Abstract
The phylogeny of the Lecanora varia group is inferred from ITS sequences using Bayesian and ML phylogenetic analysis methods. According to our data, usnic acid-containing, corticolous Lecanora species do not form a monophyletic group but occur in three major clades together with other groups of Lecanora and Protoparmeliopsis. The new combination Lecanora filamentosa (Stirt.) Elix & Palice is proposed. The new species Lecanora schizochromatica is described from northwestern North America. It produces atranorin as a major substance and is closely related to the morphologically and chemically similar L. filamentosa. The American Biatora pullula Tuck. is synonymised with Lecanora anopta Nyl., which is hereby reported for continental North America for the first time. The phylogenetic relationships between the major clades of Lecanora are still largely unresolved and require more intensive taxon and character sampling.
Similar content being viewed by others
Introduction
Lecanora Ach. is a large, crustose lichen genus traditionally characterised by colourless, non-septate ascospores in eight-spored asci, green-algal photobionts and a lecanorine margin. Since the middle of the twentieth century, Lecanora has received considerable attention from systematists (e.g. Brodo 1984; Brodo et al. 1994; Dickhäuser et al. 1995; Eigler 1969; Eigler and Poelt 1965; Fröberg 1997; Grube et al. 2004; Guderley 1999; Laundon 2003a, 2003b; Leuckert and Poelt 1989; Lumbsch 1994; Lumbsch and Elix 2004; Lumbsch et al. 1997; Miyawaki 1988; Motyka 1995, 1996a, b; Poelt 1952, 1958, 1966; Poelt et al. 1995; Printzen 2001; Ryan 1989a, b, 1998; Ryan and Nash 1997; Ryan et al. 2004; Śliwa and Wetmore 2000; Śliwa 2007; van den Boom and Brand 2008) and is probably one of the best studied crustose lichen genera. Lecanora, in its traditional circumscription (Zahlbruckner 1926), has been recognized to be an artificial assemblage of species, and several genera have been split off over the years (Choisy 1929, 1949; Hafellner and Türk 2001; Kalb 1991; Motyka 1995, 1996a; Poelt 1983). Although the notion has prevailed that the remainder of Lecanora is still heterogeneous, few authors have attempted to split the rest of the genus into more natural units. Zahlbruckner (1926) distinguished several sections within Lecanora, and Poelt (1958) published an elaborate infrageneric classification of the lobate species. Eigler (1969) accepted deviating groups, such as Aspicilia A. Massal., Haematomma A. Massal., and Harpidium Körb., which had originally been described in the nineteenth century as separate genera, and split most of these, including Lecanora s.str. into “groups”. The last comprehensive attempt at a subgeneric classification of Lecanora was published by Motyka (1995, 1996a, b) who split several new or resurrected genera from Lecanora and divided the remainder of the genus into 14 sections.
Thallus morphology and secondary metabolites have traditionally been the most important characters for the separation of groups within Lecanora. However, based on chemical data, Brodo and Elix (1993) argued against a formal taxonomic recognition of species groups within Lecanora. Although the phylogenetic relationships among species groups are still largely unresolved, recent molecular studies indeed largely confirmed the notion that some are heterogeneous. Arup and Grube (1998) found the lobate subgenus Placodium paraphyletic and intermingled with species of the L. varia (Hoffm.) Ach., L. dispersa (Pers.) Sommerf. and L. polytropa (Hoffm.) Rabenh. groups and the genus Arctopeltis Poelt. The same authors demonstrated that Rhizoplaca Zopf is not monophyletic (Arup and Grube 2000) and concluded that thallus morphology does not reflect the phylogenetic relationships within the genus.
One of the species groups that has been treated by several authors in recent years is the ‘Lecanora varia group’. Choisy (1929) assigned L. varia to a new genus Straminella M. Choisy because of its yellow thallus colour (caused by usnic acid). Based mainly on pycnospore characters, Choisy (1949) assigned the members of the Lecanora saligna group to yet another new genus, Lecanoropsis, a name which was almost never subsequently used (except by, e.g., Weber and Wittmann 1992). Eigler (1969) accepted Straminella and assigned a number of other species with usnic acid, such as L. conizaeoides Nyl. ex Crombie, L. expallens Ach., L. polytropa (Hoffm.) Rabenh., L. saligna (Schrad.) Zahlbr., L. subintricata (Nyl.) Th. Fr. and L. symmicta (Ach.) Ach., to the genus, while Motyka (1996a, 1996b) distributed these species over several genera and sections. Recently, this “Lecanora varia group” has been studied by several authors. Śliwa and Wetmore (2000) and Printzen (2001) treated all or a subset of the North American species, Laundon (2003a) discussed a set of 13 species and finally Martínez and Aragón (2004) revised the species occurring in the Iberian Peninsula. In the first two works, new, slightly deviating subgroups were proposed for the species putatively related to L. varia. Printzen (2001) doubted that the group is monophyletic and instead proposed four species groups mainly based on amphithecial characters: (1) Lecanora brucei (as L. spec. 1) and L. symmicta with a biatorine exciple, (2) L. americana (de Lesd.) Printzen, L. confusa Almb., L. perconfusa Printzen, L. strobilina (Spreng.) Kieffer and L. substrobilina with an ecorticate, lecanorine amphithecium, (3) L. albellula Nyl., L. latens Printzen, L. mughicola Nyl., L. polytropa, L. saligna and L. subintricata with an amphithecial cortex of more or less equal thickness, and (4) L. coniferarum Printzen, L. densa (Śliwa & Wetmore) Printzen, L. laxa (Śliwa & Wetmore) Printzen and L. varia with a basally thickened amphithecial cortex. The results of Arup and Grube (1998) already indicated that L. symmicta, L. conizaeoides and L. varia do not belong in the same clade within Lecanora but their study focused on lobate species of Lecanora and did not include more species of the group. In this study, we present a phylogenetic analysis of an extended sample of taxa from the “L. varia group” based on ITS sequences. Our main objective was to see whether L. burgaziae I. Martínez & Aragón, L. densa, L. laxa and L. varia are closely related species and form a monophyletic group. For this purpose, we have extended the available sequence data for Lecanora species with a focus on usnic acid-containing species. In order not to bias our taxon sampling towards the well-sampled groups within Lecanora, we left out some of the species that were included in the analyses of Arup and Grube (1998, 2000) and Grube et al. (2004). Our second intention was to see whether molecular data supported the species groups suggested by Printzen (2001). This objective was, however, somewhat impeded because we were unable to obtain fresh material or readable sequences from many of the relevant species. In the course of our work, two more species of the group from western North America came to our attention. One of them, L. anopta, had not been reported from continental North America, and a second, L. schizochromatica, is here formally described as new to science. The phylogenetic positions of both taxa were also analysed.
