Abstract
Purpose of Review
Internationally, cardiovascular disease (CVD) is the leading cause of death in women. With risk factors for CVD continuing to rise, early identification and management of chronic diseases such as hypertension, diabetes, and obstructive sleep apnea is necessary for prevention. Pregnancy is a natural stress test for women with risk factors who may be predisposed to CVD and offers a unique opportunity to not only recognize disease but also implement effective and long-lasting strategies for prevention.
Recent Findings
Prevention begins before pregnancy, as preconception screening, counseling, and optimization of chronic diseases can improve maternal and fetal outcomes. Throughout pregnancy, women should maintain close follow-up, continued reevaluation of risk factors, with counseling when necessary. Continued healthcare engagement during the “fourth trimester,” 3 months following delivery, allows clinicians to continue monitoring the evolution of chronic diseases, encourage ongoing lifestyle counseling, and connect women with primary care and appropriate specialists if needed. Unfortunately, this postpartum period represents a major care gap, as a significant proportion of most women do not attend their scheduled visits. Social determinants of health including decreased access to care and economic instability lead to increased risk factors throughout pregnancy but particularly play a role in poor compliance with postpartum follow-up. The use of telemedicine clinics and remote monitoring may prove to be effective interventions, bridging the gap between physicians and patients and improving follow-up for at-risk women.
Summary
While many clinicians are beginning to understand the impact of CVD on women, screening and prevention strategies are not often implemented until much later in life. Pregnancy creates an opportunity to begin engaging women in cardiovascular protective strategies before the development of the disease.
Similar content being viewed by others
Introduction
CVD is the leading cause of pregnancy-related maternal mortality in the USA [1, 2]. Through extraordinary efforts worldwide, global maternal mortality has been decreasing steadily over the past few decades [3,4,5,6]; however, the proportion of direct and indirect maternal mortality due to CVD is on the rise [7, 8]. The rise of CVD has been particularly notable in developed countries such as the USA, which has seen an increase in maternal mortality in recent years [5, 6]. Most concerning about the rising trend is that an estimated two-thirds of CVD-related pregnancy deaths are thought to be preventable, indicating a significant lapse in surveillance and implementation of prevention strategies [9].
Pregnant women are at particular risk for CVD complications as every organ system undergoes physiologic and anatomic stress not only while fostering the growing fetus but also during the process of labor and childbirth [10]. During pregnancy and labor, cardiac output can increase by approximately 30 to 50% as a result of increased stroke volume and heart rate [10,11,12]. Many women are able to undergo this stress without significant residual effects; however, this natural “stress test” can enhance CVD in women who have underlying risk factors leading to serious complications [10].
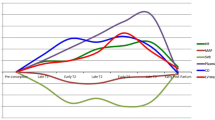
The risk of CVD has only intensified in recent years as more women are becoming pregnant later in life and traditional risk factors such as chronic hypertension, diabetes, obesity, and smoking are on the rise [13, 14]. As demonstrated in Fig. 1 below, such risk factors can increase a women’s risk for adverse pregnancy outcomes (APOs), including preterm birth, gestational diabetes mellitus, preeclampsia, gestational hypertension, small-for-gestational-age infant, and placental abruption, which are associated with increased CVD risk later in life [15, 16]. Specifically, APOs increase a women’s risk of indirect maternal adverse events such as arrhythmias, heart failure, peripartum cardiomyopathy, valvular disorders, cerebrovascular accidents (CVA), and coronary artery disease (CAD) [7, 16, 17••].
Traditional risk factors in pregnancy. Legend: SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index. Figure created via BioRender.com
The rising trends in CVD risk factors and specific preventative care strategies before, during, and after pregnancy will be the focus of our discussion below. As CVD remains the leading cause of death for women across age groups, pregnancy offers a window of opportunity for risk evaluation, stratification, and implementation of primary and secondary prevention strategies as women are more engaged with the healthcare system.
Trends of Cardiovascular Risk Factors
Chronic hypertension, diabetes mellitus, dyslipidemia, obesity, smoking, and sleep apnea are all risk factors for APOs and cardiovascular diseases in reproductive age women [10, 15, 16]. Understanding the definitions, prevalence, and associated risks of each can help women and clinicians make informed decisions about their care.
Hypertension
Chronic hypertension, or preexisting hypertension, is defined by systolic blood pressure (SBP) ≥140 and/or diastolic blood pressure (DBP) ≥ 90 mmHg before 20 weeks of gestation or having an elevated blood pressure that persists after 12 weeks or more postpartum [18]. A study by Bateman et al of over 50 million women reported that the prevalence of chronic hypertension in the USA grew from 0.9 in 1995–1996 to 1.5% in 2007–2008 [19]. Another cross-sectional analysis demonstrated almost a 13-fold increase in the prevalence of chronic hypertension since 1970, closely correlated with increasing maternal age [13]. While older maternal age is a risk factor for chronic hypertension, the rate of the disease has been increasing across all age groups [20]. Women with congenital heart defects (CHD) such as aortic coarctation are also at increased risk of preexisting hypertension and thus pregnancy-related hypertensive complications [21].
Further analysis by Cameron et al. has demonstrated that women from rural communities in the USA have a higher prevalence of preexisting hypertension than women from urban communities [22]. The rural-urban gap is compounded by other social determinants of health including racial inequity, poor health literacy, food insecurity, and economic instability, all of which are correlated with increased APOs [23]. Women with chronic hypertension are at increased risk for poor maternal and fetal outcomes [18, 19, 24]. Bateman et al. demonstrated that women with chronic hypertension are at increased risk for several adverse outcomes including preeclampsia (odds ratio [OR] 10.07; 95% confidence interval [CI] 9.68–10.48), pulmonary edema (OR 9.26; 95% CI 6.67–12.85), stillbirth (OR 2.31; 95% CI 2.11–2.53), and poor fetal growth (OR 3.00; 95% CI 2.83–3.19) [19]. This study went on to show that having concomitant comorbidities such as gestational diabetes or chronic renal disease further increased the risk of APOs [19]. A meta-analysis of 55 studies found that women with chronic hypertension have a relative risk of 7.7 (95% CI 5.7–10.1) for preeclampsia and a relative risk for preterm delivery and neonatal admission of 2.7 (95% CI 1.9 to 3.6) and 3.2 (95% CI 2.2 to 4.4), respectively [24].
Diabetes Mellitus
Approximately, 90% of diabetes cases during pregnancy are the result of gestational diabetes; however, pregestational diabetes due to type 1 and type 2 diabetes are increasing [25]. Compared to gestational diabetes, type 1 and type 2 diabetes in pregnancy have greater maternal and fetal risk, particularly when uncontrolled [26, 27]. Poor glucose control particularly during early pregnancy can lead to congenital malformations, spontaneous abortion, and stillbirths [28]. Neonates are at increased risk of a number of complications including hypoglycemia, macrosomia, respiratory distress, and hyperbilirubinemia amongst others [29]. Children born to women with diabetes are at risk of becoming obese or developing diabetes later in life [30]. Pregnancy can also lead to APOs such as preeclampsia, which can further exacerbate known diabetes complications such as retinopathy and nephropathy [25, 31, 32]. Women with pregestational diabetes are at increased risk for acute myocardial infarction and symptomatic coronary artery disease during pregnancy, particularly those with comorbidities such as nephropathy and hypertension [33, 34].
Dyslipidemia
Between 1999 and 2008, approximately 18–22% of reproductive-age women in the USA were reported to have abnormally elevated triglycerides levels [35]. During pregnancy, all plasma lipids including triglycerides, total cholesterol, and low-density lipoprotein-c (LDL-C) rise [36]. The greatest increase is typically seen in triglycerides, which can increase as much as double or triple prepregnancy levels [36]. Overall, these physiologic changes are thought to be nonatherogenic as they quickly normalize after delivery [36, 37]. However, the rise in triglycerides also has a corresponding decrease in LDL-C size creating particles that may be especially atherogenic although this is debated [38, 39]. Fetal effects of hyperlipidemia include premature birth, low birth weight, and later in life, increased risk of atherogenesis [40].
Obesity
The proportion of women who are overweight (body mass index [BMI] ≥ 25 kg/m2) or obese (BMI ≥ 30 kg/m2) has increased globally from 29.8 (CI 29.3–30.2%) in 1980 to 38.0% (CI 37.5–38.5%) in 2013 [41]. The Center for Disease Control (CDC) reports that in the USA, the number of women who were obese prior to pregnancy rose from 26.1 in 2016 to 29% in 2019; a trend that was consistent across all age groups [42]. In the USA, African American and Native American women have higher rates of obesity at 34.7% and 36.4%, respectively, compared to White women at 23.7% [43]. Obesity and excessive weight gain during pregnancy have not only been associated with increased risk of APOs (preeclampsia, gestational diabetes) [44] but also maternal mortality [45] during pregnancy. Increased neonatal and infant mortality, as well as CHDs in the fetus [46, 47], have also been associated with a higher maternal BMI [48]. The effects of maternal obesity during pregnancy extend beyond the peripartum period and have been associated with long-term complications such as major cardiovascular events (MACE), including death, myocardial infarction, stroke, and peripheral artery disease, compared to mothers with normal BMI [49].
