Abstract
Lecideoid lichen-forming fungi are a large, heterogeneous group that includes many species described during the nineteenth century that are of unclear taxonomic status. We revise such a group, the species of which have previously been treated under the much-misunderstood names Catillaria contristans or Toninia squalescens, and use a seven-locus phylogeny to determine its phylogenetic position. We found strong support for a previously unrecognized monophyletic lineage within the Sphaerophoraceae, comprising five phylogenetic species, and describe the new genus Gilbertaria to accommodate them. The new genus is characterized by a crustose growth form, 1-septate ascospores, thick ((1.5–)2–3(–4) μm wide) paraphyses and asci of the Biatora-type. We revise the nomenclature and give new delimitations and descriptions of the Northern Hemisphere species Gilbertaria contristans comb. nov., G. holomeloides comb. nov., G. squalescens comb. nov. and describe the new species G. astrapeana from the Falkland Islands.
Similar content being viewed by others
Introduction
Lecideoid lichen-forming fungi are a large and heterogeneous group of crustose lichens that is characterized by apothecia without symbiotic algal cells in the apothecial margin. In the early days of lichenological research, the majority of the then known lecideoid species (about a hundred) were referred to the genus Lecidea Ach. (Acharius 1803). Subsequent work by nineteenth century lichenologists added several hundred lecideoid species, many also described in the genus Lecidea, while at the same time other lichenologists were developing a more fine-grained classification that led to the introduction of numerous new genera, such as Bacidia De Not., Biatora Fr., Buellia De Not., Catillaria A.Massal., Lecidella Körb., Micarea Fr. and Rhizocarpon Ramond ex DC.
At the beginning of the twentieth century, the Austrian lichenologist Alexander Zahlbruckner set out to catalogue all the lichens described at that time (Zahlbruckner 1921–1940). His aim was not just to list described names but to also express his taxonomic opinion and to list all published literature treating each taxon. Such a gigantic project necessitated some simplifying shortcuts, one of which was to mechanically refer crustose lichens with hyaline ascospores, a green, non-Trentepohlia algal symbiont and lacking algal cells in the apothecial margin to either the genus Lecidea if ascospores were 1-celled, to Catillaria if ascospores were 2-celled, to Bacidia if ascospores had 3 or more transverse septa and to Rhizocarpon if they were muriform. In addition, because of the disproportionate importance placed on thalline morphology, species with a squamulose thallus were placed in Lecidea sect. Psora Schaer. if the ascospores were one-celled and in Toninia A.Massal. if they were septate. This led to species with similar apothecial characteristics being placed in different genera and, subsequently, different families. Zahlbruckner’s taxonomic system came to dominate taxonomical thinking about lichens for decades at the expense of a more natural taxonomy based on a wider suite of apothecial characters.
Beginning in the 1960s and 70s, the search for a more natural classification of lecideoid lichens was revitalized. This included revisions at the species level, requiring the revival of previously described genera as well as the description of numerous new ones (e.g. Hertel 1967; Coppins 1983; Hertel 1984; Hertel and Rambold 1987; Timdal 1991; Printzen 1995; Schmull et al. 2011; Kistenich et al. 2018). However, many species and species groups have not been revised since Zahlbruckner’s time and are still classified following his artificial taxonomy. One example of this concerns the group of small crustose species with 1-septate ascospores that are currently treated under the names Catillaria contristans (Nyl.) Zahlbr. and Toninia squalescens (Nyl.) Th.Fr. During the nineteenth century, several new species were described in this group: Catillaria sphaeralis Körb., Lecidea contristans Nyl., Lecidea dufourii Nyl., Lecidea holomeloides Nyl., Lecidea hypocyanea Stirt., Lecidea squalescens Nyl. and Thalloidima rimulosum Th.Fr. A first recognition that these names denoted very similar entities was made by Fries (1874), who included Catillaria sphaeralis, Lecidea dufourii and his own Thalloidima rimulosum as synonyms of Toninia (=Lecidea) squalescens. Zahlbruckner (1921–1940) accepted four species in this group in three different genera: Catillaria contristans (including Lecidea holomeloides), C. sphaeralis (including L. dufourii), Toninia squalescens (including Thalloidima rimulosum) and Lecidea hypocyanea, which was retained in Lecidea because, according to its protologue, it had simple ascospores. Vainio (1934) implicitly used Zahlbruckner’s taxonomic generic scheme for these species, and accepted Toninia squalescens (including Thalloidima rimulosum) and Catillaria dufourii (including C. sphaeralis), but he also described the new species Catillaria kivakkensis Vain., which he considered close to C. dufourii (Nyl.) Vain. (Vainio 1934). A further addition to this group was made from North America by Lowe (1939), who described Lecidea subramosa J. Lowe, although he did not compare his new species to other names described in this group. After the 1930’s, it seems that no further species have been added.
Although it has long been acknowledged that these species are not congeneric with the type species of the genus in which they are placed (e.g. Timdal 1991; Coppins 1992), in the absence of a modern revision of this group, modern floras (e.g. Foucard 2001; Hitch et al. 2009; Wirth et al. 2013) essentially retain Zahlbruckner’s generic classification, the only difference being a further reduction in the number of species, with only two species being currently accepted: Catillaria contristans and Toninia squalescens. The taxonomic status of these names as well as their many suggested synonyms is, however, unclear and typifications are often lacking. For example, several checklists (e.g. Hafellner and Türk 2016; Nimis 2016) list Catillaria sphaeralis as a synonym of C. contristans, although neither name has been lectotypified and C. sphaeralis is, in fact, the older name. In addition, as a consequence of the lingering Zahlbrucknerian classification, the similarity of C. contristans and T. squalescens has largely been forgotten and these species are generally not compared to each other, in spite of their many anatomical similarities (cfr. Foucard 2001; Fletcher and Coppins 2009; Hitch et al. 2009).
The confusion surrounding the names Catillaria contristans and Toninia squalescens has also had implications for molecular phylogenies. The first researchers to include C. contristans in a phylogenetic analysis were Andersen and Ekman (2005), who recovered the species in a paraphyletic Micarea, close to the Micarea peliocarpa group. However, the specimen from which the sequences were obtained was actually a species of Micarea, M. oreina Kantvilas & Coppins (Kantvilas and Coppins 2019). Next, in a phylogeny including many different families, Ekman et al. (2008) recovered a specimen identified as Protomicarea limosa (Ach.) Hafellner on a highly supported branch together with Sphaerophorus globosus (Huds.) Vain. Sequences from this specimen were subsequently used for further phylogenetic work but the specimen was redetermined first as Catillaria contristans (Ekman and Blaalid 2011) and then as Toninia squalescens (Kistenich et al. 2018). As these examples illustrate, the unclear species boundaries of C. contristans and T. squalescens mean, not only that it is difficult to distinguish these species from each other, but also that there is a clear risk that they are confused with other crustose lichens that occur in the same habitat, such as species of Micarea or Protomicarea Hafellner. Hence, there is a great need for a modern revision of this group. Our aim with this study was to perform such a revision of Catillaria contristans, Toninia squalescens and their suggested synonyms, as well as to determine their phylogenetic position.
Material and methods
Morphology and anatomy
Measurements were made under a light microscope on material mounted in water, using an oil-immersion, objective lens, with a precision of 0.5 μm for measurements of finer anatomical structures (e.g. ascospores and paraphyses). Only well-developed ascospores lying outside the asci were measured. Ascospore dimensions and length/breadth ratios are presented in the format: (minimum value observed–) mean value ± standard deviation (–maximum value observed) with n being the number of measurements. To examine colour reactions of pigments and solubility of crystals, we used a 10% solution of KOH (abbreviated K), a 4–5% solution of common commercial bleach (abbreviated C) and a 50% solution of HNO3 (abbreviated N). Anatomical features (especially paraphyses) were sometimes examined after staining of tissues with Floxine B. Apical structures of asci were examined in an iodine solution (0.3–0.4% I, abbreviated I) after pretreatment with K (abbreviated KI). We checked thallus reactions with K, C and, in addition, an ethanolic solution of para-phenylenediamine (abbreviated Pd). We performed HPTLC (following the method described by Arup et al. 1993) using solvent system C (Orange et al. 2010). Material from the following herbaria was included in the study: ASU, BM, E, H, L, MICH, MSC, O, QFA, S, TUR and UPS.
DNA extraction, amplification and sequencing
We extracted total DNA using a QIAGEN Plant DNA Mini Kit following the manufacturer’s instructions or, alternatively, with Chelex 100 (Bio-Rad, Hercules, CA, USA) following the procedure described by Ferencova et al. (2017). To achieve a 10% solution of Chelex, we used 0.1 g of Chelex per ml extract. For amplification and sequencing, we used the primers MS1, MSU7 as well as mrSSU1, mrSSU2 and mrSSU3R (used as nested primers for Austropeltum glareosum Henssen, H.Döring & Kantvilas and on their own in all other cases) for the mitochondrial ribosomal small subunit 12S, hereafter mtSSU (White et al. 1990; Zhou and Stanosz 2001; Zoller et al. 1999); NSSU97A, NSSU1088, NS8 and SR11R for nuclear ribosomal small subunit 18S, hereafter nSSU (Kauff and Lutzoni 2002; Spatafora et al. 1995; White et al. 1990); LRlecF and LRlecR for nuclear ribosomal large subunit 28S, hereafter LSU (Schneider et al. 2015); ITS1F and ITS4 for nuclear ribosomal DNA ITS1-5.8-ITS2, hereafter ITS (Gardes and Bruns 1993; White et al. 1990); the newly designed MCM7-580for (5′ ATG CCN AVR GAR CAN ADG-3′) and MCM7-1560rev (5′-GCR ACW CCD GGR TCR CCC AT-3′) as well as MCM7-709for and MCM7-1348rev (either as nested primers or on their own) for the Mini-chromosome Maintenance Complex 7 (Schmitt et al. 2009), hereafter MCM7; and fRPB2-5F and fRPB2-7cR with the nested primers RPB2-980F and RPB2-1554R for the RNA polymerase II complex subunit, hereafter RPB2 (Liu et al. 1999; Reeb et al. 2004). For the amplification of mtSSU and nSSU, we used the Phusion Green Hot Start II High-Fidelity DNA Polymerase Master Mix. For mtSSU, we used the following PCR thermal profile: an initial hold at 98 °C for 30 s followed by 35 cycles of denaturization at 98 °C for 10 s, annealing 30 s at 60.1 °C (for the primer pair MS1 + MSU7) or 60.3 °C (for the primer pair mrSSU1 + mrSSU3R) or 64.2 °C (for the primer pair mrSSU2 + mrSSU3R) and polymerization at 72 °C for 30 s, and finally a hold at 72 °C for 10 min. For nSSU, we used the following thermal profile when using the primer pair NSSU97A + NS8: an initial hold at 98 °C for 30 s followed by 35 cycles of denaturizing at 98 °C for 10 s, annealing at 66.2 °C for 30 s and polymerization at 72 °C for 50 s, and finally a hold at 72 °C for 10 min. When running a nested PCR for nSSU with the primer pair SR11R + NSSU1088, the same program was used, but the annealing temperature was set to 63.1 °C and the time for polymerization to 50 s.
For ITS, LSU, MCM7 and RPB2, we used VWR Red Taq DNA Polymerase Master Mix following the manufacturer’s protocols. For the amplification of ITS, we used this PCR thermal profile: an initial hold at 94 °C for 3 min followed by 35 cycles of denaturization at 94 °C for 30 s, annealing at 50 °C for 45 s and polymerization at 72 °C for 1 min., and finally a hold at 72 °C for 10 min. For LSU, we used the following PCR thermal profile: an initial hold at 94 °C for 3 min followed by 35 cycles of denaturization at 94 °C for 30 s, annealing at 57 °C for 45 s and polymerization at 72 °C for 1 min 45 s; and finally, a hold at 72 °C for 10 min. For MCM7, we used the following PCR thermal profile both for the primer pair MCM7-580for + MCM7-1560rev and for MCM7-709for + MCM71348rev: an initial hold at 94 °C for 3 min followed by 3 cycles of denaturization at 94 °C for 45 s, annealing at 57 °C for 45 s and polymerization at 72 °C for 1 min 45 s; then, 4 cycles of 94 °C for 40 s, 54 °C for 40 s, 72 °C for 45 s; 4 cycles of 94 °C for 40 s, 51 °C for 40 s, 72 °C for 45 s; 30 cycles of 94 °C for 30 s, 48 °C for 30 s, 72 °C for 45 s; and finally, a hold at 72 °C for 10 min. When running nested PCR for MCM7, the same thermal profile was used for the second as for the first PCR. We used a two-step nested PCR for RPB2. For the first step, using the primer pair fRPB2-5F and fRPB2-7cR, we used the following PCR thermal profile: an initial hold at 94 °C for 3 min followed by 35 cycles of denaturization at 94 °C for 30 s, annealing at 53 °C for 45 s, polymerization at 72 °C for 1 min 45 s and finally a hold at 72 °C for 10 min. For the second step, using the primer pair RPB2-980F and RPB2-1554R, we used this PCR thermal profile: an initial hold at 94 °C for 3 min followed by 15 cycles of denaturization at 94 °C for 30 s, annealing at 65 °C for 45 s (decreasing 1° each cycle), and polymerization at 72 °C for 1 min 20 s; then, 28 cycles of 94 °C for 30 s, 50 °C for 45 s, 72 °C for 1 min 20 s, and finally a hold at 72 °C for 10 min. PCR products were subsequently purified with Exo-sap-IT (USB Corporation) and sent for sequencing to Macrogen Europe Inc.
