Abstract
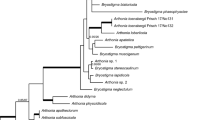
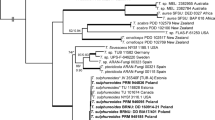
A survey was conducted in Brazil to collect fungi on ferns. Based on morphology and inferred phylogeny from DNA sequences of two loci, namely the internal transcribed spacer (ITS) regions and the large subunit nuclear ribosomal RNA gene (LSU), several species belonging to chalara-like genera and lachnoid fungi were recognized. Eighteen fungal isolates, collected from five host species, representing 10 different localities were studied. Three novel genera (Lachnopsis, Scolecolachnum and Zymochalara), and six novel species (Bloxamia cyatheicola, Lachnopsis catarinensis, Lachnopsis dicksoniae, Scolecolachnum pteridii, Zymochalara lygodii and Zymochalara cyatheae) are introduced. Furthermore, two new combinations (Erioscyphella euterpes and Erioscyphella lushanensis) are proposed. Two novel taxa (Lachnopsis catarinensis and Lachnopsis dicksoniae) may be included in the list of potentially endangered fungal species in Brazil, if proven to be restricted to their tree-fern host, Dicksonia sellowiana, which is included in the official list of endangered plant species in Brazil.











Similar content being viewed by others
References
Alfenas RF, Lombard L, Pereira OL, Alfenas AC, Crous PW (2015) Diversity and potential impact of Calonectria species in Eucalyptus plantations in Brazil. Stud Mycol 80:89–130
Almeida DAC, Barbosa FR, Gusmão LFP (2012) Alguns fungos conidiais aquáticos-facultativos do bioma Caatinga. Acta Bot Bras 26:924–932
Arambarri A, Cabello M, Cazau C (1992) A new hyphomycete from Santiago River. V. Bloxamia cremea. Mycotaxon 43:327–330
Armando EAS, Chaves ZM, Dianese JC (2015) Phaeostilbelloides and Velloziomyces – new dematiaceous genera from the Brazilian Cerrado. Mycotaxon 130:757–767
Baral HO (2015) Nomenclatural novelties. Index Fungorum no. 225
Baral HO, De Sloover JR, Huhtinen S, Laukka T, Stenroos S (2009) An emendation of the genus Hyaloscypha to include Fuscoscypha (Hyaloscyphaceae, Helotiales, Ascomycotina). Karstenia 49:1–17
Baral HO, Galán R, Platas G, Tena R (2013) Phaeohelotium undulatum comb. nov. and Phaeoh. succineoguttulatum sp. nov., two segregates of the Discinella terrestris aggregate found under Eucalyptus in Spain: taxonomy, molecular biology, ecology and distribution. Mycosystema 32:386–428
Baral HO, Haelewaters D (2015) Rommelaarsia flavovirens gen. et sp. nov. (Helotiales), a new discomycete on Equisetum with a peculiar asexual state. Ascomycete.org 7:321–330
Baranyay JA, Funk A (1969) Helotium resinicola n.sp. and its Stilbella conidial state. Can J Bot 47:1011–1014
Berthet P (1964) Formes conidiennes de divers discomycetes. Bull Trimest Soc Mycol Fr 80:125–149
Bogale M, Orr MJ, O’Hara MJ, Untereiner WA (2010) Systematics of Catenulifera (anamorphic Hyaloscyphaceae) with anassessmentofthephylogeneticposition of Phialophora hyalina. Fungal Biol 114:396–409
Cai L, Wu WP, Hyde KD (2009) Phylogenetic relationships of Chalara and allied species inferred from ribosomal DNA sequences. Mycol Prog 8:133–143. doi:10.1007/s11557-009-0585-5
Cândido TS, Silva AC, Guimarães LMS, Ferraz HGM, Júnior NB, Alfenas AC (2014) Teratosphaeria pseudoeucalypti on Eucalyptus in Brazil. Trop Plant Pathol 39:407–412
Cantrell SA, Haines JH (1997) New red species of Lachnum from the tropics. Mycol Res 101:1081–1084
Cantrell SA, Hanlin RT (1997) Phylogenetic relationships in the family Hyaloscyphaceae inferred from sequences of ITS regions, 5.8S ribosomal DNA and morphological characters. Mycologia 89:745–755
Carpenter SE, Dumont KP (1978) Los Hongos de Colombia - IV. Bisporella triseptata and its allies in Colombia. Caldasia 12:339–348