Smoking
In the USA, the prevalence of cigarette smoking during pregnancy has decreased from 9.2 in 2010 to 6.9% in 2017; though, smoking prevalence remains high among young women between ages 20 and 24 [50]. Unfortunately, e-cigarette use among pregnant women has recently increased drastically, particularly among young women [51]. In this analysis by Obisesan et al., 50% of women reported concurrent cigarette use [51]. Smoking cigarettes and the use of other nicotine-containing products during pregnancy is associated with an increased risk of stillbirth [52], preterm birth, and low-birth-weight deliveries [53]. Children whose mothers smoked are also at increased risk of asthma [54] and childhood obesity as they get older [55, 56].
Sleep Apnea
Sleep-disordered breathing (SDB) and more concerning, obstructive sleep apnea (OSA), is a common complication of pregnancy characterized by intermittent upper airway obstruction and resultant desaturation, frequent awakenings from sleep with increased systemic arterial pressures [57]. A systematic review of 33 studies estimates the global prevalence to be approximately 15% (95% CI 12–18%) [58]. OSA has been shown to increase in prevalence across gestation, with the highest prevalence in the third trimester [59]. Pregnant women with a history of type 2 diabetes, hypertension, obesity, CHDs, and increasing maternal age are at risk of developing OSA [59, 60]. The development of OSA further increases the risk of cesarian delivery, gestational hypertension, preeclampsia, and preterm delivery [61,62,63]. Maternal OSA also has a two-fold increased risk of low birth weight and small for gestational age infants [64]. OSA during pregnancy also has prolonged morbidity and leads to an increased risk of pulmonary embolism (OR 5.4; 95% 2.3–8.9), cardiomyopathy (OR 9.0; 95% CI 7.5–10.9), and congestive heart failure (OR 8.9; 95% CI 7.5–10.7) [65].
Preventive Care and Counseling Before and During Pregnancy
Pre-conception CVD Risk Evaluation
Many nonpregnant women do not know the health risks associated with pregnancy [66]. While women and providers recognize the importance of establishing regular prenatal care; preconception care is not routinely sought out or offered. Preconception counseling should be offered to all reproductive-age women whether the patient is actively planning a pregnancy or not [67]. However, women with CVD risk factors should receive prepregnancy counseling by specialized multidisciplinary cardio-obstetrics teams [68, 69]. Referrals to specialized cardio-obstetrics teams for individual risk analysis and shared decision-making should be placed at least 6 months prior to planned conception [68]. Preconception counseling offers the opportunity for screening, primary CVD prevention, and optimization of chronic health conditions prior to conception, which can prove vital in improving maternal, fetal, and later childhood outcomes [70•]. Nutritional surveys and medication reconciliation with regard to pregnancy can improve control of CVD and reduce the risk of teratogenicity effects prior to conception. As risk factors change over time, each should be reevaluated as needed prior to conception and throughout pregnancy. Recommendations for the management of CVD risk factors throughout all stages of pregnancy are summarized in Table 1 below.
Hypertension
Guidelines tend to have more liberal thresholds than the general population due to concerns for decreased fetoplacental perfusion with more aggressive blood pressure control as well as data demonstrating the increased risk of hospitalization without increased risk of other adverse effects for mothers with more tightly controlled blood pressures [18, 72]. Women with chronic hypertension taking angiotensin-converting enzyme (ACE) inhibitors or angiotensin-II receptor blockers (ARBs) should be switched to another medication at least 1 month prior to attempting to conceive [71]. If found to be pregnant while taking ACE inhibitors or ARBs, these medications should be discontinued within 2 days due to the risk of congenital abnormalities.
The American College of Cardiology (ACC) and American Heart Association (AHA) hypertension guidelines explicitly lay out treatment thresholds for hypertension in the general population with goal SBP < 120 and DBP < 80 mm Hg [73]. However, current guidelines for the management of hypertension in pregnancy do not currently take into account the AHA/ACC guidelines and are conflicting across societies due to limited and disputing data regarding the efficacy of strict blood pressure control [18, 74,75,76,77].
The European Society of Cardiology (ESC) recommends treatment for chronic hypertension when SBP ≥ 150 mm Hg or DBP ≥ 95 mm Hg [76]. However, these targets are lower for individuals with gestational hypertension or evidence of preeclampsia with target blood pressure thresholds of SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg [76]. These recommendations stem from the results of the Control of Hypertension in Pregnancy Study (CHIPS), which demonstrated that tighter control of blood pressure was more protective of severe maternal hypertension [78]. A further post hoc analysis of this study demonstrated that for those with less tight control, the development of severe hypertension was a particular risk factor for adverse maternal outcomes [79] The current treatment thresholds are outlined in Table 2 below.
A meta-analysis examining the use of anti-hypertensives for women with mild to moderate hypertension (SBP 140–169 and DBP 90–109) by Abalos et al. further demonstrated that pharmaceutical management with beta-blockers and calcium-channel blockers reduced the risk of maternal development of severe hypertension [81]. However, the data regarding poor neonatal and fetal outcomes and the development of maternal complications was inconclusive [81]. The Chronic Hypertension and Pregnancy (CHAP) trial is a recently published, multi-center, randomized, open-label trial which demonstrated that a treatment threshold of 140/90 for pregnant women with chronic hypertension led to a reduction of preeclampsia, preterm birth, neonatal fetal harm and death [82]. While previous ACOG guidelines recommended a treatment threshold of SBP ≥ 160 mm Hg or DBP ≥ 105-110 mm Hg, in April 2022, ACOG revised their guidelines to reflect the results of the CHAP trial and currently recommends a treatment threshold of 140/90 mm Hg for women with chronic hypertension. [83].
Common first-line outpatient anti-hypertensives with demonstrated safety in pregnant women are beta-blockers, specifically labetalols (initial dose 100–200 mg twice daily, increasing as needed to 800 mg every 8 to 12 h for a maximum 2400 mg per day), dihydropyridine calcium-channel blockers such as extended-release nifedipine (30–60 mg daily, maximum 120 mg per day) and methyldopa (250 mg twice or three times daily, to maximum dose 3000 mg per day) [18, 84]. Hydralazine or diuretics such as hydrochlorothiazide can be considered a second-line agent [18, 85]. In the acute inpatient setting, severe hypertension should be managed with intravenous (IV) or intramuscular (IM) therapy. ACOG recommends using IV labetalol (initial dose of 10–20 mg, followed by 20 to 80 mg every 10 to 30 min, for a maximum cumulative dose of 300 mg), IV or IM hydralazine (initial dose of 5 mg, then 5–10 mg every 20–40 min, for a maximum cumulative dose of 20 mg), or immediate-release oral nifedipine (initial dose of 10–20 mg, repeat as needed in 20 min then in 2–6 h for a maximum cumulative dose of 180 mg) [77].
Diabetes Mellitus
The American Diabetic Association (ADA) recommends that women should be counseled on strict maintenance of glycemic control for a target hemoglobin A1c <6.5% prior to conception [86]. NICE guidelines recommend that any woman with A1c above 10% should be advised to avoid pregnancy due to fetal and neonatal risks [87]. Early in pregnancy, women have increased insulin sensitivity and lower insulin requirements; however, this reverses and peaks during the second and early third trimester, after which it stabilizes [88]. During pregnancy, ACOG [25] and ADA [85] recommend the following glucose targets (as much as safely possible) for type 1 and 2 diabetes as well as gestational diabetes:
-
Fasting <95 mg/dL (5.3 mmol/L) and either
-
One-hour postprandial <140 mg/dL (7.8 mmol/L) or
-
Two-hour postprandial <120 mg/dL (6.7 mmol/L)
Tight control with A1c <6.5% has been shown to have the lowest rate of congenital defects and other adverse fetal outcomes during pregnancy [26, 27, 85]. If strict maintenance <6.5% is unsafe due to the risk of hypoglycemia, the A1c goal can be liberalized to <7% [26, 84]. Due to frequent red-cell turnover during pregnancy, A1c is more variable, requiring more frequent monitoring [25]. The management of diabetes involves both pharmacologic and nonpharmacologic therapy, including a carbohydrate-controlled diet and regular exercise [89]. With regard to pharmacologic therapy, insulin is the preferred therapy for the management of type 1 and type 2 diabetes [84]. Insulin requirements should be monitored closely due to rapid shifts in insulin resistance during pregnancy. There are limited data on the effectiveness and risk of adverse effects for most oral hypoglycemic agents; however, recent data suggest metformin may be used safely in pregnancy [25, 90]. Given that diabetes in pregnancy leads to an increased risk of preeclampsia, low-dose aspirin at 81 mg/day should be initiated at 12 weeks of gestation, ideally prior to 16 weeks and continued until delivery [25, 85].