Taxon sampling
To determine the phylogenetic position of Gilbertaria, we assembled a sequence data set with a selection of species and genera from families considered to belong to the Sphaerophorineae: the Pilocarpaceae (the genera Byssoloma Trevis., Calopadia Vězda, Fellhanera Vězda and Micarea), the Psoraceae (Brianaria S.Ekman & M.Svensson, Protoblastenia (Zahlbr.) J.Steiner, Psora Hoffm.), the Ramalinaceae (Bacidia, Biatora, Bilimbia De Not., Lecania A.Massal. and Ramalina Ach.) and the Sphaerophoraceae (Miadlikowska et al. 2014; Wijayawardene et al. 2018). As BLAST-searches, and earlier phylogenies (Ekman et al. 2008; Ekman and Blaalid 2011) indicated that Gilbertaria could belong to the latter family, we included all genera considered to belong to the Sphaerophoraceae: Austropeltum Henssen, H.Döring & Kantvilas, Bunodophoron A.Massal., Calycidium Stirt., Leifidium Wedin, Neophyllis F.Wilson, and Sphaerophorus Pers. (Prieto et al. 2013; Wedin and Döring 1999; Wedin et al. 2000; Wedin 2002; Wijayawardene et al. 2018). We found it difficult to get amplifications from the sole member of the genus Austropeltum, A. glareosum, and failed to get any other marker than mtSSU from this species. The A. glareosum terminal in our analysis, therefore, contains sequences from two different specimens: nSSU from GenBank and mtSSU from our sequenced specimen (see Table 1). Further, we included two genera from the monogeneric families Psilolechiaceae (Psilolechia A.Massal.) and Scoliciosporaceae (Scoliciosporum A.Massal.), because these have usually emerged close to the Sphaerophorineae in earlier studies (e.g. Kraichak et al. 2018; Miadlikowska et al. 2014). In a recent phylogeny (Kraichak et al. 2018), the Scoliciosporaceae appeared paraphyletic because the two representatives of the family (Scoliciosporum intrusum (Th.Fr.) Hafellner and S. umbrinum (Ach.) Lojka) ended up on different branches, with S. umbrinum seemingly belonging to the Sphaerophoraceae. The cause of this result seems to be the RPB1 sequence for S. umbrinum used in that analysis (GenBank ID AY756397). This sequence blasts with species of Bunodophoron and is, in fact, almost identical to a sequence from B. australe (Laurer) A.Massal. (MF954576). Other loci from S. umbrinum, nominally from the same voucher, are not close to Bunodophoron. We thus consider the RPB1 sequence in question as erroneous and have not used it for our analysis. Two sequences of Protomicarea limosa available in Genbank (HQ660558, HQ650655) were included in test analyses, but were found to be distant to anything else included and were thus removed from the final analysis. As an outgroup, we selected species from two genera of the Lecanoraceae (Lecanora (Ach.) and Lecidella) because this family appears on a sister branch to the Sphaerophorineae (e.g. Miadlikowska et al. 2014). For the selected genera and species, we downloaded sequences of mtSSU, nSSU, LSU, ITS, MCM7, RPB1 and RPB2 from GenBank (Table 1).
Sequence alignment
For the non-protein-coding markers (ITS, mtSSU, nSSU and LSU), we used two different alignment strategies. The first was to estimate the alignment using an iterative method (PASTA) that optimizes the alignment using maximum likelihood, which in principle implies no subsequent removal of ambiguous positions or anything else from the resulting alignments. The second strategy was to align using MAFFT and then filter out positions that contribute to guide-tree uncertainty using GUIDANCE2. This potentially means removing large parts of the alignment that have been assessed as ambiguous from the subsequent analysis.
For the first strategy, we estimated separate alignments for ITS, mtSSU, nSSU and LSU using PASTA (Mirarab et al. 2015), with the mask option activated, MAFFT (algorithm L-INS-i) as the aligner, OPAL for the pairwise merging and FastTree as the tree estimator, with GTR + Γ as the model for molecular evolution. Inspection of the alignment of mtSSU revealed problems due to the presence of a long intron. We therefore estimated the mtSSU dataset using MAFFT with the algorithm E-INS-i, which is designed to handle sequence data sets with multiple conserved domains divided by long gaps (Katoh et al. 2019). From the MAFFT alignment of mtSSU, we removed one intron of 923 bp from G. squalescens. We then estimated the mtSSU alignment using PASTA, as described above. To minimize potential problems with missing data, the ends of all the PASTA alignments were trimmed. For the second alignment strategy, we first estimated alignments for the same four non-protein coding markers with MAFFT, using the algorithm E-INS-i (Katoh et al. 2019) and then performed filtering of ambiguous positions using GUIDANCE2 (Sela et al. 2015), running 100 bootstrap-replicates. Alignment positions that fell below the default confidence score of 0.93 were removed, which meant removing 43.9% of the ITS, 8.8% of the mtSSU, 14.4% of the LSU and 2% of the nSSU alignment, respectively. As for the PASTA alignments, the ends of the GUIDANCE2 alignments were trimmed.
For the protein-coding genes (MCM7, RPB1 and RPB2), we estimated alignments with MAFFT using the algorithm E-INS-i (Katoh et al. 2019). After aligning the sequences, we identified several non-coding introns in the RPB1 and RPB2 alignments. These were very variable and not possible to unambiguously align, so we removed them before any further analysis was performed. No such introns were detected in the alignment of MCM7. A subsequent filtering with GUIDANCE2 of the alignments of the protein-coding genes did not indicate any positions falling below the confidence score of 0.93. The alignments of the three protein-coding genes are thus the same in both the PASTA alignment set and in the GUIDANCE2 alignment set.
We checked for incongruence among markers by performing a separate maximum likelihood analysis of each of the eleven alignments (four PASTA alignments, four filtered with GUIDANCE2 and three for the protein-coding genes) using IQTree with 500 non-parametric bootstrap replicates (Nguyen et al. 2015). The single marker trees from these analyses were then compared to identify any conflicting results, defined as conflicting clades with > 75% bootstrap support. We found no such conflicts and, therefore, decided to concatenate the eleven alignments into two sets of seven markers each: (I) the four PASTA alignments + the three protein coding gene alignments, and (II) the four GUIDANCE2 alignments + the three protein coding gene alignments. The final alignments are included in Supplemental Material 1.
Partitioning schemes
We made two analyses of each of the two alignment sets, one Bayesian analysis using MrBayes and one maximum likelihood analysis using IQTree. We estimated a separate partitioning scheme for each of these four analyses. For the Bayesian analysis, we assessed the division of the two concatenated alignment sets into partitions using PartitionFinder 2.1.1. (Lanfear et al. 2017), which also allows for simultaneous estimation of models of molecular evolution for the partitions. We restricted the estimation to models implemented in MrBayes 3.2.6., used BIC for model selection, assumed linked branched lengths (= edge-proportional model) and used the ‘greedy’ algorithm (Lanfear et al. 2012). For the PASTA alignments set, we assessed the division of the concatenated alignment into 15 partitions: mtSSU, nSSU, LSU, ITS1, 5.8S, ITS2 and independent 1st, 2nd, and 3rd codon positions for each of the three protein-coding genes MCM7, RPB1 and RPB2. For the GUIDANCE2 alignment set, we assessed the division of the concatenated alignment into 13 partitions: mtSSU, nSSU, LSU, ITS (including 5.8S) and independent 1st, 2nd, and 3rd codon positions for MCM7, RPB1 and RPB2. The best model fit for the PASTA alignment set was achieved when eleven of the partitions were merged into four: (1) ITS1 + ITS2; (2) the 1st codon positions of MCM7, RPB1 and RPB2; (3) the 2nd codon positions of MCM7, RPB1 and RPB2; and (4) the 3rd codon positions of MCM7, RPB1 and RPB2. For the resulting eight partitions, GTR + Γ was selected as the best model for MCM7 1st + RPB1 1st + RPB2 1st and mtSSU; RPB2 2nd; GTR + Γ + I for ITS1 + ITS2 and MCM7 2nd + RPB1 2nd + RPB2 2nd; SYM + Γ + I for LSU; K80 + Γ + I for nSSU and MCM7 3rd + RPB1 3rd + RPB2 3rd; and K80 for 5.8S. The best model fit for the GUIDANCE2 alignment set was achieved when nine of the partitions were merged into three: (1) the 1st codon positions of MCM7, RPB1 and RPB2; (2) the 2nd codon positions of MCM7, RPB1 and RPB2; and (3) the 3rd codon positions of MCM7, RPB1 and RPB2. For the resulting seven partitions, GTR + Γ was selected as the best model for MCM7 1st + RPB1 1st + RPB2 1st and mtSSU; GTR + Γ + I for MCM7 2nd + RPB1 2nd + RPB2 2nd; SYM + Γ + I for LSU; and K80 + Γ + I for ITS, nSSU and MCM7 3rd + RPB1 3rd + RPB2 3rd.
For the maximum likelihood analyses, we assessed partitioning schemes and their models of molecular evolution with ModelFinder as implemented in IQTree (Kalyaanamoorthy et al. 2017). For each of the two alignment sets, we assessed the same 15 and 13 partitions as for the Bayesian analysis. The best model fit for the PASTA alignment set was achieved when ten of the partitions were merged into three: (1) 5.8S + RPB1 2nd + RPB2 2nd + MCM7 2nd; (2) RPB1 1st + RPB2 1st + MCM7 1st; and (3) RPB1 3rd + RPB2 3rd + MCM7 3rd. For the resulting eight partitions, TIM2e+I+G4 was selected as the best model for ITS1 and RPB1 3rd + RPB2 3rd + MCM7 3rd; TPM3u+F+I+G4 for 5.8S + RPB1 2nd + RPB2 2nd + MCM7 2nd; TNe+G4 for ITS2; TIM2+F+G4 for RPB1 1st + RPB2 1st + MCM7 1st; TVM+F+G4 for mtSSU; TIM3e+I+G4 for LSU; and K2P+I+G4 for nSSU. The best model fit for the GUIDANCE2 alignment set was achieved when eleven of the partitions were merged into four: (1) the 1st codon positions of MCM7, RPB1 and RPB2; (2) the 2nd codon positions of MCM7, RPB1 and RPB2; (3) the 3rd codon positions of MCM7, RPB1 and RPB2; and (4) LSU + nSSU. For the resulting six partitions, TNe+I+G4 was selected as the best model for ITS; TIM2+F+G4 for RPB1 1st + RPB2 1st + MCM7 1st; TPM3+F+I+G4 for RPB1 2nd + RPB2 2nd + MCM7 2nd; TIM2e+I+G4 for RPB1 3rd + RPB2 3rd + MCM7 3rd; TVM+F+G4 for mtSSU; and TNe+I+G4 for LSU + nSSU.
Phylogenetic analysis
We performed phylogenetic analyses of the two concatenated, partitioned alignment sets, implementing the models of molecular evolution from PartitionFinder using MrBayes 3.2.6. (Ronquist et al. 2012). We used flat Dirichlet priors for the substitution rates and state frequencies, an exponential (1) distribution for the gamma shape parameter and uniform distributions for invariant sites and topology. In test runs using maximum likelihood analysis, the total tree length for the PASTA alignment set was found to be ≈ 3.5. We therefore adjusted the branch length prior to a compound Dirichlet prior with α = 1 and β = 0.29. Likewise, the total tree length for the GUIDANCE2 alignment set was ≈ 4, and the branch length prior for that analysis thus set to α = 1 and β = 0.25. We performed two runs of four MCMC chains each, three heated and one cold. For initial test runs, we set the temperature of the heated chains to 0.20 but reduced this to 0.10 to improve swapping rates. The sample frequency was set to every 100th generation and the fraction of trees discarded as burn-in was set to 25%. We ran the analysis for a fixed number of generations and convergence was then assessed using the convenience package in R vers. 4.2.1., both in terms of checking whether enough samples had been obtained and whether these represent the true posterior distribution (Guimarães Fabreti and Höhna 2022). If any parameter has been sampled less than 625 times, convenience will assess convergence as having failed. We considered a posterior probability (PP) of 0.95 or higher as high support.
In addition to the Bayesian analysis, we also performed maximum likelihood analyses of the concatenated alignment with IQTree vers. 1.6.12 (Chernomor et al. 2016; Nguyen et al. 2015), using the partitioning scheme and models of molecular evolution from ModelFinder. As in the Bayesian analysis, we used edge-proportional partition models. We assessed branch support by running 500 non-parametric bootstrap replicates. We considered a bootstrap support (BS) of 75 or higher as high support.
Results
We generated 68 new sequences (Table 1). The final PASTA alignment set included 65 terminals, 208 sequences and had 9228 characters, 1778 of which were parsimony-informative. The final GUIDANCE2 alignment set had 8731 characters, 1511 of which were parsimony-informative. The Bayesian analysis of the PASTA alignment set was halted after running for 25 million generations, resulting in a posterior of 375,002 samples. Assessment of convergence using the convenience package indicated that convergence had been achieved. The Bayesian analysis of the GUIDANCE2 alignment set was likewise halted after 25 million generations, but the convergence assessment indicated that convergence had failed. The analysis was therefore continued for an additional 15 million generations, after which convergence had been achieved and the resulting posterior consisted of 600,002 samples.
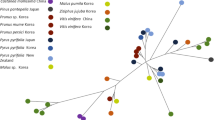
We found high support for a monophyletic new genus within the Sphaerophoraceae and describe this as the new genus Gilbertaria below. Comparisons of analysis results for the two different alignment sets showed no important differences, with the support for the node uniting the Sphaerophoraeceae being high across all four analyses (PASTA alignment set: PP = 1, BS = 99; GUIDANCE2 alignment set: PP = 1, BS = 100), as was the support for the node uniting Gilbertaria (PASTA alignment set: PP = 1, BS = 100; GUIDANCE2 alignment set: PP = 1, BS = 100). Because of the similar results, only the results from the analyses of the PASTA alignment set are presented in Fig. 1.