Coetsee C, Wingfield MJ, Crous PW, Wingfield BD (2000) Xenochalara, a new genus of dematiaceous hyphomycetes for Chalara-like fungi with apical wall building conidial development. S Afr J Bot 66:99–103
Coppins BJ, Minter DW (1980) A new hyphomycete from Northumberland. Notes R Bot Gard Edinburgh 38:363–365
Crous PW, Verkley GJM, Groenewald JZ, Samson RA (eds) (2009) Fungal biodiversity. CBS laboratory manual series no 1. Centraalbureau voor Schimmelcultures, Utrecht
Crous PW, Quaedvlieg W, Hansen K, Hawksworth DL, Groenewald JZ (2014) Phacidium and Ceuthospora (Phacidiaceae) are congeneric: taxonomic and nomenclatural implications. IMA Fungus 5:173–193
de Beer ZW, Duong TA, Barnes I, Wingfield BD, Wingfield MJ (2014) Redefining Ceratocystis and allied genera. Stud Mycol 79:187–219
Farr DF, Rossman AY (2015) Fungal databases systematic mycology and microbiology laboratory, ARS, USDA. http://nt.ars-grin.gov/fungaldatabases/. Accessed 15 May 2015
Fiuza PO, Gusmão LFP, Castañeda Ruiz RF (2015) Conidial fungi from the semiarid Caatinga biome of Brazil: a new species of Selenosporella from submerged leaves. Mycotaxon 130:601–605
Forzza R, Leitman P, Costa A, Siqueira Filho JA, Martinelli G, Monteiro RF, Santos-Silva F, Saraiva DP, Paixão-Souza B, Louzada RB (2015) Lista de espécies da flora do Brasil. http://floradobrasil.jbrj.gov.br. Accessed 16 Feb 2015
Guatimosim E, Pinto HJ, Pereira OL, Fuga CAG, Vieira BS, Barreto RW (2015) Pathogenic mycobiota of the weeds Bidens pilosa and Bidens subalternans. Trop Plant Pathol 40:298–397
Guatimosim E, Schwartsburd PB, Barreto RW, Crous PW (2016) Novel fungi from an ancient niche: cercosporoid and related sexual morphs on ferns. Persoonia 37:106–141
Haines JH (1980) Studies in the Hyaloscyphaceae I: some species of Dasyscyphus on tropical ferns. Mycotaxon 11:189–216
Haines JH (1992) Studies in the Hyaloscyphaceae VI: the genus Lachnum (ascomycetes) of the Guyana Highlands. Nova Hedwigia 54:97–112
Haines JH, Dumont KP (1984) Studies in the Hyaloscyphaceae. III: the long-spored, lignicolous species of Lachnum. Mycotaxon 19:1–39
Han JG, Hosoya T, Sung G-H, Shin HD (2014) Phylogenetic reassessment of Hyaloscyphaceae sensu lato (Helotiales, Leotiomycetes) based on multigene analyses. Fungal Biol 118:150–167
Hawksworth DL (1991) The fungal dimension of biodiversity: magnitude, significance, and conservation. Mycol Res 95:641–655
Hernández-Gutiérrez A, Dianese JC (2014) Cercosporoid hyphomycetes on malpighiaceous hosts from the Brazilian Cerrado: new Passalora and Pseudocercospora species on hosts of the genus Banisteriopsis. Mycol Prog 13:365–371
Hernández-Gutiérrez A, Braun U, Dianese JC (2014) Cercosporoid hyphomycetes on malpighiaceous hosts from the Brazilian Cerrado: species of Pseudocercospora on hosts belonging to Byrsonima. Mycol Prog 13:193–210
Hosoya T (2002) Hyaloscyphaceae in Japan (6): the genus Hyphodiscus in Japan and its anamorph Catenulifera gen. nov. Mycoscience 43:47–57
Hosoya T, Sasagawa R, Hosaka K, Sung G-H, Hirayama Y, Yamaguchi K, Toyama K, Kakishima M (2010) Molecular phylogenetic studies of Lachnum and its allies based on the Japanese material. Mycoscience 51:170–181
Hustad VP, Kucera V, Rybarikova N, Lizon P, GaislerJ BTJ, Miller AN (2014) Geoglossum simile of North America and Europe: distribution of a widespread earth tongue species and designation of an epitype. Mycol Prog 13:857–866
Izabel TSS, Almeida DAC, Monteiro JS, Castañeda-Ruiz RF (2015) Anaexserticlava caatingae, a new conidial fungus from the semi-arid Caatinga biome of Brazil. Mycotaxon 130:445–449
James TY, Kauff F, Schoch CL et al (2006) Reconstructing the early evolution of Fungi using a six-gene phylogeny. Nature 443:818–822
Johnston PR (1998) The Bloxamia anamorph of Bisporella discedens. Mycotaxon 31:345–350