Dyslipidemia
Dyslipidemia is particularly associated with APOs such as gestational diabetes and preeclampsia when triglyceride levels exceed 250 mg/dL [40]. Women with familial hypercholesterolemia (FH) and severe hypertriglyceridemia are at particularly high risk during pregnancy as there are few options for the management of dyslipidemia in pregnancy [37, 91]. Statin therapy (HMG-CoA reductase inhibitors), niacin, fibrates, and ezetimibe are all contraindicated due to evidence of teratogenicity [90]. When women are ready to conceive, guidelines recommend women discontinue statin therapy at least 4 weeks prior to discontinuing contraceptives and should continue holding it until after she has stopped breastfeeding [90, 91]. Of note, statin use, while still strongly discouraged in pregnancy, is being reevaluated as there is emerging data that hydrophilic statins (pravastatin, rosuvastatin) may not be associated with fetal malformations as seen in lipophilic statins (simvastatin, atorvastatin) [92]. For women at very high risk for CVD, The United States Food and Drug Administration (FDA) no longer lists statins as strictly contraindicated in pregnancy; however, as the data evolve, the decision to start or remain on statin therapy should be made after patient-centered risk versus benefit discussion [93].
Reproductive age women taking contraindicated medications should receive preconception counseling regarding the side effects of these medications and should be recommended to use contraceptives. If a woman discovers she is pregnant while on these medications, the medications should be stopped immediately [91]. Proprotein convertase subtilisin/kexin type 9 serine protease (PCSK-9) inhibitors have not been studied in pregnancy. However, an observational pregnancy exposure registry for evolocumab is currently underway [94]. Omega-3 fatty acids [37] and bile acid sequestrants [90] are the only two medications, which have been reported as safe during pregnancy. Thus, behavioral counseling regarding diet and exercise is crucial for additional risk mitigation. For women with FH and significant atherosclerotic disease, LDL apheresis can be considered during pregnancy [95].
Obesity
To prevent obesity-related complications, ACOG and the National Institute for Health and Clinical Excellence (NICE) guidelines recommend regular weight counseling including both diet and exercise for all women, particularly prior to pregnancy as any amount of weight loss can improve maternal and fetal outcomes [96, 97]. ACOG specifically recommends 150 min of moderate-intensity aerobic activity weekly for pregnant women; however, women should talk to their doctors if they have a specific cardiac condition that may require a tailored exercise plan [98]. ACOG further recommends an initial assessment of BMI during pregnancy and the use of the Institute of Medicine (IOM) guidelines on weight gain to prevent increasing complications [99]. The United States Preventative Services Task Force (USPSTF) released a systematic review concluding that behavioral counseling interventions by primary physicians for obesity and reducing excessive weight gain had a moderate benefit, reducing the incidence of gestational diabetes, emergency cesarian section delivery, and infant macrosomia (level B recommendation) [43]. One limitation in such reviews is the heterogeneity of behavioral interventions in terms of length and intensity of engagement [43].
Smoking
ACOG and the USPSTF recommend regular screening for tobacco use beginning with the initial visit, ideally preconceptual regular smoking cessation counseling and behavioral interventions for all women throughout pregnancy [56, 100]. The greatest advantage of smoking cessation occurs before 15 weeks of gestation; however, cessation at any point in pregnancy has benefits [101]. Women should be warned that all nicotine products including vaping, e-cigarettes, lozenges, gum, and hookahs, can all have harmful effects on the mother and fetus [56]. There are limited data regarding the risk versus benefit of nicotine replacement therapy for pregnant women [102]. However, as nicotine has known harmful effects, nicotine replacement therapy (NRT) should only be considered after a thorough discussion between patient and clinician [56]. Currently, other pharmacologic options such as varenicline and bupropion, appear to be safe in pregnancy; however current data are limited [103, 104].
Sleep Apnea
OSA and SDB are currently underdiagnosed and undertreated in pregnant women [65]. Current validated screening models for OSA including the Berlin Questionnaire and Epworth Sleepiness Scale have been shown to be poor predictors in pregnant women [103]. Facco et al. created a four-variable model, which includes frequent snoring, age, chronic hypertension, and BMI; which had higher sensitivity (88%) and specificity (86%) in identifying SDB in pregnant women [105]. Continuous positive airway pressure (CPAP) is the mainstay of treatment for mild to severe OSA as it reverses airway obstruction thus improving oxygenation and blood pressure [106].
Generational Effects of CVD
The risk of poor maternal CVD health on maternal and fetal mortality has been well-established. More recently, researchers have also sought to understand the long-term effects of uncontrolled CVD as the offspring of these mothers get older [107]. A study by Perak et al. characterized baseline maternal CVD health at 28 weeks of gestation by combining individual characteristics of BMI, blood pressure, total cholesterol, abnormal glucose, and smoking [108]. The results of this cohort study found that better maternal health was significantly associated with better cardiovascular health for their offspring during adolescence [109].
Preventive Care and Counseling Postpartum
Women with preexisting conditions including chronic hypertension, diabetes, sleep apnea, dyslipidemia, diabetes, and obesity are at increased risk of APOs and subsequently increased risk of developing cardiovascular disease in their lifetime. Thus, regular follow-up, screening, and care coordination during the postpartum “fourth trimester” is crucial for any woman with CVD risk factors or APOs. The 3 months after delivery offer an opportunity for continuity of care and reinforcement of appropriate primary and secondary prevention strategies.
Postpartum Clinics and Telemedicine
All women are encouraged to reach out to their obstetrician-gynecologists within the first 3 weeks postpartum to establish an initial assessment of individual needs [110]. However, as opposed to a single encounter, ACOG’s Presidential Task Force on Redefining Postpartum Care encourages regular longitudinal follow-up as needed, particularly for chronic diseases, lactation difficulty, or mental health concerns [111]. These visits allow the opportunity to counsel women with pregnancies complicated by APOs about their increased risk of recurrence in future pregnancies as well as overall increased risk of cardiovascular diseases. ACOG recommends a comprehensive visit within 3 months postpartum. [108].
Comprehensive postpartum care encompasses many domains including a woman’s physical, social, and psychological health, infant care, as well as chronic disease management and health maintenance [108]. With many pregnancies complicated by the rising prevalence of chronic cardiovascular risk factors and increasing maternal age, the AHA and ACOG have supported the use of multidisciplinary care by cardiologists and obstetricians [10]. A review by Jowell et al. found that referrals to transitional clinics within 0 to 6 months postdelivery improved patient education, lifestyle modifications, and appropriate specialty care referrals when necessary [109]. Referrals to specialized adult congenital disease clinics for women with CHD have also demonstrated a mortality benefit [110].
Unfortunately, an estimated 40% of women do not currently attend their postpartum visits [108]. A systemic review by Jones et al. observed that non-Hispanic White, Asian, and non-Black race/ethnicities were associated with increased follow-up rates [112]. Medicaid coverage, Medicaid eligibility, and less than a high-school education were all negative predictors of follow-up [111]. Targeted interventions improve follow-up, and care coordination may be instrumental in the prevention of further cardiovascular disease. For example, the use of telemedicine during the postpartum period is beneficial, particularly for women with limited access to transportation.
Telemedicine has shown benefits in helping women maintain breastfeeding exclusivity, manage postpartum depression, and continued blood pressure monitoring [113, 114, 115•, 116]. A feasibility study conducted by Hoppe et al. found that remote blood pressure monitoring allowed for early identification and treatment of severe hypertension and reduced readmission rates [116, 117]. As telemedicine is becoming more accessible after the COVID-19 pandemic, its use in the management of hypertensive disorders of pregnancy (HDP) in the fourth trimester is promising and may improve access to women who are unable to receive care due to time and resource constraints [118]. Telemedicine can also help bridge the gap in social determinants of health by improving health literacy and reducing the cost burden of frequent follow-up [23].
Establishing postnatal follow-up paves the way for further interventions to improve cardiovascular risk including pharmacotherapy, intensive lifestyle management, and patient and clinician education, particularly with regard to future pregnancy planning and risk of CVD [17, 109]. Further studies and interventions specifically addressing disparities in access to care across socioeconomic and racial groups are needed to change the face of postpartum care. At the health systems level, increased advocacy is needed to expand Medicare coverage and access to comprehensive care for women who lose access at 60 days postpartum [70]. The AHA in particular has been working to increase its advocacy efforts with regard to Medicaid expansion, strengthening telehealth infrastructure, and increased care coordination to mitigate the impact of the social determinants of health [70].