Majority-rule consensus tree based on a Bayesian MCMC analysis of PASTA alignment set of mrSSU, nSSU, LSU, ITS, MCM7, RPB1 and RPB2, showing the phylogenetic position of Gilbertaria in the Sphaerophoraceae. Branch support is given as posterior probability (PP)/bootstrap support (BS). Bootstrap support values are from a corresponding maximum likelihood analysis. Only BS values > 70% are shown
Within the genus Gilbertaria, we found high support for the existence of at least five distinct species (Fig. 1). Four of these correspond to entities possible to delimit anatomically, and we revise three of these species and describe one as new below. A fifth terminal (Gilbertaria sp. in Fig. 1) could represent an additional, undescribed species.
Taxonomy
Gilbertaria M. Svensson & Fryday, gen. nov. Figs. 2a–g, 3a–j
Gilbertaria astrapeana and G. contristans. a Gilbertaria astrapeana, (Fryday 10914, holotype, MSC). b Smaller (younger) form of G. contristans with sessile apothecia (Malme s.n., S F332038). c Mature form of Gilbertaria contristans with large and flat apothecia (Malme s.n., S F331995). d G. contristans, ascospores (Malme s.n., S F331975). e G. contristans, thallus with soralium (Westberg UCDLR102, MSC). f G. astrapeana, ascospores (Fryday 10914, isotype, E). g G. contristans, thallus and apothecia associated with filamentous cyanobacteria (Nordin 7451, UPS). Scale bars: a, b, c, g 1 mm; e 0.4 mm; d, f 10 μm
Gilbertaria holomeloides, G. squalescens and Moriola sp. a Gilbertaria holomeloides (Svensson 3264, UPS). b Gilbertaria squalescens (Svensson 3443, UPS). c Gilbertaria holomeloides, ascospores (Westberg PAD024, UPS). d G. holomeloides, section through apothecium (Westberg PAD324b, UPS). e G. squalescens, ascospores (Svensson 3443, UPS). f G. squalescens, section through apothecium (Nordin 7451, UPS). g G. squalescens, paraphyses after treatment with K and staining in Floxine B (Westberg PAD318, UPS). h Thallus squamules of G. squalescens covered in black hyphal net of Moriola sp. (Nordin 7561, UPS). i Section of goniocysts of Moriola sp. growing on apothecium of G. squalescens (Fryday 9175, MSC). j Degenerated apothecia of G. squalescens infected by Moriola sp. (Fryday 9175, MSC). Scale bars: a, j 0.5 mm; b 1.0 mm; c, e 5 μm; d 70 μm; f 100 μm; g 7.5 μm; h 0.25 mm; i 10 μm
MB845153
Distinguished from other genera of the Sphaerophoraceae by the crustose habit and 1-septate ascospores formed in asci of the Biatora-type.
Type species: Gilbertaria contristans (Nyl.) M. Svensson & Fryday
Thallus granular-areolate, grey or very thin, gelatinous; rarely with solitary soralia, K–, C–, UV– or UV+ faintly grey. Photobiont chlorococcoid, 6–15 μm. All species are frequently associated with tufts of filamentous cyanobacteria (Stigonema sp.) that occur between areoles or in the vicinity of apothecia. Apothecia black, lecideine, more or less flat to strongly convex, usually without any discernible margin, without pruina, without crystals refracting polarized light, without any discernible boundary tissue (sensu Döring and Wedin 2000) between vegetative and regenerative tissue. Proper exciple absent or composed of sparsely branched, radiating hyphae. Hymenium 50–70 μm tall; paraphyses comparatively broad, (1.5–)2–3(–4) μm, septate, not or only slightly thickened apically, without pigmented cap, slightly branched above, sometimes anastomosing, conglutinate in a hymenial gel. Hypothecium colourless or dilute brown, rarely darker brown, massively developed (250–350 μm deep). Asci 8-spored, cylindrical to clavate, 10–45 × 4–20 μm; tholus KI+ blue with a narrow conical masse axiale, the border of the surrounding tholus KI+ darker blue (Biatora-type; cf. Hafellner 1984, 266 for illustration). Ascospores (0–)1(–3) septate, 5–18 × 2–6 μm. Conidiomata not observed by us.
Chemistry: Standard spot tests all negative and no secondary metabolites detected by HPTLC. However, the one species with a well-developed thallus (G. squalescens) is sometimes UV+ faintly grey to white. Traces of unidentified compounds were detected in some specimens, especially of G. contristans. However, these likely emanate from the substrate, as similar compounds were detected in the same specimens when only the bryophyte substrate was analyzed by HPTLC. Two pigments occur in the apothecia of Gilbertaria: one blue, K+ brighter greenish blue, N+ magenta (Cinereorufa-green sensu Meyer and Printzen 2000) and one brown, K–, N+ magenta or N+ golden-brown.
Etymology: The genus commemorates the British lichenologist Dr Oliver Gilbert (1936–2005), with the suffix ‘-aria’ from Catillaria, the genus where the generitype species was previously placed. In a series of papers between 1982 and 1995, Dr Gilbert led the effort to rediscover and document the lichen biota of the mountainous areas of the British Isles. These included an account of the outstandingly rich lichen biota of the Ben Lawers National Nature Reserve (Gilbert et al. 1988), from where the type specimen of the generitype of the new genus was collected more than a century previously.
Ecology: All Gilbertaria species are primarily alpine and grow on dead or dying bryophytes on rock-walls or in areas of late snowlie.
Notes: Within the Sphaerophoraceae, Gilbertaria is the only known genus consisting of species with a crustose growth form. The genera Bunodophoron, Calycidium, Leifidium and Sphaerophorus all further differ from Gilbertaria by forming mazaedia, in which non-septate, often pigmented ascospores are formed in prototunicate asci without any apical structures (Wedin 1993; Wedin et al. 1998). The two remaining genera of the family both have lecideine apothecia with Lecanoralean asci but are clearly different from any species of Gilbertaria. The two known species of Neophyllis both have squamulose thalli containing lichen substances, form up to 2 mm broad apothecia on top of podetia and have broad (5–13 × 4–7 μm) unicellular ascospores (Wei and Ahti 2002; Kantvilas 2022). The sole species of the genus Austropeltum, A. glareosum, has a squamulose to foliose thallus, forms large (up to 5 mm diam.), stalked apothecia on the lobe margins, and has fusiform, unicellular ascospores (Henssen et al. 1992). Both genera also have asci with an amyloid tholus penetrated by a darker-staining tube-structure (Henssen et al. 1992; Kantvilas 2022), which is different to that found in Gilbertaria.
Gilbertaria species have sometimes been confused with the type species of the genus Protomicarea, P. limosa. However, Protomicarea limosa differs from Gilbertaria by having reddish brown pigments in the apothecia, an ascus of the Psora-type with unicellular ascospores and a white thallus usually containing pannarin (Hafellner and Türk 2001; Coppins 2009). The recently described genus Micareopsis R.C. Harris & Lendemer, with the single species M. irriguata R.C. Harris & Lendemer, is somewhat similar to Gilbertaria, but differs by having an ascus of the Lecanora-type, a reddish-brown K+ purple pigment in the hypothecium and by having a thallus containing sphaerophorin (Lendemer et al. 2013).
We have not observed pycnidia on any collection of Gilbertaria but Cannon et al. (2022) described them for ‘Catillaria’ contristans as ‘black, ± immersed, the wall dark green; conidiogenous cells in chains; conidia 3.5–4 × 0.7–1 μm, bacillar’. However, one collection of G. squalescens (Fryday 9175) supported several conidiomata resembling acervuli up to 0.2 mm across with a thick roughened, ± carbonaceous upper surface, and with bacilliform conidia, 4–6 × 1 μm. These conidiomata were concentrated in a small area of the thallus and, because we have not seen them on any other collection and acervuli are not usually produced by lichenized fungi, we suspect that they may belong to a lichenicolous fungus.
Gilbertaria astrapeana Fryday & M. Svensson, sp. nov. Fig. 2a, f
MB845154
Distinguished from G. contristans and G. squalescens by the lack of a visible thallus and from G. holomeloides by the longer ascospores. Further distinguished from all three species by its DNA sequence data.
Type: Falkland Islands, West Falkland, Port Howard, Mt Maria, Lightning Rocks, 51.619028°S, 59.601849°W, 575 m, over bryophytes on S-facing rocks, 26 January 2015, A.M. Fryday 10914 (holotype: MSC [TLC: negative]; isotype: E).
Thallus effuse, covering several cm2, at most a thin, pale brown gelatinous film may be present; apothecia arising directly from the Gymnomitrion substrate. Photobiont chlorococcoid, a few cells c. 10–15 μm diam. with a thick hyaline wall 1–2 μm wide, but not definitely associated with the fungus.
Apothecia black, lecideine, strongly convex even when immature, proper margin not apparent, 0.2–0.5 mm diam; usually ± orbicular but larger apothecia becoming irregular or even slightly lobed. In section, proper exciple not apparent. Hymenium 50–70 μm tall; paraphyses 1.5–2(–2.5) μm wide, septate, cells 15–20(–25) μm long, not constricted at the septum, slightly branched especially towards the apices, which are not swollen and are immersed in a hymenial gel; upper 5–10 μm (epihymenium) patchily dark blue- (K+ greenish-blue, N+ magenta), overlayed by a few minute granules that are POL–, K–, N+ blue, lower 30–70 μm dilute grey (K+ dilute purple-brown, N+ orange-red) with dark streaks and spots (K+ blue-black, N+ magenta); lower part (subhymenium) merging imperceptibly into the hypothecium. Hypothecium massively developed (−350 μm deep), composed of randomly organized, hyaline hyphae, c. 3 μm wide with numerous dark blue streaks and spots (K+ greenish-blue, N+ magenta). All pigments ultimately N+ magenta. Asci Biatora-type, cylindrical c. 30 × 10 μm, becoming clavate and c. 15 μm wide; ascospores hyaline, 1-septate, (14–)15.4 ± 0.9(–17) × (3–)3.3 ± 0.3(–4) μm; l/b ratio (4.3–)4.7 ± 0.3(–5.1), n = 20, narrow ellipsoid with rounded, pointed attenuated apices, but with one end more rounded and the other tapering to a more pointed apex. Conidiomata not observed.
Etymology: The epithet is derived from Astrape (ἀστραπή), the Greek goddess of lightning and the type locality ‘Lightning Rocks’.
Ecology and distribution: Known only from the type collection, which occurred on a bryophyte (Gymnomitrion sp.) mat on a siliceous rock outcrop in wind-swept, alpine tundra. Associated species: Coccotrema corallinum Messuti and Lepra argentea Fryday.
Notes: The type collection was originally determined as Catillaria contristans (Fryday et al. 2019), but the lack of a visible thallus suggested it may represent a distinct taxon. This was confirmed by DNA sequence data, which showed that it was closer to G. holomeloides, which also lacks a visible thallus but has shorter ascospores. This is the only confirmed record of a species of Gilbertaria from the Southern Hemisphere (see below under G. contristans for further discussion).
Gilbertaria contristans (Nyl.) M. Svensson & Fryday, comb. nov. Fig. 2b–e, g
MB845155
≡ Lecidea contristans Nyl., Flora 48: 354 (1865). Biatorina contristans (Nyl.) Arnold, Flora 53: 473 (1870). Catillaria contristans (Nyl.) Zahlbr., Catalog. Lich. Univ. 4: 35 (1926).—Type: [Scotland,] Ben Lawers’ top, 1864-07, I. Carroll 136 (lectotype: H-Nyl 18859 = H9510370!, designated here, MBT10008586; isolectotype: BM000974769!).
= Lecidea sabuletorum f. simplicior Nyl., Lich. Scand.: 206 (1861). Lecidea dufourii Ach. ex Nyl. [as ‘duforei’], Flora (Regensburg) 50: 373 (1867). Lecidea simplicior (Ach. ex Nyl.) Lindsay, J. Linn. Soc. 9: 372 (1867) [ICN 41.3]. Lecidea sabuletorum var. dufourii (Ach. ex Nyl.) Leight., Lich.- Flor. Great Brit.: 338 (1871). Bilimbia sabuletorum f. simplicior (Ach. ex Nyl.) Branth, Meddel. Grönland 3: 494. (1892). Bilimbia sphaeroides subspec. muscorum f. dufourii (Ach. ex Nyl.) Boist., Nouv. Flore. Lich. (2): 190 (1903). Bacidia dufourii (Ach. ex Nyl.) Lettau, Hedwigia 52: 133 (1912). Bilimbia trisepta f. simplicior (Ach. ex Nyl.) Vain., Acta. Soc. Faun. et Flor. Fennic. 53: 258 (1922). Catillaria dufourii (Ach. ex Nyl.) Vain. Acta. Soc. Faun. et Flor. Fennic. 53: 140 (1922). Thalloidima dufourii (Ach. ex Nyl.) Hav., Bergens Mus. Årbok, Naturv. Raekke 2: 41 (1936).—Type: [Sweden, Härjedalen,] Funnesdalsberget [= Funäsdalsberget], [no date,] Thedenius (lectotype: H-Nyl 18849 = H9510371!, designated here, MBT10008587).
= Lecidea hypocyanea Stirt., Scot. Natural. 5: 218 (1879), non Lecidea hypocyanea Vain. nom. illeg. [ICN Art. 53.1], Lichenogr. Fenn. 4: 300 (1934).—Type: Scotland, East Clough[?], Ben Lawers, 1874-08, [J. Stirton] (holotype: BM 000974761!).