Katoh K, Misawa K, Kuma K, Miyata T (2002) MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform.Nucleic Acids Res 30:3059–3066
Kirk PM, Cannon PF, David WM, Stalpers JA (2008) Dictionary of the fungi, 10th edn. CABI Publishing, Wallingford, UK
Koukol O (2011) New species of Chalara occupying coniferous needles. Fungal Divers 49:75–91
Liu YL, Zhang ZY (1998) A new species of the genus Bloxamia. Mycosystema 17:7–10
Macedo DM, Pereira OL, Wheeler GS, Barreto RW (2013) Corynespora cassiicola f. sp. schinii, a potential biocontrol agent for the weed Schinus terebinthifolius in the United States. Plant Dis 97:496–500
Macedo DM, Pereira OL, Hora Junior BT, Weir BS, Barreto RW (2016) Mycobiota of the weed Tradescantia fluminensis in its native range in Brazil with particular reference to classical biological control. Australas Plant Pathol 45:45–56
Mendes MAS, Urben AF (2015) Fungos relatados em plantas no Brasil, Laboratório de Quarentena Vegetal. Brasília, DF: Embrapa Recursos Genéticos e Biotecnologia. http://pragawall.cenargen.embrapa.br/-aiqweb/michtml/fgbanco01.asp. Accessed 15 May 2015
Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. Proc Gatew Comp Environ Workshop (GCE), New Orleans, LA pp 1–8
Minter DW, Holubová-Jechová V (1981) New or interesting Hyphomycetes on decaying pine litter from Czechoslovakia. Folia Geobot Phytotax 16:195–217
Nag Raj TR, Kendrick B (1975) A monograph of Chalara and allied genera. Wilfrid Laurier University Press, Waterloo
Nannfeldt JA (1932) Studien über die Morphologie und Systematic der nichtlichenisierten inoperculaten Discomyceten. Nova Act Reg Soc Sci Upsal 8:1–368
Nylander J (2004) MrModeltest v2. Program distributed by the author. Evol Biol Cent Uppsala Univ 2:1–2
Oliveira L, Harrington TC, Ferreira MA, Damacena MB, Al-Sadi AM, Al-Mahmooli IHS, Alfenas AC (2015) Species or genotypes? Reassessment of four recently described species of the Ceratocystis wilt pathogen, Ceratocystis fimbriata, on Mangifera indica. Phytopathology 105:1229–1244
Osmundson TW, Robert VA, Schoch CL, Baker LJ, Smith A, Robich G, Mizzan L, Garbelotto MM (2013) Filling gaps in biodiversity knowledge for macrofungi: contributions and assessment of an herbarium collection DNA barcode sequencing project. PLoS ONE 8:e62419
Paulin-Mahady AE, Harrington TC (2000) Phylogenetic placement of anamorphic species of Chalara among Ceratocystis species and other ascomycetes. Stud Mycol 45:209–222
Paulin-Mahady AE, Harrington TC, NcNew D (2002) Phylogenetic and taxonomic evaluation of Chalara, Chalaropsis, and Thielaviopsis anamorphs associated with Ceratocystis. Mycologia 94:62–72. doi:10.2307/3761846
Perić B, Baral HO (2014) Erioscyphella curvispora spec. nov. from Montenegro. Mycol Monten 17:89–104
Pillar VD, Müller SC, Castilhos Z, Jacques AVA (2009) Campos Sulinos: Conservação e Uso Sustentável da Biodiversidade. Ministério do Meio Ambiente, Brasilia
Pinho DB, Firmino AL, Ferreira-Junior WG, Pereira OL (2012) An efficient protocol for DNA extraction from Meliolales and the description of Meliola centellae sp. nov. Mycotaxon 122:333–345
Pirozynski KA, Morgan-Jones G (1968) Notes on microfungi. III. Trans Br Mycol Soc 51:185–206
Raitviir A (2004) Revised synopsis of the Hyaloscyphaceae. Scr Mycol 20:1–133
Rayner RW (1970) A mycological colour chart. Commonwealth Mycological Institute and British Mycological Society, Surrey, UK
Réblová M (1999) Teleomorph-anamorph connections in Ascomycetes 2. Ascochalara gabretae gen. et sp. nov. and its Chalara-like anamorph. Sydowia 51:210–222. doi:10.2307/3762067
Rocha FB, Barreto RW, Bezerra JL, Neto JAAM (2010) Foliar mycobiota of Coussapoa floccosa, a highly threatened tree of the Brazilian Atlantic Forest. Mycologia 102:1241–1252