Preeclampsia and Chronic Hypertension
For women who required antihypertensives for any HDP, the AHA recommends early blood pressure surveillance during the first 1–2 weeks postpartum for goal blood pressure SBP < 150 mm Hg and DBP < 100 mm Hg [68]. ACOG similarly recommends that all women who developed HDP should have follow-up within this same time period of 7–10 days; with earlier follow-up within 72 h after delivery for women who developed severe hypertension [119]. Approximately half of the postpartum strokes occur in the first 10 days after discharge [107]. The recommended treatment of severe-range blood pressures includes IV labetalol or hydralazine or rapid onset nifedipine, with corresponding oral agents for maintenance of blood pressure [120]. Both nifedipine and labetalol are considered compatible with breastfeeding [121, 122]. Other medication classes including non-dihydropyridine calcium channel blockers such as verapamil and certain ACE-inhibitors, such as captopril and enalapril have also been used in postpartum hypertensive management [68, 117]. The use of furosemide, a diuretic, has shown effectiveness in early blood pressure control when used in conjunction with other antihypertensive agents [123].
To combat both acute and long-term effects of HDP, Harrington et al. highlighted the importance of disease education, anticipatory guidance, and blood pressure monitoring prior to discharge from the hospital [116]. Women with HDP, particularly preeclampsia, have an increased risk of hypertension, CAD, stroke, VT, and CVD-related mortality both in the short (less than 10 years) and long term (greater than 10 years) [124, 125]. However, many women are unaware of this relationship, and thus may be less inclined to seek care after delivery. Counseling women on self-assessments with home blood pressure cuffs can also help women become more engaged in their health and follow-up [117].
Given the increased risk of future CVD, the AHA recommends contraception counseling to all women with HDP, as well as other APOs, to help women optimize risk factors prior to future pregnancies [68]. The type of contraception should be decided based on individual risk factors and comorbidities so as to not increase a woman’s risk of venous thromboembolism [68]. For women with HDP, in the long term, screening for chronic hypertension and BMI should occur twice during the first postpartum year and then spaced to annual check-ins [126, 127]. Hemoglobin A1c levels, fasting glucose, and lipid profiles should be assessed at 3 to 6 months after delivery then annually [128]. Despite its recognition as a risk-enhancing factor, there are no validated tools predicting the risk of CVD in women who develop HDP [129]. The development of such tools may be useful to help guide clinicians on the next steps in the management and follow-up recommendations.
Diabetes
Insulin sensitivity increases shortly after delivery, thus women should be warned to pay close attention to glucose readings and respective insulin requirements to reduce the risk of hypoglycemia [84]. Any woman with a recent history of gestational diabetes mellitus (GDM) should be screened 4–12 weeks postpartum using the 75-g oral glucose tolerance test as this can uncover an underlying previous unknown history of type 1 or type 2 diabetes [25, 85]. If found to have prediabetes, patients should receive intensive lifestyle counseling as there is a high risk of conversion to type 2 diabetes. Screening for prediabetes and type 2 diabetes should be continued in these women every 3 years [130]. All women with a history of diabetes should be counseled on contraceptive use to better plan for glycemic control prior to pregnancy.
Dyslipidemia
Screening for postpartum dyslipidemia is not recommended in current guidelines. However, women with a previous history of elevated cholesterol, familial hypercholesterolemia, or those who develop GDM during pregnancy are at increased risk of chronic dyslipidemia and should receive regular screening postpartum [128, 129]. Age greater than 35 years, elevated SBP before labor, A1c > 5.1%, and LDL-C > 3.56 mmol/L (137.6 mg/dL) in the second trimester have also been associated with an increased prevalence of postpartum dyslipidemia [130].
Smoking
As many as half of the women who quit smoking during pregnancy relapse within the 6 months after delivery placing themselves and their child again at risk [131]. One systematic review found that being younger, multiparous, less well educated, experiencing higher stress and anxiety, not breastfeeding, and living with a partner who smoked was the most common predictor of relapse [132]. Thus, continued counseling and identification of triggers and risk factors for relapse is of particular importance during the third trimester and should be carried out during the postpartum visits.
Sleep Apnea
While the majority of women will see their OSA resolve within the first 2 to 3 months postpartum, preliminary results by Street et al. suggest that approximately 20% of women will have persistent symptoms at 6 to 8 months [133]. These results reinforce the need for women diagnosed with OSA during pregnancy to be reevaluated postpartum. The management of OSA in the postpartum period should be continued use of CPAP to reduce long-term cardiovascular risk.
Conclusion
Cardiovascular risk factors including chronic hypertension, diabetes, sleep apnea, dyslipidemia, diabetes, and obesity have been increasing steadily over the past few decades, particularly in women of reproductive age. Each of these risk factors increases a woman’s risk for the development of APOs and higher maternal morbidity and mortality and poor fetal outcomes. Unfortunately, the compounding adverse events further increase a woman’s lifetime risk of cardiovascular disease beginning immediately after delivery. Women from rural areas, with limited access to care, poor insurance coverage, and lower socioeconomic status are at particularly high risk of developing cardiovascular risk factors and eventual diseases.
Primary prevention and risk mitigation of cardiovascular risk factors should begin prior to conception and be maintained throughout pregnancy. Continued follow-up in the fourth trimester, 3 months after delivery, can help with early identification of CVD; however, many women do not attend their postpartum visits. Health system interventions including improved care coordination, patient education, and increased digital health infrastructure can improve access to care and risk mitigation during this crucial time period. Appropriate disease screening and management can greatly improve outcomes for mother and baby throughout pregnancy and lead to overall decreased mortality from cardiovascular disease.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Anon. Pregnancy Mortality Surveillance System | Maternal and infant health | CDC. Available at: https://www.cdc.gov/reproductivehealth/maternal-mortality/pregnancy-mortality-surveillance-system.htm. Accessed November 7, 2021.
Collier AY, Molina RL. Maternal mortality in the United States: updates on trends, causes, and solutions. Neoreviews 2019;20:e561. Available at: /pmc/articles/PMC7377107/. Accessed November 7, 2021.
UNFPA, UNICEF, Organization WH, Group WB, Division the UNP. Trends in maternal mortality: 2000 to 2017 | UNFPA - United Nations Population Fund. WHO, UNICEF, UNFPA,World Bank Gr. theUnited Nations Popul. Div. 2019:104. Available at: http://apps.who.int/bookorders. Accessed October 29, 2021.
Kassebaum NJ, Bertozzi-Villa A, Coggeshall MS, et al. Global, regional, and national levels and causes of maternal mortality during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:980–1004.
Creanga AA, Syverson C, Seed K, Callaghan WM. Pregnancy-related mortality in the United States, 2011-2013. Obstet Gynecol. 2017;130:366–373. Available at: https://journals.lww.com/greenjournal/Fulltext/2017/08000/Pregnancy_Related_Mortality_in_the_United_States.15.aspx. Accessed October 31, 2021.
Knypinski J, Wolfe DS. Maternal mortality due to cardiac disease in pregnancy. Clin Obstet Gynecol. 2020;63:799–807. Available at: https://journals.lww.com/clinicalobgyn/Fulltext/2020/12000/Maternal_Mortality_Due_to_Cardiac_Disease_in.12.aspx. Accessed October 31, 2021.
Kotit S, Yacoub M. Cardiovascular adverse events in pregnancy: a global perspective. Glob Cardiol Sci Pract 2021;2021. Available at: /pmc/articles/PMC8133785/. Accessed October 29, 2021.
Nair M, Nelson-Piercy C, Knight M. Indirect maternal deaths: UK and global perspectives. Obstet Med. 2017;10:10. Available at: /pmc/articles/PMC5405948/. Accessed October 29, 2021.
Petersen EE, Davis NL, Goodman D, et al. Vital signs: pregnancy-related deaths, United States, 2011–2015, and strategies for prevention, 13 States, 2013–2017. MMWR Morb Mortal Wkly Rep. 2019;68. Available at: https://pubmed.ncbi.nlm.nih.gov/31071074/. Accessed November 7, 2021.
Sharma G, Ying W, Silversides CK. The importance of cardiovascular risk assessment and pregnancy heart team in the management of cardiovascular disease in pregnancy. Cardiol Clin. 2021;39:7–19. Available at: https://pubmed.ncbi.nlm.nih.gov/33222816/. Accessed October 29, 2021.
Ouzounian JG, Elkayam U. Physiologic changes during normal pregnancy and delivery. Cardiol Clin. 2012;30:317–29.
Sanghavi M, Rutherford JD. Cardiovascular physiology of pregnancy. Circulation. 2014;130:1003–1008. Available at: https://www.ahajournals.org/doi/abs/10.1161/CIRCULATIONAHA.114.009029. Accessed October 31, 2021.