Thallus rarely more than 1–2 cm across, light grey to lead grey, often discoloured light yellowish to light yellowish brown in old herbarium material, composed of smooth shiny granules 0.2–0.3 mm across, sometimes becoming proliferate and forming shortly branched areoles to 0.8 mm that may form a confluent crust to 1.5 mm across, K–, C–, Pd–, UV–. Cortex up to 20 μm thick, composed of hyaline, horizontally arranged cells c. 10 × 4 μm. Areoles rarely bursting open to form blue-green soralia 0.2–0.3 mm across. Photobiont chlorococcoid, cells 7–12(–14) μm diam.
Apothecia black, shiny, convex and marginless when young, becoming flatter when mature, immersed between areoles or sessile, (0.4–)0.7–0.9 mm diam, often with an irregular outline and/or appearing to be formed from several confluent apothecia, 1.2–1.5 mm across. In section, proper exciple not clearly differentiated from the hymenium, ± hyaline or pale blue internally becoming increasingly blue pigmented outwards with the outer 5–10 μm often strongly blue pigmented; composed of radiating, sparsely branched and anastomosing hyphae 1.5–2.5 μm wide laterally, becoming less well organized below the hypothecium with hyphae up to 4 μm wide. Hymenium 60–70 μm tall, ± hyaline or pale blue with the upper 5–10 μm (epihymenium) strongly blue pigmented (K+ brighter greenish blue, N+ magenta), often with a thin (< 5 μm) epipsamma; paraphyses conglutinated even in K, ± simple, sometimes branched near the apex, septate with cells 15–20 μm long, only slightly (or not at all) constricted at the septum, 1.5–3.0 μm wide, not or only slightly widened at the apex. Hypothecium hyaline to dilute brown (K–), c. 250 μm deep, composed of randomly arranged hyphae c. 3 μm wide. Asci Biatora-type, cylindrical, c. 30 × 10 μm; ascospores hyaline, (0–)1(–3)-septate, irregularly biseriate in ascus, (9.5–)13.3 ± 2.0(–18) × (2.5–)4.1 ± 0.8(–6) μm; l/b ratio (1.7–)3.41 ± 0.9(–6), n = 75, ellipsoid, often ± dacryoid with one end rounded and the other tapering to a narrow, rounded point. Conidiomata not observed.
Ecology and distribution: Gilbertaria contristans is an alpine species that grows on tufts of dead bryophytes (e.g. Andreaea Hedw., Racomitrium Brid.) on acid rocks, including seepages. Associated species include Buellia chionea (Th.Fr.) Sheard, Frutidella caesioatra (Schaer.) Kalb, and Stereocaulon spp.
Gilbertaria contristans is known from Northern and Central Europe as well as Greenland, but its status in North America is uncertain. It was first reported for the continent by Miller et al. (2005) and confirmed by Hinds et al. (2009) but we re-examined the specimens upon which those reports were based and they do not appear to represent a species of Gilbertaria. It was also reported from Alaska by Dillman et al. (2012) but the specimen upon which this report is based (Thor 8863, in S) was not available for study. Other collections from the USA (Alaska) and Canada identified as this species are included in the Consortium of North American Lichen Herbaria database (https://lichenportal.org/cnalh/). One of these collections (from Québec) is a misidentification of G. holomeloides (see below), and another from Alaska (in ASU) is Micarea turfosa (A.Massal.) Du Rietz and it is probable that G. contristans has not been correctly reported from the continent. Although Gilbertaria contristans has been reported from Australia (Tasmania) that report is based on a misidentification (see below). It has been reported from New Zealand (Galloway 2007) and although we have not seen this collection, Galloway’s description of the habitat as ‘on soil in snowbanks’ and apothecia as ‘convex to subglobose’ suggests it is more likely to be G. astrapeana or G. holomeloides. However, the description of the thallus as ‘warty granular’ excludes both of these species. It was also reported from the South Shetland Islands by Søchting et al. (2004) and, although we have not examined the specimens, macrophotographs of these collections likely represent a Micarea sp.
Notes: We located two syntypes of Lecidea contristans. The collection BM00974769 is very small and contains only 2–3 apothecia. As the collection H-Nyl 18859 is somewhat larger, we designate this collection as the lectotype.
When describing Lecidea sabuletorum f. simplicior, Nylander (1861) referred to material from at least three localities: ‘supra muscos ad Helsingfors [ipse] et in Sueciae montibus [Thedenius] atque supra terram in Lapponia’. We found two collections that are likely part of the original material under the name Lecidea sabuletorum f. simplicior in H-Nyl, namely a Thedenius collection (H-Nyl 18849) and a collection from ‘Rossia Lapponia’ made by G. Selin in 1861 (H-Nyl 18841). The material collected in Helsinki cited by Nylander (1861) was not found in H-Nyl. However, this collection was apparently examined by Fries (1874), who, based on this, reduced L. sabuletorum f. simplicior to synonymy with Lecidea verrucula Norman (= Micarea turfosa). We located a collection with the label ‘Helsingfors prope rivum Tali, W. Nylander 1851’ in UPS (L-774560!), which likely is the same collection as the one cited in the protologue of Nylander (1861). This collection indeed represents Micarea turfosa, whereas the collections H-Nyl 18849 and H-Nyl 18841 are both referable to G. contristans (see also Coppins 1983: 200). We view a lectotypification on one of the latter two collections as preferable, as that would be more in line with how the name has been used. As the Thedenius collection is the larger of the two, we designate this as the lectotype of L. sabuletorum f. simplicior.
Lecidea sabuletorum f. simplicior was subsequently raised to the level of species by Nylander (1867, as Lecidea dufourei) and possibly also by Lindsay (1867, as Lecidea simplicior). In the latter case, Lindsay did not directly cite the basionym, but the name could still be legitimate because he gave the authority of his name as ‘Nyl. MS’ and also mentioned that it could be a mere variety of Lecidea sabuletorum, which could be considered an indirect reference to the basionym thus validating the name (ICN Art 41.3). Anyway, Lindsay’s L. simplicior has, as far as we know, never been in use.
When raising Lecidea sabuletorum f. simplicior to the level of species, Nylander (1867) chose the new epithet ‘dufourei’, an unpublished name that he ascribed to Acharius (various spellings of this epithet occur in the literature; both above and in the following we have chosen to correct the spelling to ‘dufourii’ following ICN Art. 60.8). The status of Lecidea dufourii is that of a replacement name for L. sabuletorum f. simplicior, both because the wording in the protologue suggests that this was Nylander’s intention (ICN Art. 6.11), but also because L. dufourii was validated only by reference to the earlier description of L. sabuletorum f. simplicior (ICN Art. 6.12). Consequently, the designated lectotype of L. sabuletorum f. simplicior is also the lectotype of L. dufourii (ICN Art. 7.4). The material cited by Nylander (1867) when introducing the name L. dufourii (likely corresponding to H-ACH 442, its smaller duplicate UPS-ACH L-00423, and H-Nyl 18848a, b and c) is therefore not part of the original material of L. dufourii. Although it does not matter for the typification, we note that the poorly developed material in H-ACH and UPS-ACH represents a species with a dark thallus growing on calcareous rock, which is not conspecific with (or even closely related to) H-Nyl 18848a, b or c, which all are G. contristans.
In the past, there has been much confusion regarding the delimitation of Gilbertaria contristans and G. squalescens, with old herbarium collections of these two species seemingly randomly assigned to either of these two epithets (or their synonyms). We have found several examples of collections where the species occur in mixtures or are glued next to each other, apparently because the collector considered them to be of the same species. Several taxonomists have also treated them as one species, for example Fries (1874, as Toninia squalescens) and Körber (Koerber 1861, as Catillaria sphaeralis, where the original material includes both species). In contrast, modern floras (e.g. Foucard 2001; Fletcher and Coppins 2009; Hitch et al. 2009) tend to accept both species, but without pointing out that they are similar to each other. As we show here, G. contristans and G. squalescens are well separated both morphologically and genetically. Gilbertaria contristans differs from G. squalescens in having a thallus composed of smaller areoles that often forms a confluent crust, as opposed to a thallus of larger, bullate areoles in G. squalescens. Gilbertaria contristans may, unlike G. squalescens, also form soralia, a feature which we report for the first time here. However, these are too rare to be of much use for species determinations. The apothecia in G. contristans tend to be rather flat and are immersed between areoles or sessile, whereas the apothecia in G. squalescens are mostly strongly convex with a ± constricted base. Finally, the ascospores in G. contristans are on average longer (usually > 10 μm; < 10 μm in G. squalescens) and have a higher length/breadth ratio (c. 3.5; 2.5 in G. squalescens).
Gilbertaria contristans may also be confused with other crustose lichens with 1-septate ascospores and similar pigmentation. Material from Tasmania reported as Catillaria contristans, and from which sequences were used in the phylogenetic analysis of Andersen and Ekman (2005), actually represents Micarea oreina (Kantvilas and Coppins 2019). This species differs from G. contristans by, for example, having thin, anastomosing paraphyses 1–1.7(−2) μm wide, broader ascospores (−8 μm wide) and a ascus of the Micarea-type (Kantvilas and Coppins 2019). Other species of Micarea, for example M. lignaria (Ach.) Hedl. and M. viridiatra Coppins, may occur in the same habitat as G. contristans, but can also be distinguished by having thin, anastomosing paraphyses and a ‘micareoid’ (4–8 μm in diam.) photobiont. A further species with 1-septate ascospores and pigmentation similar to G. contristans is Megalaria jemtlandica (Th.Fr. & Almq.) Fryday, which differs by having larger ascospores (16–26 × 7–10 μm; Fryday 2004a).
According to Cannon et al. (2022), G. contristans may have immersed pycnidia, containing conidia 3.5–4 × 0.7–1 μm.
Selected additional specimens examined: Finland. Inari Lapland: Inari par., Pitkävuono, 1870-09, F. Silén 300 (H9221063). Utsjoki par., Kevo Subarctic Research Station c. 3 km SW, cliff Kotkapahta in Kevojoki valley, on Andreaea in seepage, 1965-08-20, T. Ahti 20913 (H 9221061). Lapponia Enontekiensis: Enontekiö par., Enontekis, Pietsovara, 1867, J.P. Norrlin (H 9221063, UPS L-707497). Enontekiö par., SW-Saena, ad rupem subcalcar. muscorum, 1949-07-21, A.J. Huuskonen (H 9221060). France. Vosgesis [= in the Vosges mountains], 1840, M. Blind (H-Nyl 18848a); Vosgesis, [no date, no collector] in herb. Lenormand (H-Nyl. 18848b). Greenland. Disko: Mellanfjorden, 1871-08-07, T.M. Fries (S F331952 [TLC: negative]). Nepisat [= Nipisat], 1871-06-21, T.M. Fries (S F331953). Iceland. Borgarfjarðarsýsla: Akrafjellet [= Akrafjall], 1937-07-28, B. Lynge (S F331958 [TLC: negative]; UPS L-797513). Vesturland: Hvammur, 1876-08-13, C. Grönlund (UPS L-797517). Snæfellsnessýsla: Fróðárheiði pass, Miðfjell and Knarrarfjall Mts, elev. 360 m, 64.50470N 23.28388W, over mosses on basaltic rock, 2009-07-22, A. Nordin 6821 (UPS L-204756). Norway. Finnmark: Werrethamn, 1802-06-18, G. Wahlenberg (UPS L-797514 [TLC: negative]). Sør-Varanger, slope E of lake Båtvatnet, elev., 120 m, UTM: NS8075 (2333 II), mossy rock in pine forest, 1986-07-22, E. Timdal 4639 (O L101196). Hordaland: Ulvik, Finse, Jomfrunut, 1916-07-19, G.E. Du Rietz (UPS L-604110). Nordland: Nordal hd, Geiranger, elev. 100 m, on moss, 1947-07-10, A.H. Magnusson 20807 (UPS L-145351). Vega par., Vega Island, Eidem, hill S of the old farm, exposed rock, 1976-06-15, G. Degelius V-1568 (UPS L-72595). Sogn og Fjordane: Flora, Levrasundet, on mosses, 1977-06-29, S.W. Sundell 11817 (UPS L-710818). Sør-Trøndelag: Oppdal, Dovre, Jerkind, Geteryggen, 1863, T.M. Fries (with G. squalescens, UPS L-797509). Ørland, Vardeheia, elev. 80-150 m, UTM (WGS84): NR 368-372 662-663 (map: 1522 II), on Andreaea sp., 1997-07-15, R. Haugan 7443 (O-L119417). Russia. Lapponia Orientalis: 1861, G. Selin (H-Nyl 18841). Sweden. Härjedalen: Ljusnedal par., Anåfjället (Ånnfjället), klipphylla nära krönet 1301, 1950-08-09, S.W. Sundell 123-50 (UPS L-709450). Tännäs par., Funnäsdalsberget, 1866, S. Almquist (with G. squalescens, S F332018). Jämtland: Åre par., Handöl, 1873, S. Almquist & E. Almquist (S F332032 [TLC: negative]); Handölsfallen, 1913-08-31, G.E. Du Rietz (UPS L-167818 [TLC: negative]); Täljstensberget, 1913-07-29, G.E. Du Rietz (UPS L-167819 [TLC: negative]). Åre par., Snasahögen, blockhav i övre regio alpina, 1913-07-12, G.E. Du Rietz (S F332036; UPS L-167817 [TLC: negative]); 1910-07-23, G.O. Malme (S F331975); 1913-06, G.O. Malme (S F332038); 1914-06-14, G.O. Malme (S F331993 [TLC: negative]); Skurdalsporten, 1914-06-25, G.O. Malme (S F331995 [TLC: negative]). Undersåker par., Vällista [= Mt. Välliste], 1913-06-15, G.O. Malme (S F331990 [TLC: negative]); 1914-06, E.P. Vrang (with G. squalescens, UPS L-797489 [TLC: negative]). Undersåker par., Mt. Middagsvalen, ca 2 km SE of Vallbo, ca 200 m S of the highest part, elev. 860 m, 63°14090N, 13°04617E, on flat, mossy rock, 2013-08-16, A. Nordin 7573 (UPS L-632796). Undersåker par., Gåsåliden, close to the path from Edsåsen, c. 7.5 km SW of Undersåker, elev. 850, 63°27854N, 13°12772E, on bryophytes on horizontal rock, 2015-08-24, A. Nordin 7877 (UPS L-722977). Åre par., Mt.Täljstensvalen, at the summit, 63°2427N, 12°4604E, on mosses on rock, 2018-07-24, M. Westberg, UCDLR102 (UPS L-931311, MSC). Lule Lappmark: Gällivare par., Dundret, elev. 800 m, 1921-08-11. A.H. Magnusson 6318 (UPS L-797498). Kvikkjokk par., in saxis muscosis infra Skärfi [= Skierfe], 1871, P.J Hellbom & E.V.M. Hellbom (with G. squalescens, S F332030). Torne Lappmark: Jukkasjärvi par., Abiskosuolo, on irrigated rocks on an islet, 1921-07-08, A.H. Magnusson 5578 (S F332028; S F332022 [TLC: negative]; UPS L-797494); on steep rock, to the north (UPS L-71954). United Kingdom. [Wales:] Cader [= Cadair] Idris, [no date, W.A. Leighton?] no. 115 (H-Nyl 18848c).