Rodrigues AL, Pinho DB, Lisboa DO, Nascimento RJ, Pereira OL, Alfenas AC, Furtado GQ (2014) Colletotrichum theobromicola causes defoliation, stem girdling and death of mini-cuttings of Eucalyptus in Brazil. Trop Plant Pathol 39:326–330
Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542
Saccardo PA (1884) Conspectus generum Discomycetum hucusque cognitorum. Bot Centralbl 18(213–220):247–255
Schoch CL, Seifert KA, Huhndorf S et al (2012) Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc Natl Acad Sci U S A 109:6241–6246
Seifert KA, Carpenter SE (1987) Bisporella resinicola comb. nov. and its Eustilbum anamorph. Can J Bot 65:1262–1267
Silva M, Pinho DB, Pereira OL, Fernandes FM, Barreto RW (2016) Naming potentially endangered parasites: foliicolous mycobiota of Dimorphandra wilsonii, a highly threatened Brazilian tree species. PLoS ONE 11:e0147895. doi:10.1371/journal.pone.0147895
Smith AR, Pryer KM, Schuettpelz E, Korall P, Schneider H, Wolf PG (2008) A classification for extant ferns. Taxon 55:705–731
Spooner BM (1987) Helotiales of Australasia: Geoglossaceae, Orbiliaceae, Sclerotiniaceae, Hyaloscyphaceae. Bibl Mycol 116:1–711
Spooren M (2014) A new species of Bloxamia from freshwater in the Netherlands. Mycosphere 5:346–349
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA 6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Untereiner WA, Naveau FA, Bachewich J, Angus A (2006) Evolutionary relationships of Hyphodiscus hymeniophilus (anamorph Catenulifera rhodogena) inferred from β-tubulin and nuclear ribosomal DNA sequences. Can J Bot 84:243–253
Vilgalys R, Hester M (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol 172:4238–4246
Wang Z, Binder M, Hibbett DS (2005) Life history and systematics of the aquatic discomycete Mitrula (Helotiales, Ascomycota) based on cultural, morphological, and molecular studies. Am J Bot 92:1565–1574
White TJ, Bruns T, Lee J, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications: 315–322. Academic, New York, pp 322–514
Windisch PG (2002) Pteridófitas do Brasil: diversidade decrescente. In: Araujo EL, Moura NA, Sampaio EVSB (eds) Biodiversidade, conservação e uso sustentável da flora do Brasil. Universidade Federal Rural de Pernambuco, Recife, pp 196–198
Zhang YH, Zhuang WY (2004) Phylogenetic relationships of some members in the genus Hymenoscyphus (Ascomycetes, Helotiales). Nova Hedwigia 78:475–484
Zhao P, Zhuang WY (2011) Evaluation of ITS region as a possible DNA barcode for the genus Lachnum (Helotiales). Mycosystema 30:932–937
Zhuang WY (1988) Notes on Lachnellula theiodea. Mycotaxon 31:411–416
Zhuang WY, Hyde KD (2001) New species of Lachnum and Perrotia from Hong Kong, China. Mycologia 93:606–611
Zhuang WY, Wang Z (1998) Some new species and new records of discomycetes in China. VIII. Mycotaxon 66:429–438
Acknowledgments
The authors would like to thank Dr Richard T. Hanlin and Dr Wen-Ying Zhuang for their helpful contributions regarding Herbarium information, and Dr Dartanhã José Soares for his collaboration during the field work. Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Conselho Nacional do Desenvolvimento Científico e Tecnológico (CNPq) and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) are thanked for financial support. Electron microscopy studies were performed at the Núcelo de Microscopia e Microanálise da Universidade Federal de Viçosa (NMM-UFV).
Author information
Authors and Affiliations
Corresponding author
Additional information
Section Editor: Gerhard Rambold
Rights and permissions
About this article
Cite this article
Guatimosim, E., Schwartsburd, P.B., Crous, P.W. et al. Novel fungi from an ancient niche: lachnoid and chalara-like fungi on ferns. Mycol Progress 15, 1239–1267 (2016). https://doi.org/10.1007/s11557-016-1232-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11557-016-1232-6