Ananth C V., Duzyj CM, Yadava S, Schwebel M, Tita ATN, Joseph KS. Changes in the prevalence of chronic hypertension in pregnancy, United States, 1970 to 2010. Hypertension. 2019;74:1089–1095. Available at: https://www.ahajournals.org/doi/abs/10.1161/HYPERTENSIONAHA.119.12968. Accessed October 31, 2021.
Wild R, Weedin EA, Wilson D. Dyslipidemia in pregnancy. Endocrinol Metab Clin N Am. 2016;45:55–63.
Shah LM, Wand A, Ying W, et al. Prevention starts in the womb: opportunities for addressing cardiovascular risk factors during pregnancy and beyond. Methodist Debakey Cardiovasc J. 2021;17:48–59. Available at: http://journal.houstonmethodist.org/articles/10.14797/mdcvj.696/. Accessed October 2, 2021.
Minhas AS, Ying W, Ogunwole SM, et al. The association of adverse pregnancy outcomes and cardiovascular disease: current knowledge and future directions. Curr Treat Options Cardiovasc Med. 2020;22:61. Available at: https://jhu.pure.elsevier.com/en/publications/the-association-of-adverse-pregnancy-outcomes-and-cardiovascular-. Accessed November 4, 2021.
•• Parikh NI, Gonzalez JM, Anderson CAM, et al. Adverse pregnancy outcomes and cardiovascular disease risk: unique opportunities for cardiovascular disease prevention in women: a scientific statement from the American Heart Association. Circulation 2021;143:E902–E916. Available at: https://www.ahajournals.org/doi/abs/10.1161/CIR.0000000000000961. Accessed October 31, 2021. This AHA statement cohesively highlights the relationship between APOs and CVD and summarizes current data regarding opportunities for prevention during pregnancy and post-partum follow up.
Anon. ACOG Practice Bulletin No. 203: Chronic hypertension in pregnancy. Obstet. Gynecol. 2019;133:e26–e50. Available at: https://journals.lww.com/greenjournal/Fulltext/2019/01000/ACOG_Practice_Bulletin_No__203__Chronic.50.aspx. Accessed October 31, 2021.
Bateman BT, Bansil P, Hernandez-Diaz S, Mhyre JM, Callaghan WM, Kuklina EV. Prevalence, trends, and outcomes of chronic hypertension: a nationwide sample of delivery admissions. Am J Obstet Gynecol. 2012;206:134.e1–8.
Bornstein E, Eliner Y, Chervenak FA, Grünebaum A. Concerning trends in maternal risk factors in the United States: 1989–2018. EClinicalMedicine 2020;29. Available at: http://www.thelancet.com/article/S2589537020304016/fulltext. Accessed November 13, 2021.
Krieger E V., Landzberg MJ, Economy KE, Webb GD, Opotowsky AR. Comparison of risk of hypertensive complications of pregnancy among women with versus without coarctation of the aorta. Am J Cardiol. 2011;107:1529–1534. Available at: https://pubmed.ncbi.nlm.nih.gov/21420058/. Accessed November 24, 2021.
Cameron NA, Molsberry R, Pierce JB, et al. Pre-pregnancy hypertension among women in rural and urban areas of the United States. J Am Coll Cardiol. 2020;76:2611–2619. Available at: https://pubmed.ncbi.nlm.nih.gov/33183896/. Accessed November 13, 2021.
Shah LM, Varma B, Nasir K, et al. Reducing disparities in adverse pregnancy outcomes in the United States. Am Heart J. 2021;242:92–102. Available at: https://pubmed.ncbi.nlm.nih.gov/34481757/. Accessed November 14, 2021.
Bramham K, Parnell B, Nelson-Piercy C, Seed PT, Poston L, Chappell LC. Chronic hypertension and pregnancy outcomes: systematic review and meta-analysis. BMJ. 2014;348. Available at: https://www.bmj.com/content/348/bmj.g2301. Accessed November 1, 2021.
Anon. ACOG Practice Bulletin No. 201: Pregestational diabetes mellitus. Obstet Gynecol. 2018;132:E228–E248. Available at: https://journals.lww.com/greenjournal/Fulltext/2018/12000/ACOG_Practice_Bulletin_No__201__Pregestational.48.aspx. Accessed November 4, 2021.
Jensen DM, Korsholm L, Ovesen P, et al. Peri-conceptional A1C and risk of serious adverse pregnancy outcome in 933 women with type 1 diabetes. Diabetes Care. 2009;32:1046–1048. Available at: https://care.diabetesjournals.org/content/32/6/1046. Accessed November 4, 2021.
Guerin A, Nisenbaum R, Ray JG. Use of maternal GHb concentration to estimate the risk of congenital anomalies in the offspring of women with prepregnancy diabetes. Diabetes Care. 2007;30:1920–1925. Available at: https://pubmed.ncbi.nlm.nih.gov/17446531/. Accessed November 4, 2021.
Mills JL. Malformations in infants of diabetic mothers. Teratol Birth Defects Res A Clin Mol Teratol. 2010;88:769–778. Available at: /pmc/articles/PMC4158942/. Accessed November 4, 2021.
Mitanchez D, Yzydorczyk C, Simeoni U. What neonatal complications should the pediatrician be aware of in case of maternal gestational diabetes? World J Diabetes. 2015;6:734. Available at: /pmc/articles/PMC4458502/. Accessed November 4, 2021.
Hummel S, Pflüger M, Kreichauf S, Hummel M, Ziegler A-G. Predictors of overweight during childhood in offspring of parents with type 1 diabetes. Diabetes Care. 2009;32:921–925. Available at: https://care.diabetesjournals.org/content/32/5/921. Accessed November 4, 2021.
Temple RC, Aldridge VA, Sampson MJ, Greenwood RH, Heyburn PJ, Glenn A. Impact of pregnancy on the progression of diabetic retinopathy in type 1 diabetes. Diabetes Med. 2001;18:573–577. Available at: https://pubmed.ncbi.nlm.nih.gov/11553188/. Accessed November 4, 2021.
Purdy LP, Phelps RL, Hantsch CE, et al. Effect of pregnancy on renal function in patients with moderate-to- severe diabetic renal insufficiency. Diabetes Care. 1996;19:1067–1074. Available at: https://pubmed.ncbi.nlm.nih.gov/8886551/. Accessed November 4, 2021.
Ladner HE, Danielsen B, Gilbert WM. Acute myocardial infarction in pregnancy and the puerperium: a population-based study. Obstet Gynecol. 2005;105:480–484. Available at: https://journals.lww.com/greenjournal/Fulltext/2005/03000/Acute_Myocardial_Infarction_in_Pregnancy_and_the.6.aspx. Accessed November 4, 2021.
Gordon MC, Landon MB, Boyle J, Stewart KS, Gabbe SG. Coronary artery disease in insulin-dependent diabetes mellitus of pregnancy (class H): a review of the literature. Obstet Gynecol Surv. 1996;51:437–444. Available at: https://journals.lww.com/obgynsurvey/Fulltext/1996/07000/Coronary_Artery_Disease_in_Insulin_Dependent.23.aspx. Accessed November 4, 2021.
Laz TH, Rahman M, Berenson AB. Trends in serum lipids and hypertension prevalence among non-pregnant reproductive-age women: United States National Health and Nutrition Examination Survey 1999-2008. Matern. Child Health J. 2013;17:1424–1431. Available at: https://pubmed.ncbi.nlm.nih.gov/23054453/. Accessed November 6, 2021.
Hadden DR, McLaughlin C. Normal and abnormal maternal metabolism during pregnancy. Semin Fetal Neonatal Med. 2009;14:66–71. Available at: https://pubmed.ncbi.nlm.nih.gov/18986856/. Accessed November 6, 2021.
Mukherjee M. Dyslipidemia in pregnancy. Am Coll Cardiol. 2014. Available at: https://www.acc.org/latest-in-cardiology/articles/2014/07/18/16/08/dyslipidemia-in-pregnancy. Accessed November 6, 2021.
Brizzi P, Tonolo G, Esposito F, et al. Lipoprotein metabolism during normal pregnancy. In: American Journal of Obstetrics and Gynecology. Vol 181. Am J Obstet Gynecol. 1999:430–434. Available at: https://pubmed.ncbi.nlm.nih.gov/10454696/. Accessed November 6, 2021.
Belo L, Caslake M, Gaffney D, et al. Changes in LDL size and HDL concentration in normal and preeclamptic pregnancies. Atherosclerosis. 2002;162:425–432. Available at: https://pubmed.ncbi.nlm.nih.gov/11996963/. Accessed November 6, 2021.
Grimes SB, Wild R. Effect of pregnancy on lipid metabolism and lipoprotein levels. Endotext 2018. Available at: https://www.ncbi.nlm.nih.gov/books/NBK498654/. Accessed November 6, 2021.