Exsiccata: Iceland. Arnesýsla: valley of the Sauoá river, N of the Gafudalur farmstead 2 km N of the village of Hveragerdi, lat/long 64°01N, 21°12E, grid: RN 7016800 154230, alt. 80 m, growing on cushions of Andreaea on steep faces of big boulders situated at the edge of the stream, 1979-06-28, H. Hertel 21165 (Hertel, Lecid. Exs. No 342, UPS L-125823). Slovakia. Carpati, Tatra Magna, in valle ‘Velická dolina’, supra lacum ‘Velické pleso’ dictum, alt. 1850 m.s.m., ad rupes graniticas, supra pulvinos musorum, 1969-09-10, A. Vêzda (as Catillaria dufourei, Vêzda, Lich. Sel. Exs. No 856, S F330939; UPS L-024587). Sweden. Jämtland: Snasahögen, 1912-07-12, G.O.A. Malme (as Toninia squalescens, Malme, Lich. Suec. Exs. No 314, H; UPS L-108908).
Gilbertaria holomeloides (Nyl.) M. Svensson & Fryday, comb. nov. Fig. 3a, c, d
MB845156
≡ Lecidea holomeloides Nyl., Flora 49: 369 (1866). Biatorina holomeloides (Nyl.) Arnold, Flora 53 (30–31): 474 (1871).—Type: [United Kingdom, Scotland,] Ben Lawers, [no date], [T.] Jones (holotype or syntype: H-Nyl 19139 = H9510514!).
Thallus widespreading but effuse, at most a thin, pale greyish to brown gelatinous film may be present; apothecia arising directly from the Gymnomitrion substrate. Photobiont chlorococcoid, 8–12(–16) μm with a thick hyaline wall 1–2 μm; thin-walled chlorococcoid algae 4–6 μm sometimes also occur.
Apothecia black, lecideine, sessile with a ± constricted base, strongly convex even when immature, orbicular 0.2–0.3(–0.5) mm diam., proper margin not apparent. In section, proper exciple not apparent. Hymenium 45–70 μm tall, hyaline or rarely light blue-green; paraphyses mostly (1–)1.5–2.5 μm wide, occasionally with solitary thicker paraphyses (− 3.5 μm wide) interspersed among the narrower, more numerous ones, septate (cells 10–15 μm long), not or slightly constricted at the septa, often forked towards the apices, which are not swollen and immersed in a hymenial gel, sometimes anastomosing in their lower part; upper 5–10 μm (epihymenium) patchily blue (K+ brighter greenish-blue, N+ magenta), the pigment often confined to a gel-matrix above the paraphyse apices; lower part (subhymenium) merging imperceptibly into the hypothecium. Hypothecium massively developed (−250 μm deep), composed of randomly organized, hyaline hyphae c. 3 μm wide, patchily light-brown, rarely darker brown (K–), often with numerous dark blue-black streaks and spots (K+ blue-black, N+ magenta). All pigments ultimately N+ magenta. Asci Biatora-type, cylindrical c. 45 × 10 μm, becoming clavate when mature, 25 × 20 μm; ascospores hyaline, (0–)1-septate, (9–)11.7±1.7(–15) × (2.0–)3.4 ± 0.7(–4.5) μm; l/b ratio (2.2–)3.6 ± 0.3(−4.7), n = 37, straight or slightly bent, narrow ellipsoid with bluntly pointed apices. Conidiomata not observed.
Ecology and distribution: Gilbertaria holomeloides is a pioneer species of arctic-alpine environments, typically growing on moribund Gymnomitrion mats in areas of late snow-lie. Accompanying lichen species are few or absent but may include Ameliella grisea Fryday & Coppins and small thalli of, for example, Solorina crocea (L.) Ach. and Thamnolia vermicularis (Sw.) Schaer. Gilbertaria holomeloides is currently known from the UK (Scotland), Sweden and Canada (Québec). In both Canada and the UK, it is known from only a single locality but in Sweden the species appears to be quite common. There may also be other collections that have been incorrectly identified as Catillaria (or Lecidea) contristans, such as the exsiccata specimen from the U.K. cited below. However, G. holomeloides has likely been overlooked in the past due to its small size and because of growing on a substrate where few other lichens occur. The species is likely to be much more widely distributed than the records listed below indicate.
Notes: Nylander (1865, 1866) described Lecidea contristans and L. holomeloides from the same locality (Ben Lawers), but without comparing the two species. This, taken together with the anatomical similarity of the apothecia, probably contributed to L. holomeloides soon being treated as a synonym of L. contristans. As is clear from our phylogeny (Fig. 1), the two species are indeed closely related but well separated genetically. The most obvious morphological differences between G. contristans and G. holomeloides are the absence of a well-developed thallus and the smaller apothecia and ascospores in G. holomeloides. The ecology of the two species is also different as G. contristans grows in more dry situations on dying bryophytes on, for example, sloping rock-walls, whereas G. holomeloides tends to grow in more humid situations on the ground. Gilbertaria astrapeana is very similar to G. holomeloides but differs by having larger ascospores.
With its small, black and immarginate apothecia, Gilbertaria holomeloides is possible to mistake for a species of Micarea. The most similar species in that genus is Micarea viridiatra, which may grow in the same habitat as G. holomeloides and has similar pigmentation as well as 1-septate ascospores of about the same dimensions (Coppins 1985). It differs by having thin (1.3–1.7 μm wide), more richly branched and anastomosing paraphyses, by having pycnidia (unknown in G. holomeloides), by the absence of any brown pigment in the apothecia (Coppins 1985) and an ascus of the Micarea-type (our observations). Megalaria jemtlandica is also superficially similar to G. holomeloides and may have a thin and varnish-like thallus when growing on dead Gymnomitrion but differs by having much larger ascospores (16–26 × 7–10 μm; Fryday 2004a).
Additional specimens examined: Canada. Québec: Nunavik, Detroit d’Hudson, 5.9 km au sud-est du fond de l’anse Erik, 62°493000N, 77°30599W, 263 m., avec Gymnomitrion, paroi ferrugineuse d’exposition ouest, 2015-08-10, J. Gagnon 127.15c (QFA). Sweden. Jämtland: Undersåker par., about 3 km NW of Storulvån Mountain Lodge, the E top of Mt. Getryggen (Tjiejhtenrudtje), 63°183227N, 12°321799E, SWEREF99: 7008818 365221, elev. 1375 m, wind-swept alpine mountain top, on mosses on the ground, 2017-08-14, M. Svensson 3264 (UPS [TLC: negative]). Undersåker par., Mt. Välliste, just N of the track on the NE part of the mountain, 63°27221N, 13°14537E, SWEREF99: 7017262 406946, elev. 965 m, on dead or dying bryophytes on the ground, 2019-06-26, M. Svensson 3493 (MSC; UPS [TLC: negative]). Undersåker par., Sylarna, about 400 m NW of Sylarna Mountain Station, 63°04496N, 12°26758E, SWEREF99: 6993534 361838, elev. 1040 m, on soil and mosses in area of late snow-lie, 2019-07-03, M. Svensson 3513 (UPS [TLC: negative]). Lule Lappmark: Jokkmokk par., Padejlanta National Park, 11 km WNW of Staloluokta, N of the river Duvggejåhkå (Tukijåkkå, Tokijokk), between Mt. Stuorra Duvgge (Stuor Tokivare, Stuorra Tuki) and Mt. Unna Duvgge (Unna Toki, Unna Tuki), bare mountain region, 210 m E of small cabin, 67.34502, 16.4521, elev. 678 m, on bryophytes on small hill, 2020-07-31, M. Westberg PAD024 (UPS L-1019033); ibid., E slope of Mt. Unna Duvgge, bare mountain region, 1040 m NE of small cabin, 67.35321, 16.45813, elev. 791 m, on bryophytes on sloping siliceous rock, 2020-08-04, M. Westberg PAD324b (UPS L-1019036). Pite Lappmark: Arjeplog par., ridge along the NW part of the lake Ikkesjávrre close to the border to Norway, on mosses on boulder in a late snow area, 66°53N, 16°01E, 2017-08-25, M. Westberg, L. Hedenäs & G. Odelvik PL336 (in collection of Ameliella grisea, UPS L-930873). Torne Lappmark: Jukkasjärvi par., Mt. Vassitjåkka (= Vássečohkka), the top plateau, elev. 1490 m, 68°38138N, 18°27508E, på jord och klipphyllor på toppskulten, 1943-08-22, G.E. Du Rietz (UPS L-953210).
Exsiccatum: United Kingdom. Scotland: [Mid Perthshire (VC 88):] supra terram prope cacumen montis Scotiae Ben Lawers, J.M. Crombie (as Lecidea contristans, Crombie, Lich. Brit. Exs. No. 177, UPS L-724606).
Gilbertaria squalescens (Nyl.) M. Svensson & Fryday, comb. nov. Fig. 3b, e–j
MB845157
≡ Lecidea squalescens Nyl., Öfvers. Kgl. Vetensk.-Akad. Förhandl. 17: 297 (1860). Toninia squalescens (Nyl.) Th.Fr., Lich. Scand.: 340 (1874). Thalloedema [sic] squalescens (Nyl.) Stein in Cohn, Kryptog.-Flora von Schlesien 2 (2H): 174 (1879).—Type: [Norway] Dovre, [no date; on the isolectotype in UPS given as 1844] W.P. Schimper (lectotype: H-Nyl 18840 = H9510353!, designated by Timdal 1991 [ICN Art. 9.10]; isolectotypes: TUR-V 20271!, UPS L-797502!).
= Thalloidima rimulosum Th.Fr., Lich. Arct.: 174 (1860).—Type: [Norway,] Finnmark, Syd-Varanger, Jarfjorden, 1857-08-21, T.M. Fries (lectotype: UPS L-22985!, designated by Timdal 1991 [ICN Art. 9.10]; isolectotype: S F331961!).
= Catillaria sphaeralis Körb., Parerg. Lich.: 196 (1861). Buellia sphaeralis (Körb.) Anzi, Atti Soc. Ital. Sc. Natur. 9: 252 (1866). Biatorina sphaeralis (Körb.) Jatta, Sylloge Lich. Ital. Cryptog. Pars III: 565 (1911). Bacidia sphaeralis (Körb.) Erichsen, Flechtenfl. Nordwestdeutschl.: 154 (1957).—Type: [Czech Republic or Poland,] Sudetis, Schneekoppe, [no date,] [G.W.] Körber (lectotype: L-0056595, envelope marked ‘A’!. designated here, MBT10008588; isolectotypes: L-0063110!, UPS L-670616!).
= Catillaria kivakkensis Vain., Acta Soc. Faun. et Flor. Fennic. 57: 453 (1934).—Type: Rossia [= Russia], Karelia keretina, Kivakka, 1877, E. Wainio (holotype: TUR-V 22310! [TLC: negative]).
= Lecidea subramosa J. Lowe, Lloydia 2: 297 (1939).—Type: [U.S.A., New York, Adirondack Region,] Mount Marcy (near Lake Placid), elev. 4900 ft, on soil, 1933-08-23, J. L. Lowe 3130 (holotype: MICH!; Coppins and Fryday 2005).
Thallus of grey, bullate squamules 0.3–0.5(−0.8) mm diam. forming a ± contiguous crust up to 2–3 cm across, surface of squamules usually covered by a network of fine brown lines of a lichenicolous fungus, often miscoloured light yellowish to light yellowish brown in old herbarium material, K–, C–, Pd–, UV– or UV+ faintly grey or white. Cortex up to 20 μm thick, composed of hyaline, horizontally arranged cells c. 10 × 4 μm, larger squamules becoming hollow with a crust c. 0.1 mm thick. Photobiont chlorococcoid, cells 6–12 μm diam.