Materials and methods
Morphology and anatomy
Characteristics of the apothecia and thallus were investigated by light microscopy on hand-cut sections mounted in water and 10% KOH, and stained with I Lugol (Merck 1.09261) or lactophenol cotton blue (LCB; Merck 1.13741). Photos were taken with a Zeiss Axioskop digital camera. Microscopic measurements were made at ×1,000 magnification in water and are given as (smallest single measurement)–smallest mean–largest mean–(largest single measurement). The small and large means are based on ≥10 measurements on individual specimens. Nomenclature of non-soluble pigments follows Meyer and Printzen (2000). Thin layer chromatography was carried out according to Culberson (1972) and Culberson and Johnson (1982), and high performance liquid chromatography according to Elix et al. (2003).
Molecular analysis: DNA-isolation, PCR-amplification and sequencing
Specimens used for DNA extraction and sequencing are kept in FR (Herbarium Senckenbergianum), unless otherwise noted. DNA extraction was extracted from 1 or 2 apothecia per specimen. Apothecia were cut from the thallus with the help of a razor blade, moistened in a sterile water drop on a microscope slide and examined under the dissecting microscope in order to remove all possible contaminations such as detritus or epiphytic fungal hyphae. Samples were placed in 1.5-ml microcentrifuge tubes and DNA was extracted using either DNEasy Plant Mini Kit® (Qiagen) or NucleoSpin® Plant II (Macherey-Nagel) following the manufacturer’s protocols with minor modifications. Sequences of the nuclear ribosomal ITS region were obtained using the primers ITS1F (Gardes and Bruns 1993) and ITS4 (White et al. 1990). PCR reactions were prepared for a final volume of 25 μl, containing 2 μl of each primer, 2 μl of dNTPs (10 μM), 2.5 μl of Herculase® reaction buffer, 1.25 U of Herculase® enhanced polymerase (Stratagene), and 14.25 μl of distilled water.
PCR amplifications were set up under the following conditions: one initial denaturing step at 94°C for 4 min followed by 6 touchdown cycles of 1 min at 94°C, 1 min at 62°C and 1 min 45 s at 72°C, decreasing the annealing temperature 1°C each cycle; 34 cycles of 30 s at 94°C, 30 s at 56°C and 1 min 45 s at 72°C; ending with an extension step of 10 min at 72°C, after which the samples were kept at 4°C. PCR products were extracted from the agarose gel and purified using either peqGOLD MicroSpin Gel Extraction Kit (Peqlab, Biotechnologie) or QIAquick® PCR purification Kit (QIAGEN 28106) following the manufacturer’s instructions. PCR products were labelled using the Quick Start Kit (Beckman Coulter) and run on a CEQ™ 8800 Genetic Analysis System (Beckman Coulter).
Sequence alignment and phylogenetic analysis
Sequence fragments obtained were checked, assembled and manually adjusted in SeqManII v.5.07© (DNASTAR). A total of 29 newly obtained sequences were aligned together with 24 sequences retrieved from GenBank (Table 1). Sequence alignment was performed with Muscle v3.6 (Edgar 2004). Ambiguously aligned regions were removed from the alignment using Gblocks 0.91b (Castresana 2000), in which all parameters were set to fit minimal restrictions, yielding a final alignment of 441 bp. The species of the L. subfusca group were chosen as outgroup based on previous phylogenetic studies (Arup and Grube 1998, 2000).
Phylogenetic analyses were carried out using Metropolis coupled Bayesian Markov Chain Monte Carlo (MC)3 and Maximum Likelihood approaches, implemented in the software MrBayes v.3.1.2 (Huelsenbeck and Ronquist 2003) and PHYML v 2.4.4 (Guindon and Gascuel 2003), respectively. Separate nucleotide substitution models for each part of the nrITS region (ITS1, 5.8S, ITS2) were selected with the help of MrModelTest v2 (Nylander 2004) using the AIC as optimality criterion. The following substitution models were selected: GTR+Γ for ITS1 and ITS2 and K80 for 5.8S.
For the (MC)3 analysis, the following settings were used. Substitution model parameters were unlinked across data partitions. Five parallel runs were started beginning with a random tree. Each run consisted of 6 incrementally heated chains using the default settings of MrBayes. Run length was preset to 6M generations sampling every 30th tree. To ensure that runs had converged, the average standard deviation of split frequencies for the five parallel runs was calculated every 100K generations discarding the first 25% of the tree sample as burn-in. Runs were stopped after 2M generations, when the standard deviation had dropped below the threshold of 0.01. A 50% majority-rule consensus tree with branch lengths, including all compatible groups with PP < 0.5 was obtained from the 50K post-burn-in trees. The ML analysis was run with 1,000 bootstrap resamples using the \( {\hbox{GTR + }}\Gamma {\hbox{ + I}} \) substitution model (the closest approximation to the three models inferred for the three partitions of the ITS). The likelihood parameter scores were obtained in TRACER v1.4 (Rambaut and Drummond 2007). Phylogenetic trees were drawn with the program TreeView (Page 1996).
Results and discussion
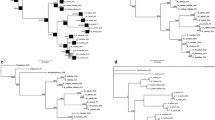
We produced 29 new ITS sequences. The final alignment contained 53 sequences, with a total of 441 unambiguously aligned characters. The likelihood parameters of the Bayesian analysis are available upon request. The majority-rule consensus tree based on 50,000 trees from the (MC)3 sample is shown in Fig. 1. The ML analysis yielded a similar topology; branch support values for the (MC)3 (PP ≥ 0.95) and the ML bootstrap analysis (BP ≥ 70) are indicated above the branches. Bold branches have both PP and BP values above these thresholds and are considered to be well supported.
Three species of the L. subfusca group were chosen as outgroup based on previous phylogenetic analyses (Arup and Grube 1998, 2000). These species (L. epibryon, L. allophana and L. campestris) form a monophyletic group (PP = 1, BP = 100). The rest of the species are distributed over three well supported clades but the relationships among these clades are unresolved (Fig. 1). The new species L. schizochromatica belongs to clade 1 (PP = 1, BP = 100), which also comprises L. filamentosa and L. expallens. The relationship between clade 1 and the L. symmicta group (clade 2) has no support.