Collaboration TG 2013 O, Ng M, Fleming T, et al. Global, regional and national prevalence of overweight and obesity in children and adults 1980-2013: a systematic analysis. Lancet (London, England) 2014;384:766. Available at: /pmc/articles/PMC4624264/. Accessed November 1, 2021.
Driscoll AK GE. Increases in prepregnancy obesity: United States, 2016–2019. NCHS Data Br. no 392. Natl. Cent. Heal. Stat. 2020. Available at: https://www.cdc.gov/nchs/products/databriefs/db392.htm#Suggested_citation. Accessed November 1, 2021.
Davidson KW, Barry MJ, Mangione CM, et al. Behavioral counseling interventions for healthy weight and weight gain in pregnancy: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325:2087–2093. Available at: https://jamanetwork.com/journals/jama/fullarticle/2780318. Accessed November 1, 2021.
Schummers L, Hutcheon JA, Bodnar LM, Lieberman E, Himes KP. Risk of adverse pregnancy outcomes by prepregnancy body mass index : a population-based study to inform prepregnancy weight loss counseling. In: Obstetrics and Gynecology. Vol 125. Obstet Gynecol. 2015:133–143. Available at: https://pubmed.ncbi.nlm.nih.gov/25560115/. Accessed November 1, 2021.
Saucedo M, Esteves-Pereira AP, Pencolé L, et al. Understanding maternal mortality in women with obesity and the role of care they receive: a national case-control study. Int J Obes. 2020 451 2020;45:258–265. Available at: https://www.nature.com/articles/s41366-020-00691-4. Accessed November 1, 2021.
Persson M, Razaz N, Edstedt Bonamy AK, Villamor E, Cnattingius S. Maternal overweight and obesity and risk of congenital heart defects. J Am Coll Cardiol. 2019;73:44–53. Available at: https://pubmed.ncbi.nlm.nih.gov/30621950/. Accessed November 24, 2021.
Hedermann G, Hedley PL, Thagaard IN, et al. Maternal obesity and metabolic disorders associate with congenital heart defects in the offspring: a systematic review. PLoS One. 2021;16:e0252343. Available at: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0252343. Accessed November 25, 2021.
Chen A, Feresu SA, Fernandez C, Rogan WJ. Maternal obesity and the risk of infant death in the United States. Epidemiology 2009;20:74. Available at: /pmc/articles/PMC2674328/. Accessed November 1, 2021.
Lee KK, Raja EA, Lee AJ, et al. Maternal obesity during pregnancy associates with premature mortality and major cardiovascular events in later life. Hypertension. 2015;66:938–944. Available at: https://www.ahajournals.org/doi/abs/10.1161/HYPERTENSIONAHA.115.05920. Accessed November 1, 2021.
Azagba S, Manzione L, Shan L, King J. Trends in smoking during pregnancy by socioeconomic characteristics in the United States, 2010–2017. BMC Pregnancy Childbirth 2020 201 2020;20:1–7. Available at: https://bmcpregnancychildbirth.biomedcentral.com/articles/10.1186/s12884-020-2748-y. Accessed November 2, 2021.
Obisesan OH, Osei AD, Uddin SMI, et al. E-cigarette use patterns and high-risk behaviors in pregnancy: behavioral risk factor surveillance system, 2016–2018. Am J Prev Med. 2020;59:187–195. Available at: https://jhu.pure.elsevier.com/en/publications/e-cigarette-use-patterns-and-high-risk-behaviors-in-pregnancy-beh. Accessed November 13, 2021.
Pineles BL, Hsu S, Park E, Samet JM. Systematic review and meta-analyses of perinatal death and maternal exposure to tobacco smoke during pregnancy. Am J Epidemiol. 2016;184:87–97. Available at: https://academic.oup.com/aje/article/184/2/87/2236646. Accessed November 2, 2021.
Dietz PM, England LJ, Shapiro-Mendoza CK, Tong VT, Farr SL, Callaghan WM. Infant morbidity and mortality attributable to prenatal smoking in the U.S. Am J Prev Med. 2010;39:45–52. Available at: https://pubmed.ncbi.nlm.nih.gov/20547278/. Accessed November 2, 2021.
Gilliland FD, Li YF, Peters JM. Effects of maternal smoking during pregnancy and environmental tobacco smoke on asthma and wheezing in children. Am J Respir Crit Care Med. 2001;163:429–436. Available at: www.atsjournals.org. Accessed November 2, 2021.
Maessen SE, Ahlsson F, Lundgren M, Cutfield WS, Derraik JGB. Maternal smoking early in pregnancy is associated with increased risk of short stature and obesity in adult daughters. Sci Reports. 2019 91 2019;9:1–9. Available at: https://www.nature.com/articles/s41598-019-39006-7. Accessed November 2, 2021.
Anon. Tobacco and nicotine cessation during pregnancy. ACOG Comm. Opin. 2020. Available at: https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2020/05/tobacco-and-nicotine-cessation-during-pregnancy. Accessed November 2, 2021.
Dominguez JE, Street L, Louis J. Management of obstructive sleep apnea in pregnancy. Obstet Gynecol Clin N Am. 2018;45:233. Available at: /pmc/articles/PMC5995135/. Accessed November 1, 2021.
Liu L, Su G, Wang S, Zhu B. The prevalence of obstructive sleep apnea and its association with pregnancy-related health outcomes: a systematic review and meta-analysis. Sleep Breath. 2018;23:399–412. Available at: https://link.springer.com/article/10.1007/s11325-018-1714-7. Accessed November 1, 2021.
Pien GW, Pack AI, Jackson N, Maislin G, Macones GA, Schwab RJ. Risk factors for sleep-disordered breathing in pregnancy. Thorax. 2014;69:371–377. Available at: https://thorax.bmj.com/content/69/4/371. Accessed November 1, 2021.
Drake M, Ginde S, Cohen S, et al. Prevalence, risk factors, and impact of obstructive sleep apnea in adults with congenital heart disease. Pediatr Cardiol. 2020;41:724–728. Available at: https://pubmed.ncbi.nlm.nih.gov/32002577/. Accessed November 24, 2021.
Spence DL, Allen RC, Lutgendorf MA, Gary VR, Richard JD, Gonzalez SC. Association of obstructive sleep apnea with adverse pregnancy-related outcomes in military hospitals. Eur J Obstet Gynecol Reprod Biol. 2017;210:166–172. Available at: https://pubmed.ncbi.nlm.nih.gov/28040612/. Accessed November 1, 2021.
Facco FL, Parker CB, Reddy UM, et al. Association between sleep-disordered breathing and hypertensive disorders of pregnancy and gestational diabetes mellitus. Obstet Gynecol. 2017;129:31–41. Available at: https://journals.lww.com/greenjournal/Fulltext/2017/01000/Association_Between_Sleep_Disordered_Breathing_and.5.aspx. Accessed November 1, 2021.
Pamidi S, Pinto LM, Marc I, Benedetti A, Schwartzman K, Kimoff RJ. Maternal sleep-disordered breathing and adverse pregnancy outcomes: a systematic review and metaanalysis. Am J Obstet Gynecol. 2014;210:52.e1-52.e14. Available at: https://pubmed.ncbi.nlm.nih.gov/23911687/. Accessed November 1, 2021.
Ding XX, Le Wu Y, Xu SJ, et al. A systematic review and quantitative assessment of sleep-disordered breathing during pregnancy and perinatal outcomes. Sleep Breath. 2014;18:703–713. Available at: https://pubmed.ncbi.nlm.nih.gov/24519711/. Accessed November 2, 2021.
Louis JM, Mogos MF, Salemi JL, Redline S, Salihu HM. Obstructive sleep apnea and severe maternal-infant morbidity/mortality in the United states, 1998-2009. Sleep. 2014;37. Available at: https://pubmed.ncbi.nlm.nih.gov/24790262/. Accessed November 1, 2021.
Esposito G, Ambrosio R, Napolitano F, Di Giuseppe G. Women’s knowledge, attitudes and behavior about maternal risk factors in pregnancy. PLoS One. 2015;10. Available at: https://pubmed.ncbi.nlm.nih.gov/26714032/. Accessed November 7, 2021.
Anon. Prepregnancy Counseling . ACOG 2019. Available at: https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2019/01/prepregnancy-counseling. Accessed November 7, 2021.
Mehta LS, Warnes CA, Bradley E, et al. Cardiovascular considerations in caring for pregnant patients: a scientific statement from the American Heart Association. Circulation. 2020;141:e884–e903. Available at: https://www.ahajournals.org/doi/abs/10.1161/CIR.0000000000000772. Accessed October 2, 2021.
Davis MB, Walsh MN. Cardio-obstetrics: team-based care to improve maternal outcomes. Circ Cardiovasc Qual Outcomes. 2019;12. Available at: https://www.ahajournals.org/doi/abs/10.1161/CIRCOUTCOMES.118.005417. Accessed November 26, 2021.