Apothecia black, strongly convex and marginless even when young, with a more or less constricted base; mature apothecia (0.3–)0.5–0.8 mm diam., often appearing to be formed from several confluent apothecia, 1.0–1.2 mm across. In section, proper exciple not apparent. Hymenium 50–70 μm tall; paraphyses 2–3(–4) μm wide, septate (cells 10–15 μm long), not constricted at the septum, slightly branched only towards the apices, which are not swollen and are immersed in a hymenial gel; upper 5–10 μm (epihymenium) patchily blue (K+ brighter greenish-blue, N+ magenta), the pigment extending down through the hymenium in broad streaks, usually with numerous hyphae of the lichenicolous fungus on the surface and these are also abundant at the base of the hypothecium. Hypothecium merging imperceptibly with the hymenium, massively developed (−350 μm deep), composed of randomly organized, hyaline hyphae, c. 3 μm wide, upper 50–75 μm dilute brown (K–, N+ dilute golden-brown) with darker spots, lower section ± hyaline. Asci Biatora-type, cylindrical c. 20–35 × 10 μm becoming somewhat clavate and 15 μm wide; ascospores hyaline, (0–)1(–3)-septate, (5–)8.5 ± 3.6(–14) × (2.5–)3.6 ± 0.6(–5) μm; l/b ratio (1.5–)2.5 ± 0.7(–4.7), n = 80, broadly ellipsoid, often with one end rounded and the other narrowing to a rounded point, sometimes ± dacryoid. Conidiomata not observed.
Ecology and distribution: Gilbertaria squalescens is primarily an alpine species, typically growing on tufts of bryophytes (especially Andreaea spp.) on slightly sloping, acid rock surfaces (including seepages) in open to somewhat shaded situations. Accompanying species include Frutidella caesioatra, Gilbertaria contristans, Lecanora leptacina Sommerf. and Lepraria neglecta (Nyl.) Erichsen. Gilbertaria squalescens is known from North America and far eastern Russia as well as Central and Northern Europe. It may have a northern, circumpolar distribution.
Notes: Lecidea squalescens and Thalloidima rimulosum were both described in 1860 (Fries 1860; Nylander 1860). Part 6 of Öfversigt af Kongl. Vetenskaps-akademiens förhandlingar, which contains the description of L. squalescens, has the publication date June 1860, whereas the publication of Lichenes Arctoi, in which T. rimulosum was described, took place between ‘Mai–Dec. 1860’ (Stafleu and Cowan 1976). It is thus not clear which of the two names has priority. However, in his later treatment of the species, Fries (1874) gave L. squalescens priority and listed T. rimulosum as a synonym. As long as the precise dates of publication are unknown, we accept Fries’ decision (see also Timdal 1991).
As mentioned by Liška (2013), four specimens of Catillaria sphaeralis that likely are part of the original material are available in L, one in Körber’s Typenherbar and three in his Stammherbar. In addition, we located a specimen in UPS that is likewise likely to be part of the original material (UPS L-670616). The specimen from the Typenherbar (L-0056594) is labelled ‘Lectotype’, but we are not aware of any earlier lectotypification of C. sphaeralis. It is a typical specimen of Gilbertaria contristans. Of the three specimens from the Stammherbar, one (L-0063110) is G. squalescens, one (L-0056595) contains unsorted bryophyte tufts with both G. contristans and G. squalescens and the last (L-0837153) may represent G. squalescens but is not in good condition. The very small specimen in UPS is also G. squalescens. The rather short protologue of C. sphaeralis (Koerber 1861) is consistent with both G. contristans and G. squalescens and it is likely that Körber included both species in his concept of C. sphaeralis.
Since its description in 1861, Catillaria sphaeralis has mostly been treated as a synonym, either of Catillaria contristans/dufourii (Hafellner and Türk 2016; Nimis 2016; Vainio 1934;) or of Toninia squalescens (Fries 1874). A typification of C. sphaeralis on a collection containing either G. contristans or G. squalescens would thus, in both cases, be consistent with the protologue and with how the name has been used. Since C. sphaeralis is an older name than Lecidea contristans, a typification of C. sphaeralis on that species would mean reducing the epithet contristans to synonymy. In the interests of nomenclatural stability, we view the designation of a lectotype containing G. squalescens as more appropriate. The collections L0063110 and UPS L-670616 are both very small, and we have therefore chosen to designate part of the collection L0056595 as the lectotype of C. sphaeralis. As mentioned above, this collection contains both G. contristans and G. squalescens. We have therefore sorted the two species into separate envelopes, marked ‘A’ for G. squalescens (designated as lectotype) and ‘B’ for G. contristans.
Gilbertaria squalescens has often been confused with G. contristans, see discussion under the latter species. When growing on very small tufts of Andreaea or other bryophytes, G. squalescens may sometimes occur as smaller morphs, with a less developed thalli and smaller apothecia. Such a specimen was described as the new species Catillaria kivakkensis by Vainio (1934), who noted the similarities to C. dufourii (= Gilbertaria contristans). Small specimens of G. squalescens can be distinguished from G. contristans by the shape and colour of the areoles and by the smaller (especially shorter) ascospores.
Gilbertaria squalescens could also be mistaken for Frutidella caesioatra, which likewise grows on dying bryophytes on acid rock in alpine situations. However, the thallus of the latter species is not composed of bullate areoles but of more or less isidioid granules and the apothecia are usually blue-grey pruinose. In addition, F. caesioatra clearly differs from G. squalescens anatomically by, for example, having simple ascospores, by having a red-brown pigment in the hypothecium and by having a secondary metabolite chemistry with sphaerophorin and xanthones (Gilbert 2009; Kalb 1994). Protomicarea limosa is also somewhat similar, but differs by having unicellular ascospores, a reddish brown pigment in the hypothecium, an ascus of the Psora-type and a thallus that usually contains pannarin (Coppins 2009; Hafellner and Türk 2001). Species of Micarea with similar pigmentation and well-developed thallus occurring in the same habitat (e.g. Micarea lignaria) may be distinguished by thin (ca. 1.5 μm wide), usually richly branched and anastomosing paraphyses and by other ascospore characters.
The lichenicolous fungus that occurs as a superficial, dark hyphal net on the thallus of many collections of G. squalescens appears to be a species of Moriola Norman. This fungus also infects the apothecia of the lichen, which become brown-coloured and, if the infection is heavy, distorted (Fig. 3j). The fungus exhibits a simple form of lichenisation with the hyphae forming hollow spherical structures up to 100 μm diam. that are filled with algal cells (Fig. 3i). Norman (1872) coined the name ‘goniocysts’ for these structures but this name has subsequently also been applied to a variety of other structures that comprise both fungal and algal cells (e.g. Sérusiaux 1985; Spribille et al. 2014). Similar fungi, growing on Stereocaulon spp., were previously reported as lichenicolous by Zhurbenko and Triebel (2008), and have been suggested to be species of the pyrenomycete lichenicolous genus Merismatium Zopf (Triebel 1989). However, they are also quite frequent, apparently non-lichenicolous, on damp soil and among bryophytes in alpine environments (Fryday 1997, 2004b) but are rarely collected or reported. Several species have been described (Bachmann 1925; Norman 1872) that are separated by their perithecial characters but, unfortunately, we have yet to discover any fruiting bodies on the species associated with G. squalescens. Fryday (1997), however, reported ascospores with the dimensions 15–18 × 5.5–6 μm for the free-living alpine species.
Selected additional specimens examined: Austria. Tirol: Kühthai, 1872-08 [, F. Arnold] (UPS L-170621). Canada. Québec: Nunavik, Detroit d’Hudson, 10.8 km au sud-sud-ouest du fond de la baie Kangirsukutaq, 62°38797N, 76°92493W, 415 m., dépôt glaciaire mi-versant, 2015-08-12, J. Gagnon 142.2f (QFA [TLC: negative]). Norway. Finnmark: Sør-Varanger, Elvenaes, 1864-07-28, T.M. Fries (UPS L-797515). Hordaland: Ulvik, Finse, Flakavassnuten, elev. 1700 m, 1916-07-20, G.E. Du Rietz (UPS L-599025); Flakvassnutens topp, 1916-07-20, G.E. Du Rietz (UPS L-598810). Møre og Romsdal: Rauma, Grytten hd, Trollstien, alt. 850 m, on moss, 1947-07-08, A.H. Magnusson 20717 (UPS L-797500 [TLC: negative]). Nord-Trøndelag: Rognvik hd, Mellingsfjeld, häll i reg. subalp., 1939-06-21, S. Ahlner (S F331959 [TLC: negative]). Sør-Trøndelag: Oppdal, Dovre, Vaarstien, 1863-08-06, T.M. Fries (with G. contristans, UPS L-797503, L-797504); Dovre, Jerkind, Geteryggen, 1863, T.M. Fries (with G. contristans, UPS L-797509). Nordland: Vega par., Vega Island, Sundsvoll, S of the farms, on moss on big boulder, 1973-06-24, G. Degelius V-312 (UPS L-797512 [TLC: negative]). Russia. Sibiria Septentrionalis: sinus Kongam ad fretum Bering, 64°50 lat. bor., 173° long. occid. (Greenw.), 1879-07-28-30, E. Almquist (S F331956). Sweden. Härjedalen: Storsjö par., Helagsfjällets topp, alt. 1796 m, 1913-07-18, G.E. Du Rietz (UPS L-167816 [TLC: negative]). Tännäs par., Funäsdalsberget, 1866, S. Almquist (UPS L-797487 [TLC: negative]; with G. contristans, S F332018). Jämtland: Kall par., Anjeskutan, 1914-07-07, A.H. Magnusson (UPS L-797491[TLC: negative]). Kall par., St. Mjölkvattnet, Tjouren [= Tjovren], klippa N om bron strax nedanför bron, ‘Rhizocarpon-bältet’, 1942-08-24, S. Ahlner, (S F332011 [TLC: negative]). Kall par., Sösjöfjällen, north of Näverhön, 63.96919N 12.98386E, on moss cushions on rocky ground, 2020-08-24, M. Westberg JMT131 (UPS L-1026518). Undersåker par., Mt Middagsvalen, SW slope, ca 1.5 km NW of Vallbo, elev. 855 m, 63°13983N, 13°04343E, on bryophytes, 2015-08-21, A. Nordin 7797 (UPS L-721939). Undersåker par., Vällista [= Mt. Välliste], 1914-06, E.P. Vrang (UPS L-167820 [TLC: negative]; with G. contristans, UPS L-797489). Åre par., 3.5 km SW of Oppdalsvallen, by the water fall Silverfallet, just above the tree limit, elev. 750 m, 63°25120N, 12°32646E, 2017-08-15, M. Svensson 3272 (UPS). Åre par., Åreskutan, in summe montis, 1917-08-08, G. O. Malme (S F331982 [TLC: negative]); close to path c. 150 m S of the summit, elev. 1380 m, 63°42999N, 13°09542E, on bryophytes in crevice, 2012-08-31, A. Nordin 7451 (UPS L-587792); c. 500 m SW of the summit, elev. 1325 m, 63°42836N, 13°08603E, 2013-08-15, A. Nordin 7561 (UPS L-632750 [TLC: negative]); c 570 m SW of the summit, elev. 1260 m, 63°42720N, 13°08566E, 2018-07-26, on mosses over siliceous rock, M. Westberg UCDLR149 (UPS L-931311); about 640 m S of Toppstugan, south-facing, alpine mountain slope, elev. 1265 m, 63°42539N, 13°09219E, on bryophytes on the ground, 2018-07-26, M. Svensson 3443 (UPS [TLC: negative], MSC); about 575 m SW of Toppstugan, elev. 1300 m, 63.42712N 13.08563E, on bryophytes, 2018-07-26, M. Svensson 3440 (UPS); about 570 m S of Toppstugan, elev 1275 m, 62.42544N 13.09416E, on bryophytes, 2018-07-26, M. Svensson 3442 (UPS). Åre par., Snasahögen, 1914-06-24, A.H. Magnusson (with G. contristans, UPS L-797490). Lule Lappmark: Kvikkjokk par., in saxis muscosis infra Skärfi [= Skierfe], 1871, P.J Hellbom & E.V.M. Hellbom (with G. contristans, S F332030). Torne Lappmark: Jukkasjärvi par., the valley Kärkevagge (= Gearggevággi), ca 2.5 km NNW of Lake Trollsjön, elev. 750 m, 68°24217N 18°19253E, on bryophytes on vertical rock wall of large boulder, 2019-08-01, M. Svensson 3581 (UPS [TLC: negative]). Jukkasjärvi par., 3 km N of Riksgränsen, about 750 m E of Riksröse 266c, elev. 540 m, 68°27169N 18°7425E, on bryophytes on vertical rock wall, 2019-07-31, M. Svensson 3633 (UPS [TLC: negative]). Jukkasjärvi par., Vassitjåkko [= Vássečohkka], regio alpina, on moss, 1921-07-24, A.H. Magnusson 5786a (UPS L-797496). Värmland: Gustav Adolf par., S of Stjärnudden, over thin earth on a boulder near a brook, 1980-06-09, S.W. Sundell 13882 (UPS L-122572 [TLC: negative]). Norra Finnskoga par., ca 10 km N of Höljes, 61°00N, 12°37E, elev. 320 m, on big boulders in open situation, 1986-05-31, G. Thor 6473 (UPS L-174177 [TLC: negative]). Ångermanland: Härnösand par., Härnön, 1873, S. Almquist & E. Almquist (UPS L-797488 [TLC: negative]). Åsele Lappmark: Vilhelmina par., 15 km WNW of Klimpfjäll, 120 m S of the bridge over river Saxån, the N slope of Mt Stihke, elev. 763 m, 65.11755N 14.50871E, alpine heath on rather calcareous soil, on mosses on stone slab, 2017-07-26, M. Westberg ULR128 (UPS L-880141). Switzerland. Monte Susten, ad terram, [no date,] Gisler (UPS L-170618). USA Alaska: Hoonah-Angoon District, Glacier Bay National Park, Dundas Bay, 58.3437°N, 136.3990°W, 350 m, snow bed in gully, granitic rock, 2012-07-25, A. Fryday 10156 (MSC). New Hampshire: Coos Co., Mt. Washington, low point on trail to Mt Clay, 44°16.75′N, 71°18.74′W, 1400 m, over bryophytes on east-facing rock with seepage, 2008-08-27, A. Fryday 9172 [TLC: negative], 9175 [TLC: negative], 9177 [TLC: negative] (MSC).