Clade 2 (PP = 1, BP = 100) consists of the saxicolous species L. orosthea and L. sulphurea, as well as the epiphytic L. aitema, L. confusa, L. flavoleprosa, L. symmicta and a sister clade made up of the two epiphytic species L. cf. fulvastra collected in western Cuba and characterised by the presence of calycin, and L. austrocalifornica Lendemer & K. Knudsen. The third highly supported clade (PP = 1, BP = 94) combines the rest of the species, and falls into Protoparmeliopsis s. lat. and the L. varia, L. dispersa, L. saligna, Placodium, and L. varia groups.
Species belonging to the dispersa group (L. semipallida, L. perpruinosa and L. pruinosa) form a strongly supported clade (PP = 1, BP = 99) sister to Protoparmeliopsis (PP = 1; BP = 88), agreeing with results in Grube et al. (2004). Protoparmeliopsis is also well supported (PP = 1; BP = 96) and includes the lobate species P. muralis, P. achariana and L. garovaglii. The ‘L. varia clade’ is also well supported (PP = 1; BP = 89), but the relationships of this group within clade 3 lacked statistical support. Previous studies (Arup and Grube 1998, 2000) also failed in assessing the natural relationship of this group to others within Lecanora.
None of the other groups receives statistical support, indicating that more genetic loci and perhaps a more comprehensive taxon sampling are necessary to resolve their relationships. Nevertheless, there is independent morphological evidence to support some of the groups. For example, the two lobate species, L. novomexicana and L. opiniconensis included in the subgenus Placodium, group together, as do two species of L. saligna, L. anopta and L. saligna, both with isousnic acid. Lecanora subintricata also produces isousnic acid but it is unclear whether it belongs in the same group. In a preliminary analysis based on a slightly different taxon sample, it grouped with L. anopta, although also with low support.
Finally, two different clades can be distinguished in the ‘L. varia group’. The first of them (PP = 1, BP = 0.98) comprises four taxa closely related to L. varia. Lecanora conizaeoides, a species that is morphologically very similar to L varia but distinguished among other things by containing fumarprotocetraric acid, is basal to the rest of the species within the clade. GenBank sequences of Lecanora varia appear paraphyletic with L. densa and two specimens of L. burgaziae, a recently described species from the Iberian Peninsula, intermixed. The second clade combines five collections of the epiphytic L. laxa.
Our results illustrate that the taxonomy and systematics of the Lecanora varia group are still in need of revision. This involves species delimitation as well as the distinction of phylogenetic groups. A number of recently described species (e.g. L. burgaziae) are probably synonymous with known species. The nomenclatural implications arising from our analysis will be treated in a separate contribution. However, there are not only new synonymies but also new species to science that have come to light recently, including Lecanora schizochromatica, described below, and L. austrocalifornica (Lendemer and Knudsen 2009).
The distinction of species groups within the large genus Lecanora is a long-standing problem, as the previous attempts by Eigler (1969) and Motyka (1995, 1996a, b) illustrate, and seems at least partly due to highly homoplasious morphological characters. Clades one and two of our analyses separate L. symmicta from the morphologically very similar L. schizochromatica and L. filamentosa. Because of their similar apothecial anatomy, L. filamentosa (as L. ramulicola) was regarded as a member of the L. symmicta group by Printzen and May (2002). However, it was noted that this species produced atranorin as major secondary compound and usnic acid only in trace amounts. Atranorin is also produced in L. schizochromatica, which indicates that secondary chemistry could be a good character to separate phylogenetically related groups within Lecanora. However, L. expallens which is sister to L. filamentosa produces usnic acid as major compound. Further, the only species of the Lecanora varia group s. lat. that produce fumarprotocetraric acid, L. conizaeoides and L. austrocalifornica, belong to two different highly supported groups of species: L. conizaeoides is closely related to L. varia whereas L. austrocalifornica is sister to L. cf. fulvastra from Cuba. Then again, L. varia, L. densa, and L. burgaziae, the three species in our dataset that produce psoromic acid as major secondary compound, form a well-supported monophyletic group. The distinction between L. burgaziae, L. densa and L. varia will form part of a future study. In our analysis, L. burgaziae appears as sister to two sequences of L. varia, but a third GenBank sequence of L. varia appears basal to the clade formed by L. burgaziae, L. densa and L. varia. Finally, five sequences of Lecanora laxa, described as a subspecies of L. varia, form a well-supported clade outside L. densa, L. varia and L. conizaeoides, contradicting the statement in Laundon (2003a) that L. laxa is a psoromic acid-deficient chemomorph of L. densa, and hence it merits species rank. A closer relationship between L. laxa and L. coniferarum than between L. laxa and L. varia was previously anticipated by Printzen (2001). Unfortunately, L. coniferarum is lacking from our dataset.
To conclude, the species groups distinguished recently within the Lecanora varia group (Śliwa and Wetmore 2000, Printzen 2001) are in need of revision. As the backbone of clade three is still poorly supported, more gene loci should be included in future studies. Finally, taxon sampling may have to be improved to come to a stable systematic solution for the Lecanora varia group, as has been demonstrated in other taxonomic groups (Lumbsch et al. 2007). In order to achieve this, intense field work, even in the comparatively well-explored regions of Europe and western North America, will be necessary.
Taxonomy
Lecanora anopta Nyl., Flora 56: 292 (1873) (Fig. 2)
Syn. Biatora pullula Tuck., Synopsis North Amer. Lich. II: 129 (1888). Type: Washington Terry. [sic], 1882, W.N. Suksdorf 200 (FH!, holotype). Isousnic acid was detected by HPLC.
Syn.
Lecidea pullula (Tuck.) Zahlbr., Cat. Lich. Univ. 3: 814.