• Mehta LS, Sharma G, Creanga AA, et al. Call to action: maternal health and saving mothers: a policy statement from the American Heart Association. Circulation. 2021;144:251–269. Available at: https://www.ahajournals.org/doi/abs/10.1161/CIR.0000000000001000. Accessed October 29, 2021. This AHA statement succintly underscores the social inequities driving CVD risk factors and maternal mortality and features current recommendations for health system reform including expanding access to care and digital health services.
Lu Y, Chen R, Cai J, Huang Z, Yuan H. The management of hypertension in women planning for pregnancy. Br Med Bull. 2018;128:75. Available at: /pmc/articles/PMC6289217/. Accessed November 7, 2021.
Nabhan AF, Elsedawy MM. Tight control of mild-moderate pre-existing or non-proteinuric gestational hypertension. Cochrane Database Syst Rev. 2011. Available at: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD006907.pub2/full. Accessed November 1, 2021.
Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American college of cardiology/American Heart Association task . Hypertension. 2018;71:1269–1324. Available at: https://pubmed.ncbi.nlm.nih.gov/29133354/. Accessed September 25, 2021.
Anon. Recommendations | Hypertension in pregnancy: diagnosis and management | Guidance | NICE. Natl Inst Health Care Excell. 2019.
Brown MA, Magee LA, Kenny LC, et al. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2018;13:291–310.
Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, et al. 2018 ESC guidelines for the management of cardiovascular diseases during pregnancy: the Task Force for the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:3165–3241. Available at: https://academic.oup.com/eurheartj/article/39/34/3165/5078465. Accessed September 30, 2021.
Anon. Gestational hypertension and preeclampsia: ACOG Practice Bulletin, Number 222. Obstet. Gynecol. 2020;135:e237–e260. Available at: https://journals.lww.com/greenjournal/Fulltext/2020/06000/Gestational_Hypertension_and_Preeclampsia__ACOG.46.aspx. Accessed September 19, 2021.
Magee LA, von Dadelszen P, Rey E, et al. Less-tight versus tight control of hypertension in pregnancy. https://doi.org/10.1056/NEJMoa1404595 2015;372:407–417. Available at: https://www.nejm.org/doi/full/10.1056/nejmoa1404595. Accessed October 6, 2021.
Magee LA, Dadelszen P von, Singer J, et al. The CHIPS randomized controlled trial (Control of Hypertension in Pregnancy Study): is severe hypertension just an elevated blood pressure? Hypertens (Dallas, Tex. 1979). 2016;68:1153. Available at: /pmc/articles/PMC5058640/. Accessed November 1, 2021.
Thakkar A, Sharma G. Hypertensive disorders of pregnancy: considerations for cardiologists. In: Ram CVS, editor. Hypertension Reviews 2021, Global Perspectives. Noida: Incessant Nature Science Publishers Pvt Ltd; 2021. p. 185–201.
Abalos E, Duley L, Steyn DW, Gialdini C. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev. 2018;10. Available at: https://pubmed.ncbi.nlm.nih.gov/30277556/. Accessed November 13, 2021.
Tita AT, Szychowski JM, Boggess K, Dugoff L, Sibai B, Lawrence K, et al. Treatment for mild chronic hypertension during pregnancy. Chronic Hypertension and Pregnancy (CHAP) Trial Consortium. N Engl J Med. Published online April 2, 2022. https://doi.org/10.1056/NEJMoa2201295
Anon. Clinical Guidance for the Integration of the Findings of the Chronic Hypertension and Pregnancy (CHAP) Study. American College of Obstetricians and Gynecologists. 2022
Khedagi AM, Bello NA. Hypertensive disorders of pregnancy. Cardiol Clin. 2021;39:77–90.
Association AD. 13. Management of diabetes in pregnancy: Standards of Medical Care in Diabetes—2018. Diabetes Care. 2018;41:S137–S143. Available at: https://care.diabetesjournals.org/content/41/Supplement_1/S137. Accessed November 4, 2021.
Anon. Guidelines: Management of diabetes from preconception to the postnatal period: summary of NICE guidance. BMJ Br Med J. 2008;336:714. Available at: /pmc/articles/PMC2276266/. Accessed November 4, 2021.
Nielsen LR, Ekbom P, Damm P, et al. HbA1c levels are significantly lower in early and late pregnancy. Diabetes Care. 2004;27:1200–1201. Available at: https://pubmed.ncbi.nlm.nih.gov/15111545/. Accessed November 4, 2021.
Verҫoza Viana L, Gross JL, Azevedo MJ. Dietary intervention in patients with gestational diabetes mellitus: a systematic review and meta-analysis of randomized clinical trials on maternal and newborn outcomes. Diabetes Care. 2014;37:3345–3355. Available at: https://pubmed.ncbi.nlm.nih.gov/25414390/. Accessed November 4, 2021.
Rowan JA, Hague WM, Gao W, Battin MR, Moore MP. Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med. 2008;358:2003–2015. Available at: https://pubmed.ncbi.nlm.nih.gov/18463376/. Accessed November 4, 2021.
Lundberg G, Mehta L. Familial hypercholesterolemia and pregnancy. Am Coll Cardiol. 2018. Available at: https://www.acc.org/latest-in-cardiology/articles/2018/05/10/13/51/familial-hypercholesterolemia-and-pregnancy. Accessed November 6, 2021.
Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:E1082–E1143. Available at: http://ahajournals.org. Accessed January 22, 2021.
Morton S, Thangaratinam S. Statins in pregnancy. Curr Opin Obstet Gynecol. 2013;25:433–440. Available at: https://journals.lww.com/co-obgyn/Fulltext/2013/12000/Statins_in_pregnancy.3.aspx. Accessed November 6, 2021.
Anon. Statins: Drug Safety Communication - FDA requests removal of strongest warning against using cholesterol-lowering statins during pregnancy . U.S. Food Drug Adm. 2021. Available at: https://www.fda.gov/safety/medical-product-safety-information/statins-drug-safety-communication-fda-requests-removal-strongest-warning-against-using-cholesterol. Accessed November 27, 2021.
Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. Available at: https://www.nejm.org/doi/10.1056/NEJMoa1615664. Accessed January 22, 2021.
Goldberg AC, Hopkins PN, Toth PP, et al. Familial hypercholesterolemia: screening, diagnosis and management of pediatric and adult patients: clinical guidance from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol. 2011;5:S1–S8. Available at: https://pubmed.ncbi.nlm.nih.gov/21600525/. Accessed November 23, 2021.
Buschur E, Kim C. Guidelines and interventions for obesity during pregnancy. Int J Gynaecol Obstet. 2012;119:6. Available at: /pmc/articles/PMC4151459/. Accessed November 1, 2021.
Anon. Obesity in pregnancy: ACOG Practice Bulletin Summary, Number 230. Obstet Gynecol. 2021;137:1137–9.
Anon. Exercise during pregnancy. ACOG. Available at: https://www.acog.org/womens-health/faqs/exercise-during-pregnancy. Accessed November 23, 2021.
Anon. Weight gain during pregnancy | ACOG. Available at: https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2013/01/weight-gain-during-pregnancy. Accessed November 1, 2021.
Siu AL. Behavioral and pharmacotherapy interventions for tobacco smoking cessation in adults, including pregnant women: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;163:622–634. Available at: https://pubmed.ncbi.nlm.nih.gov/26389730/. Accessed November 3, 2021.
McCowan LME, Dekker GA, Chan E, et al. Spontaneous preterm birth and small for gestational age infants in women who stop smoking early in pregnancy: prospective cohort study. BMJ. 2009;338:1552. Available at: https://pubmed.ncbi.nlm.nih.gov/19325177/. Accessed November 2, 2021.
Turner E, Jones M, Vaz LR, Coleman T. Systematic review and meta-analysis to assess the safety of bupropion and varenicline in pregnancy. Nicotine Tob Res. 2019;21:1001–1010. Available at: https://pubmed.ncbi.nlm.nih.gov/29579233/. Accessed November 3, 2021.
Rockhill KM, Tong VT, Farr SL, Robbins CL, D’Angelo D V., England LJ. Postpartum smoking relapse after quitting during pregnancy: pregnancy risk assessment monitoring system, 2000-2011. J Women’s Health. 2016;25:480–488. Available at: https://pubmed.ncbi.nlm.nih.gov/26717489/. Accessed November 3, 2021.
Facco FL, Ouyang DW, Zee PC, Grobman WA. Development of a pregnancy-specific screening tool for sleep apnea. J Clin Sleep Med. 2012;8:389. Available at: /pmc/articles/PMC3407257/. Accessed November 2, 2021.