Exsiccata: Norway. Hordaland: Granvin par., Nesheimshorgen i Granvin, alt. 1100 m, på tyndt, delvis mosegrodd jordlag som hviler på skarvgrunn, 1937-08-02, J.J. Havaas (Havaas, Lich. Exs. Norv. No. 617, UPS L-137518). Poland. Montes Tatri Alti, vallis Dolina Roztoki, ad saxa granitica prope Siklawa, 1610 m.s.m., 1959-08-24, Z. Tobolewski (Tobolewski, Lichenotheca Polonica no. 237, S F331955 [TLC: negative]; UPS L-574300). Slovakia. Carpati, Tatra Magna, in alpe ‘Velká Svišťovka’, alt. 1800 m.s.m, 1964-05-28, supra Grimmias et Andreaeas in rupibus graniticis, A. Vězda (Vězda, Lich. Sel. Exs. No. 259, UPS L-23990).
Key to Gilbertaria
1 Thallus granular-areolate to squamulose............................ 2
1* Thallus at most a thin, gelatinous film ............................ 3
2 Thallus of grey, bullate squamules; ascospores usually < 10 μm long............................................................ G. squalescens
2* Thallus granular-areolate; ascospores usually > 10 μm long.............................................................................G. contristans
3 Ascospores 14–17 × 3–4 μm ....................... G. astrapeana
3* Ascospores 9–15 × 2–4.5 μm ................. G. holomeloides
Discussion
The taxonomic status of Catillaria contristans and Toninia squalescens has been unclear for a long time. As we demonstrate here, more than two species were hidden under these names, and they represent a previously unrecognized, but well-defined genus. The monophyly and phylogenetic position of Gilbertaria within the Sphaerophoraceae were both highly supported in all the Bayesian and maximum likelihood analyses, regardless of which alignment method was used. This indicates that our results are robust to different alignment strategies and modelling choices.
The phylogenetic position of Gilbertaria may seem surprising since no anatomical character seems to suggest an affinity to the Sphaerophoraceae. The traditional circumscription of this family includes macrolichens with prototunicate asci formed within mazaedia, such as occur in the genera Bunodophoron, Leifidium and Sphaerophorus (e.g. Wedin 1993), but studies of anatomy (Döring et al. 1999; Döring and Wedin 2000) and molecular phylogenies (Wedin and Döring 1999; Wedin et al. 2000) have both supported the emendation of the family to include the two non-mazaediate, Southern Hemipshere genera Austropeltum and Neophyllis. This enlargement made the Sphaerophoraceae more heterogeneous, including species of various growth forms and with different ascus types, different apothecial development types and different types of apothecial stalks (e.g. podetia, pseudopodetia; Döring and Wedin 2000). However, detailed developmental and morphological studies identified the presence of a trait possibly common to all members of the family: the presence of ‘boundary tissue’; that is, a tissue of sometimes pigmented, plasma-rich hyphae that forms a boundary between generative and vegetative tissue (Döring et al. 1999; Döring and Wedin 2000). The layers forming this boundary tissue have been assessed as homologous within the Sphaerophoraceae and this character was consequently suggested to be a synapomorphy for the family (Döring and Wedin 2000; Wedin et al. 2000). We have failed to identify any structure that would qualify as boundary tissue in Gilbertaria. This can at least partly be attributed to scale: in contrast to the large macrolichens of the rest of the family, Gilbertaria is a genus of small crustose lichens with a comparatively poorly developed thallus. This is especially true of two of the species (G. astrapeana and G. holomeloides) that have very poorly developed, film-like thalli. In such cases, boundaries between generative tissue (apothecia) and vegetative tissue (thalli) are a question of individual hyphae, rather than well-defined and well-differentiated layers of hyphae. The two larger species (G. contristans and G. squalescens) both have a more differentiated thallus with a discernible cortex. However, in these species, the structures are comparatively very thin (i.e. up to 20 μm thick) and there is little scope for the development of complex, layered tissue. The presence of boundary tissue within the Sphaerophoraceae may thus be connected to growth form, either as a prerequisite, or as a consequence, of the evolution of large, complex thalli.
The basal position of Gilbertaria in the Sphaerophoraceae, as well as its deviating anatomy from the other genera of the family, suggests that a separate family could be described to accommodate it. However, quite apart from the dubious benefits of erecting a new family consisting of one genus of four species, this would also leave the question of what to do with the other basal genera of the Sphaerophoraceae. Neophyllis, and especially Austropeltum, like Gilbertaria, are not only clearly different from the mazaediate genera of the rest of the Sphaerophoraceae, but also from each other. At the present time, we consider that retaining all three genera in the Sphaerophoraceae is the most prudent course of action, but this may need to be revisited in the future. Generally, a larger taxon sampling and an increased number of markers are needed to fully evaluate the boundaries and relationship of the families currently considered to belong in the Sphaerophorineae, along with others that may be shown to be referrable there by future research.
This revision formally recognizes four species of Gilbertaria, but our phylogeny indicates that more species may be involved. The ‘Catillaria contristans’ used by Kistenich et al. (2018), corresponding to Gilbertaria sp. in Fig. 1, is apparently closely related but at the same time well separated genetically from G. astrapeana, G. contristans and G. holomeloides. We have examined the specimen from which the sequences were generated (Timdal 11347, O-L163408!) and found the specimen to be similar to G. contristans, but possibly differing by having somewhat larger thallus areoles that partly appear almost coralloid. Since we have only seen one specimen that could be clearly attributed to this entity, we refrain from describing this as a new species.
There are also indications of genetic structuring within Gilbertaria squalescens, with three different sub-clades (Fig. 1): one comprising G. squalescens 1–5, one G. squalescens 7–14 and one with G. squalescens 6. The genetic differences between these three groups are consistent but very small, usually just a few bp per marker, and in the protein-coding genes restricted to the third codon position. There is no apparent correlation between the genetic pattern and geographical distribution: although most of the sequenced collections of G. squalescens are from Scandinavia, specimens from North America are present in both the larger sub-clades (G. squalescens 2 and 14). Despite thorough morphological and anatomical investigations of the sequenced specimens, we have not found any clear differences between the sub-clades, and thus prefer to treat them as one species pending further studies.
Data availability
Sequence data are deposited in GenBank. The collections cited, including all types, have been deposited in public herbaria, as indicated by herbarium acronyms. The alignments used for the phylogenetic analyses have been submitted as Supplementary material.
References
Acharius E (1803) Methodus Lichenum. C.F. Marquard, Stockholm
Andersen HL, Ekman S (2005) Disintegration of the Micareaceae (lichenized Ascomycota): a molecular phylogeny based on mitochondrial rDNA sequences. Mycol Res 109:21–30
Arup U, Ekman S, Lindblom L, Mattsson J-E (1993) High performance thin layer chromatography (HPTLC), an improved technique for screening lichen substances. Lichenologist 25:61–71
Bachmann E (1925) Die Moriolaceen. Nyt Mag Naturvid 64:170–228
Cannon P, Orange A, Aptroot A, Coppins B, Fletcher A, Fryday A, Sanderson N, Simkin J, Van den Boom P (2022) Caliciales: Catillariaceae, including the genera Catillaria and Solenopsora. Revisions of British and Irish Lichens 22:1–13
Chernomor O, von Haeseler A, Minh BQ (2016) Terrace aware data structure for phylogenomic inference from supermatrices. Syst Biol 65:995–1008
Coppins BJ (1983) A taxonomic study of the lichen genus Micarea in Europe. Bull Brit Mus (Nat Hist) Bot 11(2):17–214
Coppins BJ (1985) A new Micarea from the Scottish Highlands. Lichenologist 17:99–101
Coppins BJ (1992) Catillaria A. Massal. 1852. In: Purvis OW, Coppins BJ, Hawksworth DL, James PW, Moore DM (eds) The lichen flora of Great Britain and Ireland. Natural History Museum Publications & British Lichen Society, London, pp 166–171
Coppins BJ (2009) Protomicarea Hafellner (2001). In: Smith CW, Aptroot A, Coppins BJ, Fletcher A, Gilbert OL, James PW, Wolseley PA (eds) The lichens of Great Britain and Ireland. The British Lichen Society, London, p 751
Coppins BJ, Fryday A (2005) Reassessment of some lichen species described by Josiah Lowe, and notes on some other North American lecideoid lichens. Bryologist 109:9–17
Dillman K, Ahti T, Björk CR, Clerc P, Ekman S, Goward T, Hafellner J, Pérez-Ortega S, Printzen C, Savić S et al (2012) New records, range extensions and nomenclatural innovations for lichens and lichenicolous fungi from Alaska, U.S.A. Herzogia 25(2):177–210
Döring H, Wedin M (2000) Homology assessment of the boundary tissue in fruiting bodies of the lichen family Sphaerophoraceae (Lecanorales, Ascomycota). Plant Biol 2:361–367
Döring H, Henssen A, Wedin M (1999) Ascoma development in Neophyllis melacarpa (Lecanorales, Ascomycota), with notes on the systematic position of the genus. Aust J Bot 47:783–794
Ekman S, Blaalid R (2011) The devil in the details: interactions between the branch length prior and likelihood model affect model support and branch length in the phylogeny of the Psoraceae. Syst Biol 60:541–561
Ekman S, Andersen HL, Wedin M (2008) The limitations of ancestral state reconstruction and the evolution of the ascus in the Lecanorales (lichenized Ascomycota). Syst Biol 57:141–156
Ferencova Z, Rico VJ, Hawksworth DL (2017) Extraction of DNA from lichen-forming and lichenicolous fungi: a low-cost fast protocol using Chelex. Lichenologist 49:521–525
Fletcher A, Coppins BJ (2009) Catillaria A. Massal. (1852). In: Smith CW, Aptroot A, Coppins BJ, Fletcher A, Gilbert OL, James PW, Wolseley PA (eds) The lichens of Great Britain and Ireland. The British Lichen Society, London, pp 282–288
Foucard T (2001) Svenska skorplavar och svampar som växer på dem. Interpublishing, Stockholm
Fries TM (1860) Lichenes Arctoi Europae Groenlandiaeque hactenus cogniti. CA Leffler, Uppsala
Fries TM (1874) Lichenographia Scandinavica sive disposito lichenum in Dania, Suecia, Norvegica, Fennia, Lapponia Rossia hactenus collectorum. Pars Secunda, Berling, Uppsala
Fryday AM (1997) Ecology and taxonomy of montane lichen vegetation in the British Isles. PhD thesis, University of Sheffield
Fryday A (2004a) A new species of Fuscopannaria with a green photobiont, and other taxonomic innovations and new records of lichenized fungi from Alaska. Bryologist 107:173–179
Fryday AM (2004b) The Fifth Dougal Swinscow Memorial Lecture—Lichens of the Scottish Mountains. British Lichen Society Bulletin 94:4–7
Fryday AM, Orange A, Ahti T, Øvstedal DO, Crabtree DE (2019) Checklist of lichenized and lichenicolous fungi reported from the Falkland Islands. Glalia 8(1):1–100
Galloway DJ (2007) Flora of New Zealand. Lichens. Revised second edition including lichen-forming and lichenicolous fungi. Volume one. *Abrothallus–Pachyphiale. Manaaki Venua Pres, Lincoln
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes—application to the identification of mycorrhizae and rusts. Mol Ecol 2:113–118
Gilbert OL (2009) Frutidella Kalb (1994). In: Smith CW, Aptroot A, Coppins BJ, Fletcher A, Gilbert OL, James PW, Wolseley PA (eds) The lichens of Great Britain and Ireland. The British Lichen Society, London, p 405
Gilbert OL, Coppins BJ, Fox BW (1988) The lichen flora of Ben Lawers. Lichenologist 20:201–243
Guimarães Fabreti L, Höhna S (2022) Convergence assessment for Bayesian phylogenetic analysis using MCMC simulation. Meth Ecol Evol 13:77–90
Hafellner J (1984) Studien in Richtung einer naturlicheren Gliederung der Sammelfamilien Lecanoraceae und Lecideaceae. Beih Nova Hedwigia 79:241–371
Hafellner J, Türk R (2001) Die lichenisierten Pilze Österreichs – eine Checkliste der bisher nachgewiesenen Arten mit Verbreitungsangaben. Stapfia 76:1–167
Hafellner J, Türk R (2016) Die lichenisierten Pilze Österreichs – eine neue Checkliste der bisher nachgewiesenen Taxa mit Angaben zu Verbreitung und Substratökologie. Stapfia 104:1–184
Henssen A, Döring H, Kantvilas G (1992) Austropeltum glareosum gen. et. spec. nov., a new lichen from mountain plateaux in Tasmania and New Zealand. Bot Act 105:457–467