Description
Thallus immersed, rarely superficial in small patches, then thin and creamish white, soredia and isidia absent; photobiont trebouxioid, (8.0–)10.7–12.5(–17.0) µm diam.; apothecia rounded, mostly single or in groups of 2–3, to 68–184 per cm2, broadly adnate, (0.38–)0.45–0.53(–0.64) mm diam.; disc matt, epruinose to strongly pruinose, flat to strongly convex, pale to dark reddish brown (Fig. 2a) to greyish/greenish black; thalline margin distinctly present only on young apothecia, in mature apothecia usually poorly developed, quickly receding but rarely preserved until maturity; proper margin inconspicuous when viewed from above, matt, creamish, receding in older apothecia; proper exciple in section (40–)45.5–59(–75) µm wide laterally, (27–)34–68(–190) µm measured at thickest point at base, strongly gelatinised, with POL+ interhyphal crystals that vary in quantity, sometimes only found at base of stipe (Fig. 2e), these dissolving in K; algae occasionally present in same area; exciple of thick-walled, branched, radiating hyphae with lumina 1.5–2.0 µm, terminal cell with lumen occasionally up to 5.0 µm, unpigmented or pale brownish to rarely dark greenish (HNO3+ reddish cinereorufa-green pigment occasionally present), IKI-; hypothecium 50–130 µm tall, colourless; subhymenium consisting of thickened ascogenous hyphae, well differentiated, 20–37 µm tall, colourless; hymenium (40–)44–51(–55) µm tall, strongly inspersed with yellowish brown grana (guttulae sensu Hedlund 1892) apparently derived from lumina of old ascospores or ascogenous hyphae (Fig. 2c), sclerotised parts dissolving in K; hymenium also with brown to green-black pigment streaks in upper 1/3–1/5 and 5–12 µm of K + olivaceous, greyish-olivaceous pigments; granular epihymenium ± present; paraphyses commonly branched, sometimes anastomosed, colourless or with faint brown to blue-green pigment in outer gel sheath at tips, to 2 µm, lumina ca. 0.5–1.5 µm wide, apically to 2.0 µm wide, lumina to 1.3–2.0 µm; asci similar to Micarea-type, 30–41 × 10.0–12.2 µm (Fig. 2d); ascospores 8 per ascus, colourless, simple, ellipsoid, (7.0–)8.5–10.0(–12.0) × (3.0–)4.2–5.4(–6.0) µm. Pycnidia infrequent, black, half immersed, globose to pyriform, 60–90 µm in diam., walls brown to dark greenish, HNO3+ reddish/purplish (due to the presence of cinereorufa green pigment), more intensively coloured apically to almost colourless basally, paraplectenchymatous; conidia bacilliform, gently curved, 7–9 × 1 µm (Fig. 2F).
Chemistry
Isousnic acid (major).
Additional specimens examined
CANADA. Alberta. Kananaskis area, Rocky Creek, east of Hwy 40 (Kananaskis Trail Hwy.) and north of Peter Lougheed Provincial Park, 50°51'N, 115°10'W, calcareous cliffs along the creek in a lush Pinus contorta, Picea glauca, Populus balsamifera, Shepherdia canadensis forest, 18 Jun 1986, J.E. Marsh 1958 (CANL, filed under Xylographa parallela); British Columbia. Purcell Mountains, Toby Creek canyon, on wood of abandoned bridge, 20 Jul 2004, T. Spribille 15313 & T. Goward (CANL); East Kootenays, E of Canal Flats, Moscow Road, 50°13.373’N, 115°28.556’W, on rotten log, 1,170 m, 27 Jul 2005, T. Spribille 16546 (UBC); ibid., on wood of stump, T. Spribille 16580 (hb. Spribille); ibid., Moscow Road fen, on lignum in fen, 27 Jul 2005, T. Spribille 16509 & C.R. Björk (CANL); Liard Plain, Dease River area, 5 mi N of Baking Powder Creek, 14 mi N of Boya Lake along Cassiar Hwy., 59°32.780’N, 129°14.067’W, on rotten log in open sandy Pinus contorta forest, 710 m, 9 Oct 2007, T. Spribille 25131 (CANL, FR, PRA); Yukon. ALCAN Highway, S of Johnson’s Landing near Teslin Lake, 60°28.641'N, 133°16.527'W, on decorticated Picea glauca branch, 719 m, 8 Oct 2007, T. Spribille 27075 (CANL, FR, PRA); same locality and day, T. Spribille 25077 (GZU). U.S.A. Montana. Lincoln Co., S of Trego, Brimstone Creek, on decorticated log, 2 Jun 2004, T. Spribille 13828 (hb. Spribille).
Lecanora anopta was recently reported from Greenland (Hansen 2004), and is here reported as new to continental North America. It is a locally common species found on conifer wood in sub-continental forests of the Rocky Mountain Trench and the southern Yukon south to Washington (where it appears to be rare) and northwestern Montana. Lecanora anopta could easily be confused with a species of Lecidea s. lat., because it often lacks the thalline margin normally associated with Lecanora species. It is similar to Lecanora saligna but likewise differs in the evanescent thalline margin. The ascus is also somewhat atypical of Lecanora (Fig. 2D). The species can be recognized most readily by the diagnostic presence of sclerotised grains (old spores) in the hymenium (only rarely absent) and the presence of isousnic acid. In habit it somewhat resembles Lecanora cadubriae, but that species can readily be distinguished by the presence of norstictic acid (K+ orange to red on the thallus, K+ red needles in section). Specimens with pruinose apothecia may be reminiscent of Lecanora sarcopidoides (A. Massal.) A.L. Sm., a species thus far known only from Europe which, however, has narrower ascospores and contains pseudoplacodiolic acid as the major constituent (van den Boom and Brand 2008). Small specimens with reddish apothecia can resemble Lecidea rubrocastanea (Spribille and Printzen 2007), but differ in the longer, narrowly ellipsoid ascospores (8.5–10 µm vs 6–8 µm in L. rubrocastanea), the nearly Micarea-like as opposed to Lecidella-type ascus, and the presence of isousnic acid.
Notes
The description given above refers to examined North American material. In Fennoscandia and the Alps, L. anopta has been found to be a very variable species in terms of thallus development, pigmentation, and size of apothecia/ascospores as well as apothecial anatomy (both lecanorine and lecideine may occur) (Hedlund 1892). Well-developed European specimens issued in various exsiccata often contain cinereorufa-green pigment in apothecia and pycnidia. Large dark pycnidia are often present and contain characteristic shorter and slightly curved pycnospores (?mesoconidia), which have so far not been detected in material from North America. Also, the ascospores of European specimens may be slightly larger (see Hedlund 1892, but see also description of Lecanora anopta by Nylander 1873). Hedlund (1892) described several morphotypes, but North American specimens collected to date seem to display only part of the known variability of the species and might be referrable to f. 1 sensu Hedlund (1892). However, our ITS sequence data indicate that samples from the two continents differ in only a few nucleotides. The nomenclatural status of Lecanora anopta is not yet resolved. Several names are applicable. L. anopta is the same as the species mentioned as ‘Lecanora cadubrioides ined.’ by Spribille et al. (2008, in supplementary material).