Laposky AD, Pemberton VL. Sleep-disordered breathing and pregnancy-related cardiovascular disease. https://home.liebertpub.com/jwh 2021;30:194–198. Available at: https://www.liebertpub.com/doi/abs/10.1089/jwh.2020.8869. Accessed November 1, 2021.
Palinski W. Effect of maternal cardiovascular conditions and risk factors on offspring cardiovascular disease. Circulation. 2014;129:2066–2077. Available at: https://www.ahajournals.org/doi/abs/10.1161/CIRCULATIONAHA.113.001805. Accessed November 27, 2021.
Perak AM, Lancki N, Kuang A, et al. Associations of maternal cardiovascular health in pregnancy with offspring cardiovascular health in early adolescence. JAMA. 2021;325:658–668. Available at: https://jamanetwork.com/journals/jama/fullarticle/2776329. Accessed November 27, 2021.
Anon. Optimizing postpartum care. ACOG 2016. Available at: https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2018/05/optimizing-postpartum-care. Accessed November 6, 2021.
Jowell AR, Sarma AA, Gulati M, et al. Interventions to mitigate risk of cardiovascular disease after adverse pregnancy outcomes: a review. JAMA Cardiol. 2021. Available at: https://jamanetwork.com/journals/jamacardiology/fullarticle/2785585. Accessed November 14, 2021.
Mylotte D, Pilote L, Ionescu-Ittu R, et al. Specialized adult congenital heart disease care: the impact of policy on mortality. Circulation. 2014;129:1804–1812. Available at: https://www.ahajournals.org/doi/abs/10.1161/CIRCULATIONAHA.113.005817. Accessed November 23, 2021.
Jones EJ, Hernandez TL, Edmonds JK, Ferranti EP. Continued disparities in postpartum follow-up and screening among women with gestational diabetes and hypertensive disorders of pregnancy: a systematic review. J Perinat Neonatal Nurs. 2019;33:136. Available at: /pmc/articles/PMC6485948/. Accessed November 6, 2021.
Brown HL, DeNicola N. Telehealth in maternity care. Obstet Gynecol Clin N Am. 2020;47:497–502. Available at: https://pubmed.ncbi.nlm.nih.gov/32762934/. Accessed November 7, 2021.
Hanach N, de Vries N, Radwan H, Bissani N. The effectiveness of telemedicine interventions, delivered exclusively during the postnatal period, on postpartum depression in mothers without history or existing mental disorders: a systematic review and meta-analysis. Midwifery. 2021;94. Available at: https://pubmed.ncbi.nlm.nih.gov/33360589/. Accessed November 7, 2021.
• DeNicola N, Grossman D, Marko K, et al. Telehealth interventions to improve obstetric and gynecologic health outcomes: a systematic review. Obstet Gynecol. 2020;135:371–382. Available at: https://pubmed.ncbi.nlm.nih.gov/31977782/. Accessed November 7, 2021. The findings of this paper highlight the effectiveness of telehealth interventions in improving obstetric outcomes while decreasing the need for high-risk office visits.
Hoppe KK, Williams M, Thomas N, et al. Telehealth with remote blood pressure monitoring for postpartum hypertension: a prospective single-cohort feasibility study. Pregnancy Hypertens. 2019;15:171–176. Available at: https://pubmed.ncbi.nlm.nih.gov/30825917/. Accessed November 7, 2021.
Hoppe KK, Thomas N, Zernick M, et al. Telehealth with remote blood pressure monitoring compared with standard care for postpartum hypertension. Am J Obstet Gynecol. 2020;223:585–588. Available at: https://pubmed.ncbi.nlm.nih.gov/32439388/. Accessed November 7, 2021.
Harrington CM, Sorour N, Troy S, et al. Postpartum hypertension and the role of postpartum clinics and digital health. Curr Treat Options Cardiovasc Med. 2021;23:1–13. Available at: https://link.springer.com/article/10.1007/s11936-021-00937-y. Accessed October 29, 2021.
Anon. ACOG Committee Opinion No. 736: Optimizing postpartum care. Obstet. Gynecol. 2018;131:e140–e150. Available at: https://journals.lww.com/greenjournal/Fulltext/2018/05000/ACOG_Committee_Opinion_No__736__Optimizing.42.aspx. Accessed October 5, 2021.
Sharma KJ, Kilpatrick SJ. Postpartum hypertension: etiology, diagnosis, and management. Obstet Gynecol Surv. 2017;72:248–252. Available at: https://pubmed.ncbi.nlm.nih.gov/28426127/. Accessed October 6, 2021.
Colaceci S, Giusti A, Chapin EM, et al. The difficulties in antihypertensive drug prescription during lactation: is the information consistent? Breastfeed. Med. 2015;10:468. Available at: /pmc/articles/PMC4683560/. Accessed October 6, 2021.
Ascarelli MH, Johnson V, McCreary H, Cushman J, May WL, Martin JN. Postpartum preeclampsia management with furosemide: a randomized clinical trial. Obstet Gynecol. 2005;105:29–33. Available at: https://pubmed.ncbi.nlm.nih.gov/15625138/. Accessed November 14, 2021.
Funai EF, Friedlander Y, Paltiel O, et al. Long-term mortality after preeclampsia. Epidemiology. 2005;16:206–215. Available at: https://pubmed.ncbi.nlm.nih.gov/15703535/. Accessed September 30, 2021.
McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. 2008;156:918–930. Available at: https://pubmed.ncbi.nlm.nih.gov/19061708/. Accessed September 30, 2021.
Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. Br Med J. 2007;335:974–977. Available at: https://pubmed.ncbi.nlm.nih.gov/17975258/. Accessed September 30, 2021.
Ogunwole SM, Chen X, Mitta S, et al. Interconception care for primary care providers: consensus recommendations on preconception and postpartum management of reproductive-age patients with medical comorbidities. Mayo Clin Proc Innov Qual Outcomes. 2021;5:872. Available at: /pmc/articles/PMC8452893/. Accessed November 14, 2021.
Benschop L, Duvekot JJ, Lennep JER van. Future risk of cardiovascular disease risk factors and events in women after a hypertensive disorder of pregnancy. Heart. 2019;105:1273–1278. Available at: https://heart.bmj.com/content/105/16/1273. Accessed September 30, 2021.
Sharma G, Hays AG, Blumenthal RS. Can we reduce premature mortality associated with hypertensive disorders of pregnancy?: a window of opportunity. J Am Coll Cardiol 2021;77:1313–1316. https://doi.org/10.1016/j.jacc.2021.01.021. Accessed October 29, 2021.
O’Dwyer V, O’Higgins A, Kent E, Daly S, Kingsley B, Turner M. 245: Postpartum dyslipidemia is highly prevalent in women with gestational diabetes mellitus. Am J Obstet Gynecol. 2013;208:S113. Available at: http://www.ajog.org/article/S0002937812014937/fulltext. Accessed November 7, 2021.
Xiao H, Pei L, Li Z, Huang X, Yue S, Cao X. Pregnant dyslipidemia affects postpartum metabolism in gestational diabetes mellitus patients. Diabetes. 2020;69:1370-P. Available at: https://diabetes.diabetesjournals.org/content/69/Supplement_1/1370-P. Accessed November 7, 2021.
Pei L, Xiao H, Lai F, et al. Early postpartum dyslipidemia and its potential predictors during pregnancy in women with a history of gestational diabetes mellitus. Lipids Health Dis. 2020;19:1–8. Available at: https://lipidworld.biomedcentral.com/articles/10.1186/s12944-020-01398-1. Accessed November 7, 2021.
Colman GJ, Joyce T. Trends in smoking before, during, and after pregnancy in ten states. Am J Prev Med. 2003;24:29–35. Available at: https://pubmed.ncbi.nlm.nih.gov/12554021/. Accessed November 3, 2021.
Orton S, Coleman T, Coleman-Haynes T, Ussher M. Predictors of postpartum return to smoking: a systematic review. Nicotine Tob Res. 2018;20:665–673. Available at: https://pubmed.ncbi.nlm.nih.gov/29065203/. Accessed November 3, 2021.
Street LM, Aschenbrenner CA, Houle TT, Pinyan CW, Eisenach JC. Gestational obstructive sleep apnea: biomarker screening models and lack of postpartum resolution. J Clin Sleep Med. 2018;14:549. Available at: /pmc/articles/PMC5886432/. Accessed November 7, 2021.
Funding
Dr. Sharma is supported by R03HD104888 and Blumenthal Scholarship in Preventive Cardiology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Evidence-Based Medicine, Clinical Trials and Their Interpretations
Rights and permissions
About this article
Cite this article
Thakkar, A., Hailu, T., Blumenthal, R.S. et al. Cardio-Obstetrics: the Next Frontier in Cardiovascular Disease Prevention. Curr Atheroscler Rep 24, 493–507 (2022). https://doi.org/10.1007/s11883-022-01026-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11883-022-01026-6