Hertel H (1967) Revision einiger calciphiler Formenkreise der Flechtengattung Lecidea. Beih Nova Hedwigia 24:1–155
Hertel H (1984) Über saxicole, lecideoide Flechten der Subantarktis. Beih Nov Hedw 79:399–499
Hertel H, Rambold G (1987) Miriquidica genus novum Lecanoracearum (Ascomycetes lichenisati). Mitt Bot Staatss Münch 23:377–392
Hinds JW, Fryday AM, Dibble AC (2009) Lichens and bryophytes of the alpine and subalpine zones on Katahdin, Maine, II: Lichens. Bryologist 112(4):673–703
Hitch CJB, Timdal E, James PW (2009) Toninia A. Massal. (1852). In: Smith CW, Aptroot A, Coppins BJ, Fletcher A, Gilbert OL, James PW, Wolseley PA (eds) The lichens of Great Britain and Ireland. The British Lichen Society, London, pp 895–903
Kalb K (1994) Frutidella, eine neue Flechtengattung für Lecidea caesioatra Schaerer. Hoppea 55:581–586
Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS (2017) ModelFinder: Fast model selection for accurate phylogenetic estimates. Nature Methods 14:587–589
Kantvilas G (2022) The trouble with Neophyllis pachyphylla (lichenized Ascomycetes). Swainsonia 36:1–7
Kantvilas G, Coppins BJ (2019) Studies on Micarea in Australasia II. A synopsis of the genus in Tasmania, with the description of ten new species. Lichenologist 51:431–481
Katoh K, Rozewicki J, Yamada KD (2019) MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform 20:1160–1166
Kauff F, Lutzoni F (2002) Phylogeny of the Gyalectales and Ostropales (Ascomycota, Fungi): among and within order relationships based on nuclear ribosomal RNA small and large subunits. Mol Phyl Evol 25:138–156
Kistenich S, Timdal E, Bendiksby M, Ekman S (2018) Molecular systematics and character evolution in the lichen family Ramalinaceae (Ascomycota: Lecanorales). Taxon 67:871–904
Koerber GW (1861) Parerga Lichenologica. Ergänzungen zum Systema Lichenum Germaniae. Verlag von Eduard Trewendt, Breslau
Kraichak E, Huang J-P, Nelsen M, Leavitt SD, Lumbsch HT (2018) A revised classification of orders and families in the two major subclasses of Lecanoromycetes (Ascomycota) based on a temporal approach. Bot J Linn Soc 188:233–249
Lanfear R, Calcott B, Ho SY, Guindon S (2012) PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol Biol Evol 29:1695–1701
Lanfear R, Frandsen PB, Wright AM, Senfeld T, Calcott B (2017) PartitionFinder 2: new methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol Biol Evol 34:772–773
Launis A, Pykälä J, v.d. Boom P, Sérusiaux E, Myllys L (2019) Four new epiphytic species in the Micarea prasina group from Europe. Lichenologist 51:7–25
Leavitt SD, Westberg M, Nelsen MP, Elix JA, Timdal E, Sohrabi M, St Clair LL, Williams L, Wedin M, Lumbsch HT (2018) Multiple, distinct intercontinental lineages but isolation of Australian populations in a cosmopolitan lichen-forming fungal taxon, Psora decipiens (Psoraceae, Ascomycota). Front Microbiol 9:283
Lendemer JC, Harris RC, Tripp EA (2013) The lichens and allied fungi of Great Smoky Mountains National Park: an annotated checklist with comprehensive keys. Mem New York Bot Gard 104:1–152
Lindsay WL (1867) Contributions to the lichen-flora of Northern Europe (being an enumeration of lichens collected in Iceland, the Färö islands and Norway (the Norwegia alps) in 1857 and 1860. J Linn Soc 11:391–416
Liška J (2013) The significance of Körber's “Typenherbar”, with an explanation of the locality abbreviations on his labels. Lichenologist 45:25–33
Liu YJ, Whelen S, Hall BD (1999) Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Mol Biol Evol 16:1799–1808
Lowe J (1939) The genus Lecidea in the Adirondack Mountains of New York. Lloydia 2:225–304
Meyer B, Printzen C (2000) A proposal for a standardized nomenclature and characterization of insoluble lichen pigments. Lichenologist 32:571–583
Miadlikowska J, Kauff F, Hofstetter V, Fraker E, Grube M, Hafellner J, Reeb V, Hodkinson BP, Kukwa M, Lücking R et al (2006) New insights into classification and evolution of the Lecanoromycetes (Pezizomycotina, Ascomycota) from phylogenetic analyses of three ribosomal RNA- and two protein-coding genes. Mycologia 98:1088–1103
Miadlikowska J, Kauff F, Högnabba F, Oliver JC, Molnár K, Fraker E, Gaya E, Hafellner J, Hofstetter V, Gueidan C et al (2014) A multigene phylogenetic synthesis for the class Lecanoromycetes (Ascomycota): 1307 fungi representing 1139 infrageneric taxa, 317 genera and 66 families. Mol Phyl Evol 79:132–168
Miller NG, Fryday AM, Hinds JW (2005) Bryophytes and lichens of a calcium-rich spring seep isolated on the granitic terrain of Mt. Katahdin, Maine, U.S.A. Rhodora 107:339–358
Mirarab S, Nguyen N, Guo S, Wang L-S, Kim J, Warnow T (2015) PASTA: ultra-large multiple sequence alignment for nucleotide and amino-acid sequences. J Comp Biol 22:377–386
Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ (2015) IQ-TREE: a fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Mol Biol Evol 32:268–274
Nimis PL (2016) The lichens of Italy. A second annotated catalogue. Edizioni Univ. de Trieste, Grafiche Filacorda s.r.l., Undine
Norman JM (1872) Fuligines lichenosae eller Moriolei. Bot Not 1872:9–20
Nylander W (1860) Novitiae quaedam Licheneae Norvegicae. Öfvt Kong Vet-akad Förhandl 17:295–297
Nylander W (1861) Lichenes Scandinaviae (sive Prodromus Lichenographiae Scandinaviae). Not Sälls Faun Fl Fenn Förhandl 5 (=NS 2):1–313
Nylander W (1865) Adhuc novitiae quaedam Lichenum Europae variarum tribuum. Flora (Regensburg) 48:353–358
Nylander W (1866) Addenda nova ad Lichenographiam Europæam. Continuatio altera. Flora (Regensburg) 49:369–374
Nylander W (1867) Addenda nova ad Lichenographiam Europæam. Continuatio sexta. Flora (Regensburg) 50:369–374
Orange A, James PW, White FJ (2010) Microchemical methods for the identification of lichens, Second edn. British Lichen Society, London
Prieto M, Baloch E, Tehler A, Wedin M (2013) Mazaedium evolution in the Ascomycota (Fungi) and the classification of mazaediate groups of formerly unclear relationship. Cladistics 29:296–308
Printzen C (1995) Die Flechtengattung Biatora in Europa. Bibl Lich 60:1–275
Reeb V, Lutzoni F, Roux C (2004) Contribution of RPB2 to multilocus phylogenetic studies of the Pezizomycotina (euascomycetes, Fungi) with special emphasis on the lichen-forming Acarosporaceae and evolution of polyspory. Mol Phyl Evol 32:1036–1060
Ronquist F, Teslenko M, Mark P, Ayres DL, Höhna S, Larget B, Liu L, Huelsenbeck J (2012) MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542
Schmitt I, Crespo A, Divakar PK, Fankhauser JD, Herman-Sackett E, Kalb K, Nelsen MP, Nelson NA, Rivas-Plata E, Shimp AD, Widhelm T, Lumbsch HT (2009) New primers for promising single-copy genes in fungal phylogenetics and systematics. Persoonia 23:35–40
Schmull M, Miadlikowska J, Pelzer M, Stocker-Wörgötter E, Hofstetter V, Fraker E, Hodkinson BP, Reeb V, Kukwa M, Lumbsch HT, Kauff F, Lutzoni F (2011) Phylogenetic affiliations of members of the heterogeneous lichen-forming fungi of the genus Lecidea sensu Zahlbruckner (Lecanoromycetes, Ascomycota). Mycologia 103(5):983–1003
Schneider K, Resl P, Westberg M, Spribille T (2015) A new, highly effective primer pair to exclude algae when amplifying nuclear large ribosomal subunit (LSU) DNA from lichens. Lichenologist 47:269–275
Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, Levesque CA, Chen W (2012) Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc Nat Acad Sci 109:6241–6246
Sela I, Ashkenazy H, Katoh K, Pupko T (2015) GUIDANCE2: accurate detection of unreliable alignment regions accounting for the uncertainty of multiple parameters. Nucl Ac Res 43:W7–W14
Sérusiaux E (1985) Goniocysts, goniocystangia and Opegrapha lambinonii and related species. Lichenologist 17:1–25
Søchting U, Ovstedal DO, Sancho LG (2004) The lichens of Hurd Peninsula, Livingston Island, South Shetlands, Antarctica. Bibl Lich 88:607–658
Soto EM, Prieto M, Wedin M (2018) A new Bunodophoron species (Sphaerophoraceae) from the Neotropics. Lichenologist 50:255–266
Spatafora JW, Mitchell TG, Vilgalys R (1995) Analysis of genes coding for small-subunit rRNA sequences in studying phylogenetics of dematiaceous fungal pathogens. J Clin Microbiol 33:1322–1326
Spribille T, Resl P, Ahti T, Pérez-Ortega S, Tønsberg T, Mayrhofer H, Lumbsch HT (2014) Molecular systematics of the wood-inhabiting, lichen-forming genus Xylographa (Baeomycetales, Ostropomycetidae) with eight new species. Acta Univ Upsal/Symb Bot Upsal 37(1):1–87
Spribille T, Fryday AM, Pérez-Ortega S, Svensson M, Tønsberg T, Ekman S, Holien H, Resl P, Schneider K, Stabentheiner E et al (2020) Lichens and associated fungi from Glacier Bay National Park, Alaska. Lichenologist 52:61–181
Stafleu FA, Cowan RS (1976) Taxonomic literature. A selective guide to botanical publications and collections with dates, commentaries and types, Volume I: A–G, 2nd edn. Bohn, Scheltema, & Holkema, Utrecht
Timdal E (1991) A monograph of the genus Toninia (Lecideaceae, Ascomycetes). Opera Bot 110:1–137
Triebel D (1989) Lecideicole Ascomyceten. Eine Revision der obligat lichenicolen Ascomyceten auf lecideoiden Flechten. Bibl Lichen 35:1–278
Vainio EA (1934) Lichenographia Fennia IV. Lecideales II. Act Soc Faun Fl Fenn 57(2):1–531
Wedin M (1993) A phylogenetic analysis of the lichen family Sphaerophoraceae (Caliciales); a new generic classification and notes on character evolution. Plant Syst Evol 209:75–83
Wedin M (2002) The genus Calycidium Stirt. Lichenologist 34:63–69
Wedin M, Döring H (1999) The phylogenetic relationship of the Sphaerophoraceae, Austropeltum and Neophyllis (lichenized Ascomycota) inferred by SSU rDNA sequences. Mycol Res 103:1131–1137
Wedin M, Tehler A, Gargas A (1998) Phylogenetic relationships of Sphaerophoraceae (Ascomycetes) inferred from SSU rDNA sequences. Plant Syst Evol 209:75–83
Wedin M, Döring H, Ekman S (2000) Molecular phylogeny of the lichen families Cladoniaceae, Sphaerophoraceae, and Stereocaulaceae (Lecanorales, Ascomycotina). Lichenologist 32:171–187
Wei J-C, Ahti T (2002) Cetradonia, a new genus in the new family Cetradoniaceae (Lecanorales, Ascomycota). Lichenologist 34:19–31
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M, Gelfand D, Sinsky JJ, White T (eds) PCR protocols: a guide to methods and applications. Academic Press, New York, pp 315–332
Wijayawardene NN, Hyde KD, Lumbsch HT, Liu JK, Maharachchikumbura SSN, Ekanayaka AH, Tian Q, Phookamsak R (2018) Outline of Ascomycota: 2017. Fung Div 88:167–263
Wirth V, Hauck M, Schultz M (2013) Die Flechten Deutschlands. Eugen Ulmer, Gmbh & Co, Stuttgart
Zahlbruckner A (1921–1940) Catalogus Lichenum Universalis. Band I–X. Gebrüder Bornträger, Berlin
Zhao X, Leavitt SD, Zhao ZT, Zhang LL, Arup U, Grube M, Pérez-Ortega S, Printzen C, Śliwa L, Kraichak E et al (2016) Towards a revised generic classification of lecanoroid lichens (Lecanoraceae, Ascomycota), based on molecular, morphological and chemical evidence. Fungal Divers 78:293–304
Zhou S, Stanosz GR (2001) Primers for amplification of mt SSU rDNA, and a phylogenetic study of Botryosphaeria and associated anamorphic fungi. Mycol Res 105:1033–1044
Zhurbenko MP, Triebel D (2008) Three new species of Stigmidium and Sphaerellothecium (lichenicolous ascomycetes) on Stereocaulon. Mycol Prog 7:137–145
Zoller S, Scheidegger C, Sperisen C (1999) PCR primers for the amplification of mitochondrial small subunit ribosomal DNA of lichen-forming ascomycetes. Lichenologist 31:511–516
Acknowledgements
We thank Jean Gagnon (Québec) for sharing his lichen collections from Nunavik with us, and Gintaras Kantvilas (Hobart) for providing us with fresh material of Austropeltum glareosum. We also thank Christian Lange and Nina Lundholm (Copenhagen) for the photographs of the G. contristans specimens from the South Shetland Islands, Stefan Ekman and Juan Carlos Zamora for helpful discussions and two anonymous reviewers for comments on the manuscript.
Funding
Open access funding provided by Uppsala University. Research by MS is financially supported by the Swedish Taxonomy Initiative (grant no. 2016-206 4.3).
Author information
Authors and Affiliations
Contributions
Both authors contributed to the study conception and design. Both authors collected specimens, did morphological and anatomical work, and contributed to the descriptions. MS did the molecular lab work and performed the phylogenetic analyses. The manuscript was written by both authors together. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Section Editor: Gerhard Rambold
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Svensson, M., Fryday, A.M. Gilbertaria, a first crustose genus in the Sphaerophoraceae (Lecanoromycetes, Ascomycota) for Catillaria contristans, Toninia squalescens and related species. Mycol Progress 21, 90 (2022). https://doi.org/10.1007/s11557-022-01838-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11557-022-01838-5