Lecanora filamentosa (Stirt.) Elix & Palice comb. nov
Basionym
Lecidea filamentosa Stirt., Scottish Naturalist 5: 218 [“1879”] (1880).
Type
Scotland, Glen Lochay, Killin, 21 July 1879, Stirton (GLAM—NHB. 1927. 8.01010!—lectotype selected here—atranorin, usnic acid, paraensic acids C and D detected by HPLC; BM 731241!—isolectotype)
Lecidea filamentosa Stirt. appears to be the oldest valid name for Lecanora ramulicola (H. Magn.) Printzen & P.F. May (Printzen and May 2002), hence a new combination is introduced here.
Lecanora schizochromatica Pérez-Ortega, T.Sprib. & Printzen, sp. nov. (Fig. 3)
Mycobank accession number
MB 513164
Diagnosis
Species Lecanorae filamentosae similis sed ascosporis minoribus (9.7–11.1 × 3.5–4.1 µm). Margo apotheciorum saepe nigrescens. A L. symmicta differt substantia major atranorinum continens.
Type
Canada. British Columbia. Purcell Mountains, St. Mary’s Alpine Provincial Park, trail to Mortar Lake, 49°51.421’N, 116°24.433’W, corticolous on twigs and branches of Picea engelmannii and Abies lasiocarpa s.lat. in upper subalpine parkland, 2,380 m, 6 Jul 2006, T. Spribille 19965 & V. Wagner (CANL, holotype; FR, GZU, UBC, isotypes).
Description
Thallus crustose, rimose to cracked-rimose or verrucose areolate, rarely immersed (on wood), soredia and isidia absent; areoles roundish, 0.14–0.2 mm diam., weakly to strongly convex, surface white or whitish grey or greenish white, roughened; hypothallus rarely present, if present then black; photobiont trebouxioid, (6.0−)10.1–14.0(−17.0) µm diam.; apothecia rounded except when grouped, single or paired, less frequently aggregated in groups of 3–12, sessile with constricted base, (0.3−)0.5–0.9(−1.4) mm; disc matt, epruinose to finely pruinose, colour variable, from ‘usnic yellow’ to slate grey to bluish grey to greenish black to jet black or reddish brown (Fig. 3a–d), flat to concave in young apothecia, weakly to strongly convex in mature apothecia; proper margin prominent when young, translucent grey to jet black, shiny, receding when older or retained as a thin black line; proper exciple in section (40−)49–69(−90) µm wide laterally, (60−)68–119(−150) µm measured at thickest point at base, strongly gelatinised, of thick-walled, dichotomously branched and anastomosing hyphae with narrow, linear lumina ca. 1 µm wide, exciple filled with fine, less than 0.5 µm in diam, colourless, POL+ granular crystals (Fig. 3e), usually hazy brown within, with or without blue-green, HNO3+ reddish pigment in outer parts, IKI-; algae present or absent around base of inner exciple; exciple externally sometimes with colourless, cutical-like coating to 25 µm thick; hypothecium 50–150 µm tall, colourless; subhymenium consisting of thickened ascogenous hyphae, well differentiated, (10–)30–80 µm tall, colourless, sometimes with small oily droplets; hymenium (37–)40–58 µm tall, colourless or with pale bluish-green, K+ greenish, HNO3+ reddish mauve pigmentation in upper 1/4 or 1/5, occasionally hazy brownish; epihymenium 3–5(–7) µm thick, granular, POL+, rarely streaking into hymenium; paraphyses simple or weakly branched, colourless or with faint brown pigment in outer gel sheath at tips, lumina 0.8–1.0 µm wide, apically often unthickened or 2–3 µm wide, lumina to 1.5–2.0 µm wide; asci Lecanora-type, 32–41 × 9.3–12.0 µm (Fig. 3f); ascospores 8 per ascus, colourless, simple, narrowly ellipsoid, (8.0–)9.7–11.1(–12.0) × (3.0–)3.5–4.1(–5.0) µm. Pycnidia infrequent, black, immersed in part, pyriform, to 220 µm diam., walls brown, paraplectenchymatous; conidia filiform, straight or slightly curved, 10–16 × ca. 0.7 µm.
Chemistry
Atranorin (major), usnic acid (major) and paraensic acids C and D (minor) (HPLC). (The last two substances of aliphatic character seem to be confined to apothecia and apparently form tiny droplets in exciple.)
Etymology
The name makes a reference to the highly variable colour of the apothecial disc and proper exciple (Fig. 3a–d).
