for this study has been received by FRD from Florence University.
Open access funding provided by Università degli Studi di Firenze within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
Elisa Monterastelli, Marino Quaranta and Francesca Romana Dani contributed to the study conception and design. Material and data collection were performed by Elisa Monterastelli, Alfonso Orlotti, Giulia Calderai, Marino Quaranta and Francesca R. Dani. Material and data analyses were performed by all authors. The first draft of the manuscript was written by Francesca R. Dani and Elisa Monterastelli; all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10841_2023_519_MOESM1_ESM.pdf
Suplementary File 1: File SF1. Analysis of the nest structure, and pollen loads from M. parietina abdominal brushes and contribution of M. parietina to fruit development of Sulla (Hedysarum coronarium L.)
10841_2023_519_MOESM2_ESM.pdf
Suplementary File 2: File SF2. Identification and description of the identified species according to ecological and taxonomic relationships
10841_2023_519_MOESM5_ESM.mp4
Suplementary File 5: Video SV1. Females of Anthrax anthrax (Diptera: Bombyliidae) releasing eggs while hovering in front of the opened cells of Megachile parietina nests
10841_2023_519_MOESM7_ESM.mp4
Suplementary File 7: Video SV3. A Female of Leucospis dorsigera laying eggs in cells within the Megachile parietina nesting site
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Monterastelli, E., Orlotti, A., Calderai, G. et al. What’s in the bee nest holes? A single aggregation of Megachile parietina reveals and helps to fill up Eltonian shortfalls. J Insect Conserv 28, 15–25 (2024). https://doi.org/10.1007/s10841-023-00519-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-023-00519-2