Additional specimens examined
CANADA. British Columbia. East Kootenays, Moyie Lake SW of Cranbrook, corticolous on Larix occidentalis, 25 Aug 2003, T. Spribille 12760 & C.R. Björk (GZU); Central Interior, Upper Clearwater Valley, road to Phillips Creek, on fencepost, 6 Sep 2004, T. Spribille 15823 & C.R. Björk (CANL); Rocky Mountains, Albert River, 50°36.575’N, 115°33.229’W, corticolous on branches of Betula, 1,190 m, 30 Jul 2005, T. Spribille 16580-A (CANL); Skeena River valley, Keynton Lake, 55°12.332’N, 127°46.136’W, on Pinus contorta twigs, 375 m, 26 Aug 2006, T. Spribille 22575 (CANL); East Kootenay region, near Yahk, on Pseudotsuga menziesii twigs, 12 Jun 2004, T. Spribille 13986 (CANL); Skookumchuck area, 49°57.826’N, 115°49.204’W, on Pseudotsuga menziesii twigs, 1,275 m, 02 Aug 2005, T. Spribille 17016 (UBC); Glacier National Park, Bald Mountain, 2,315 m, on branch of Abies [lasiocarpa], T. Goward 05-526 (UBC); Shallow Lake, near Fraser border crossing, 59°43.478’N, 135°00.542’W, corticolous on Abies lasiocarpa, 835 m, 8 Oct 2007, T. Spribille 25019 (CANL); U.S.A. Alaska. Near Skagway, on bark of Betula, T. Tønsberg 32809 (BG); east side of White Pass, 59°36.754’N, 135°07.056’W, corticolous on Abies lasiocarpa, 966 m, 11 Sep 2008, T. Spribille 29396 (BG, hb. Klondike Gold Rush Natl Historic Park); Idaho. Bonner Co., on Gisborne Mtn., Priest River Experimental Forest, 48°21’N 116°46’W, bark of Abies lasiocarpa, 4,900 ft. 18 Jul 1969, T.L. Esslinger 1937 (ID); Montana. Flathead Co., Salish Mtns., near confluence of Lime and Magnesia Creeks, Collins Ranch, 48°38.793’N, 114°53.180’W, on Pinus ponderosa twigs, 1010 m, 20 Jul 2006, T. Spribille 20252 & S. Pérez-Ortega (to be issued in exsiccate Dupla Graecensia Lichenum); Lincoln Co., Fortine Creek, on Betula glandulosa twigs, 18 Aug 1997, T. Spribille 7360 & H.G. Stroh (GZU); ibid., Salish Mtns. near Mineral Mountain, 01 Jun 2004, T. Spribille 13804 (GZU); Missoula Co., Jocko Falls, 47°13.876’N, 113°48.130’W, corticolous and lignicolous on Pseudotsuga twigs, 1,330 m, 25 Jul 2006, T. Spribille 20621, S. Pérez-Ortega & T. Wheeler (GZU); Ravalli Co., Bitterroot Range, S shore of Lake Como near W end, on Pinus contorta twigs, 1370 m, 20 Jul 1981, B. McCune 11259 (hb. McCune); Oregon. Josephine Co., Siskiyou Mtns., Klamath Range, Grayback Creek near road crossing, 19 km E of Cave Junction, 42°09.22’N 123°24.15’W, on evergreen Quercus branch, 670 m, Jun 1996, B. McCune 23085 (hb. McCune); Linn Co., Cascade Range, E ridge of Squaw Mt., 44°20’ N, 122°09’ W, on Abies trunk, 1,500 m, Sep 1996, B. McCune 23210 (FR); Washington. Lewis Co., Chehalis, on Douglas-fir, L. Berglund s.n. OSC-86795; Skamania Co., Wind River Canopy Crane Research Facility, 45°49’N, 121°57’W, on bark, 355 m, 1996, B. McCune 25137 (hb. McCune).
Notes
Lecanora schizochromatica is one of the most common epiphytic lichen species in mesic forests of the inland Pacific Northwest of USA and adjacent southwestern Canada, known from around 41°N to over 60°N. It is also one of the most commonly misidentified lichens, having been labelled and reported as Lecanora symmicta (Noble 1982, pro parte), Lecidea carnulenta (Fink 1935), Lecidea paddensis (misapplied, in herb.), and Ramboldia elabens (syn. Lecidea elabens; e.g, McCune 1982, DeBolt and McCune 1993). Ramboldia elabens is only distantly related and, after review of hundreds of specimens, is still not known to occur in North America west of the Black Hills (Spribille, unpublished). More recently, L. schizochromatica has also been reported under the names Lecanora spec. A (Hauck and Spribille 2005), Lecanora ‘meliocarpella’ nom. nud. (Spribille 2006), Lecanora sp. 1 (Houde et al. 2007) and Lecanora ramulicola s. lat. (Bunnell et al. 2008).
Amazingly, L. schizochromatica does not appear to have been assigned a name in the early days of Pacific Northwest lichen taxonomy. All the species described by Tuckerman (1888, i.a.) based on material collected by Wilhelm Suksdorf in the then Washington Territory have turned out to refer to other species of lecideoid lichens, including Biatora pullula, which is a later synonym of Lecanora anopta (see that species). Lecidea carnulenta, described from New Hampshire (type: FH!) and widely applied based on its inclusion in the keys of Fink (1935), is possibly related to L. symmictella and is distinguished from L. schizochromatica amongst other things by its Porpidia-type ascus s.lat. Young apothecia of L. schizochromatica can also be confused with those of Cliostomum griffithii, which likewise have a greyish exciple and beige disc, but that species can be easily distinguished by its 1-septate ascospores and Bacidia-type asci, and it frequently possesses large pycnidia with a K+ purple pigment. The most likely species to be confused with L. schizochromatica is L. filamentosa. This species differs in having larger ascospores averaging 10–16 × 3.5–5.5 μm. Young apothecia of both species are usually pale beige to ochre and darken with age until they may eventually become completely black. In L. schizochromatica, the margin is usually darker than the disc and the first part to become black (Fig. 1 a, c, d), whereas margin and disc in L. filamentosa usually do not differ markedly or the margin is paler. Young apothecia of L. filamentosa usually have a lecanorine amphithecium that is lacking in L. schizochromatica. Specimens of L. schizochromatica with black apothecia contain higher concentrations of the pigment cinereorufa green (K+ greenish, HNO3+ reddish in section) and are usually found in highly exposed sites. Cinereorufa green is also present in shade specimens but is then often confined to the margins.
Several specimens of L. schizochromatica exposed to sunlight, particularly on exposed wood at high elevations, are aberrant in either lacking fine crystals in the excipulum (McCune 12721, hb. McCune) or in having slightly larger ascospores 11–14 × 3.5–5.0 µm and a thicker lateral exciple, 90–170 µm wide (Spribille 20071, CANL). Some specimens from southern areas are also aberrant, as with two specimens from California that can only tentatively be assigned here on account of the presence of fine crystals in the hymenium (Trinity Co., Spribille 18405, GZU) or strongly swollen paraphyses (Del Norte Co., Muggia s.n., TSB-38880). More specimens and study is required to assess variability at the geographical and ecological fringes of this species group.
Distribution and ecology
Lecanora schizochromatica occurs in open, well-ventilated habitats on bark and wood of conifers from sea level (in Alaska) to the timberline (to 2,400 m asl). It is a reliable member of a well characterized and colourful species assemblage comprised to a large degree of species endemic to western North America or with their main global distribution there, including Bryoria fremontii (Tuck.) Brodo & D. Hawksw., Kaernefeltia merrillii (Du Rietz) A. Thell & Goward, Vulpicida canadensis (Räsänen) J.-E. Mattsson & M.J. Lai, Tuckermannopsis platyphylla (Tuck.) Hale, T. orbata (Nyl.) M.J. Lai, Hypogymnia imshaugii Krog s.lat., H. occidentalis L.H. Pike, Lecanora laxa (Śliwa & Wetmore) Printzen, Ramboldia gowardiana (T. Sprib. & Hauck) Kalb, Lumbsch & Elix, and Lecidea rubrocastanea T. Sprib. & Printzen.
References
Arup U, Grube M (1998) Molecular systematics of Lecanora subgenus Placodium. Lichenologist 30:415–425
Arup U, Grube M (2000) Is Rhizoplaca (Lecanorales, lichenized Ascomycota) a monophyletic genus? Can J Bot 78:318–327
Brodo IM (1984) The North American species of the Lecanora subfusca group. In: Hertel H, Oberwinkler F (eds) Beiträge zur Lichenologie. Festschrift J. Poelt. Beiheft zur Nova Hedwigia 79. Cramer, Vaduz, pp 63–185
Brodo IM, Elix JA (1993) Lecanora jamesii and the relationship between Lecanora s. str. and Straminella. Bibl Lichenol 53:19–25
Brodo IM, Owe-Larsson B, Lumbsch HT (1994) The sorediate, saxicolous species of the Lecanora subfusca group in Europe. Nord J Bot 14:451–461
Bunnell FL, Spribille T, Houde I, Goward T, Björk C (2008) Lichens on down wood in logged and unlogged forest stands. Can J For Res 38:1033–1041
Castresana J (2000) Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol 17:540–552
Choisy M (1929) Genres nouveaux pour la lichénologie dans le groupe des Lécanoracées. Bull Soc Bot Fr 76:521–527
Choisy M (1949) Catalogue des lichens de la region lyonnaise. [Fasc. 2]. Bull Mens Soc Linn Lyon 18:137–152
Culberson CF (1972) Improved conditions and new data for the identification of lichen products by a standardized thin-layer chromatographic method. J Chromatogr 72:113–125
Culberson CF, Johnson A (1982) Substitution of methyl tert. butyl ether for diethyl ether in standarzided thin-layer chromatographic method for lichen products. J Chromatogr 238:438–487
DeBolt A, McCune B (1993) Lichens of Glacier National Park, Montana. Bryologist 96:192–204
Dickhäuser A, Lumbsch HT, Feige GB (1995) A synopsis of the Lecanora subcarnea group. Mycotaxon 56:303–323
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797
Eigler G (1969) Studien zur Gliederung der Flechtengattung Lecanora. Diss Bot 4:1–195
Eigler G, Poelt J (1965) Flechtenstoffe und Systematik der lobaten Arten der Flechtengattung Lecanora in der Holarktis. Österr Bot Z 112:285–294
Elix JA, Giralt M, Wardlaw JH (2003) New chloro-depsides from the lichen Dimelaena radiata. Bibl Lichenol 86:1–7
Fink B (1935) The lichen flora of the United States. University of Michigan Press, Ann Arbor
Fröberg L (1997) Variation in the Lecanora dispersa group in South Sweden. Symb Bot Ups 32:29–34
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes - aplication to the identification of mycorrhizae and rust. Mol Ecol 2:113–118
Grube M, Baloch E, Arup U (2004) A phylogenetic study of the Lecanora rupicola group (Lecanoraceae, Ascomycota). Mycol Res 108:506–514
Guderley R (1999) Die Lecanora subfusca-Gruppe in Süd-und Mittelamerika. J Hattori Bot Lab 87:131–257
Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52:696–704
Hafellner J, Türk R (2001) Die lichenisierten Pilze Österreichs—eine Checkliste der bisher nachgewiesenen Arten mit Verbreitungsangaben. Stapfia 76:1–167
Hansen ES (2004) Notes on some new and interesting Greenland lichens VI. Graph Scr 15:1–6
Hauck M, Spribille T (2005) The significance of precipitation and substrate chemistry for epiphytic lichen diversity in spruce-fir forests of the Salish Mountains, northwestern Montana. Flora 200:547–562
Hedlund T (1892) Kritische Bemerkungen über einige Arten der Flechtengattungen Lecanora (Ach.), Lecidea (Ach.) und Micarea (Fr.). Bihang till Kungliga Svenska Vetenskaps-Akademiens Handlingar, ser. 3, 18:1–104
Houde I, Leech S, Bunnell FL, Spribille T, Björk C (2007) Old forest remnants contribute to sustaining biodiversity: the case of the Albert River valley. B C J Ecosyst Manage 8:43–52
Huelsenbeck JP, Ronquist F (2003) MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Kalb K (1991) Lichenes Neotropici: Fascicle XX (No. 451-475). Neumarkt/ Opf
Laundon JR (2003a) Six lichens of the Lecanora varia group. Nova Hedwigia 76:83–111
Laundon JR (2003b) The status of Lecanora zosterae in the British Isles. Lichenologist 35:97–102
Lendemer JC, Knudsen K (2009) Two new usnic acid containing species of Lecanora from western North America. Opusc Philolichenum 6:73–80
Leuckert C, Poelt J (1989) Studien über die Lecanora rupicola-Gruppe in Europa (Lecanoraceae). Nova Hedwigia 49:121–167
Lumbsch HT (1994) Die Lecanora subfusca-Gruppe in Australasien. J Hattori Bot Lab 77:1–175
Lumbsch HT, Elix JA (2004) Lecanora. In: McCarthy PM, Mallett K (eds) Flora of Australia, vol 56A, Lichens 4. ABRS, CSIRO Australia, Melbourne, pp 12–62
Lumbsch HT, Plümper M, Guderley R, Feige GB (1997) The corticolous species of Lecanora sensu stricto with pruinose apothecial discs. Symb Bot Ups 32:131–161
Lumbsch HT, Schmitt I, Lücking R, Wiklund E, Wedin M (2007) The phylogenetic placement of Ostropales within Lecanoromycetes (Ascomycota) revisited. Mycol Res 111:257–267
Martínez I, Aragón G (2004) The Lecanora varia group in Spain: species with amphithecial cortex. Bryologist 107:222–230
McCune B (1982) Lichens of the Swan Valley, Montana. Bryologist 85:13–21
Meyer B, Printzen C (2000) Proposal for a standardized nomenclature and characterization of insoluble lichen pigments. Lichenologist 32:571–583
Miyawaki H (1988) Studies on the Lecanora subfusca group in Japan. J Hattori Bot Lab 64:271–326
Motyka J (1995) Porosty (Lichenes). Tom I. Rodzina Lecanoraceae, Lubelskie Towarzystwo Naukowe, Lublin
Motyka J (1996a) Porosty (Lichenes). Tom II. Rodzina Lecanoraceae.—Lubelskie Towarzystwo Naukowe, Lublin
Motyka J (1996b) Porosty (Lichenes). Tom III. Rodzina Lecanoraceae.—Lubelskie Towarzystwo Naukowe, Lublin
Noble WJ (1982) The Lichen Flora of the Coastal Douglas-fir Dry Subzone of British Columbia. PhD Thesis, University of British Columbia, Vancouver
Nylander W (1873) Addenda nova ad Lichenographiam europaeam. Continuatio sexta decima. Flora 56:289–300
Nylander JAA (2004) MrModeltest v2. Evolutionary Biology Centre, Uppsala University, Program distributed by the author
Page RDM (1996) TreeView: An application to display phylogenetic trees on personal computers. Comput Appl Biosci 12:357–358
Poelt J (1952) Die Lecanora subfusca-Gruppe in Süddeutschland. Ber Bayer Bot Ges 29:58–69
Poelt J (1958) Die lobaten Arten der Flechtengattung Lecanora Ach. sensu ampl. in der Holarktis. Mitt Bot Staatssamml Mün 2:411–573
Poelt J (1966) Die lobaten Arten der Sammelgattung Lecanora. Lichenes, Lecanoraceae. (Flechten des Himalaya 1). Khumbu Himal 1:187–202
Poelt J (1983) Bryonora, eine neue Gattung der Lecanoraceae. Nova Hedwigia 38:73–111
Poelt J, Leuckert C, Roux C (1995) Die Arten der Lecanora dispersa-Gruppe (Lichenes, Lecanoraceae) auf kalkreichen Gesteinen im Bereich der Ostalpen-eine Vorstudie. Bibl Lichenol 58:289–333
Printzen C (2001) Corticolous and lignicolous species of Lecanora (Lecanoraceae, Lecanorales) with usnic or isousnic acid in the Sonoran Desert Region. Bryologist 104:382–409
Printzen C, May P (2002) Lecanora ramulicola (Lecanoraceae, Lecanorales), an overlooked lichen species from the Lecanora symmicta group. Bryologist 105:63–69
Rambaut A, Drummond AJ (2007) Tracer v1.4, Available from http://beast.bio.ed.ac.uk/Tracer
Ryan BD (1989a) Lecanora sect. Petrasterion (lichenized Ascomycotina) in North America: Lecanora weberi Ryan, sp. nov. (subsect. Pseudocorticatae), from Colorado. Mycotaxon 36:9–14
Ryan BD (1989b) A monograph of Lecanora subgen. Placodium sect. Endochloris (lichenized Ascomycotina). Bryologist 92:513–522
Ryan BD (1998) A monograph of Lecanora subg. Placodium sect. Arctoxanthae (lichenized Ascomycotina). In: Glenn MG, Harris RC, Dirig R, Cole MS (eds) Lichenographia Thomsoniana: North American Lichenology in Honor of John W. Thomson. Mycotaxon, Ithaca, pp 105–131
Ryan BD, Nash TH III (1997) Systematics of Lecanora subgenus Placodium (lichenized Ascomycotina) in North America: an overview, with keys. Nova Hedwigia 64:111–127
Ryan BD, Lumbsch HT, Messuti MI, Printzen C, Śliwa L, Nash TH III (2004) Lecanora. In: Nash TH III, Ryan BD, Diederich P, Gries C, Bungartz F (eds) Lichen Flora of the Greater Sonoran Desert Region, vol 2, Lichens Unlimited. Arizona State University, Tempe, Arizona, pp 176–286
Śliwa L (2007) A revision of the Lecanora dispersa complex in North America. Polish Bot J 52:1–70
Śliwa L, Wetmore CM (2000) Notes on the Lecanora varia group in North America. Bryologist 103:475–492
Spribille T (2006) Materials for an epiphytic crustose lichen flora of northwest North America. Diplom thesis, University of Göttingen
Spribille T, Printzen C (2007) Lecidea rubrocastanea, a new lichen species from conifer bark and wood in interior western North America (Lecanorales, lichenized Ascomycetes). Lichenologist 39:339–347
Spribille T, Thor G, Bunnell FL, Goward T, Björk CR (2008) Lichens on dead wood: species-substrate relationships in the epiphytic lichen floras of the Pacific Northwest and Fennoscandia. Ecography 31:741–750
Tuckerman E (1888) A synopsis of the North American lichens. Part. II. Comprising the Lecideacei, and (in part) the Graphidacei. Anthony, New Bedford, MA
van den Boom PPG, Brand AM (2008) Some new Lecanora species from western and central Europe, belonging to the L. saligna group, with notes on related species. Lichenologist 40:465–497
Weber WA, Wittmann R (1992) Catalog of the Colorado Flora: a Biodiversity Baseline. University Press of Colorado, Boulder
White TJ, Bruns T, Lee S, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic, New York, pp 315–322
Zahlbruckner A (1926) Lichenes (Flechten). Spezieller Teil. In: Engler A (ed) Die natürlichen Pflanzenfamilien. Engelmann, Leipzig, pp 61–263
Acknowledgments
Our thanks are due to the curators of BM, CANL, FH and GLAM and to Curtis Björk, Trevor Goward, Bruce McCune, and Lucia Muggia for allowing us to cite their material. We are also grateful to Lucia Muggia and an anonymous referee for their valuable comments. S.P.O. is indebted to the Ministerio de Educación y Ciencia (Spain) for a postdoctoral grant and C.P. to the Deutsche Forschungsgemeinschaft for funding the project PR 567/9-1. Z.P. acknowledges the Grant Agency of the Academy of Sciences of the Czech Republic (KJB600050635), and continuing support by the Academy of the Czech Republic (AV0Z60050516) and by the Ministry of Education, Youth and Sports of the Czech Republic (0021620828). Z.P. also thanks Fredrik Jonsson who first showed him the species in the field.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s11557-010-0723-0
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Pérez-Ortega, S., Spribille, T., Palice, Z. et al. A molecular phylogeny of the Lecanora varia group, including a new species from western North America. Mycol Progress 9, 523–535 (2010). https://doi.org/10.1007/s11557-010-0660-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11557-010-0660-y